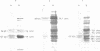Abstract
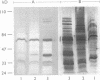
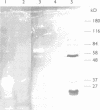
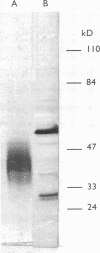
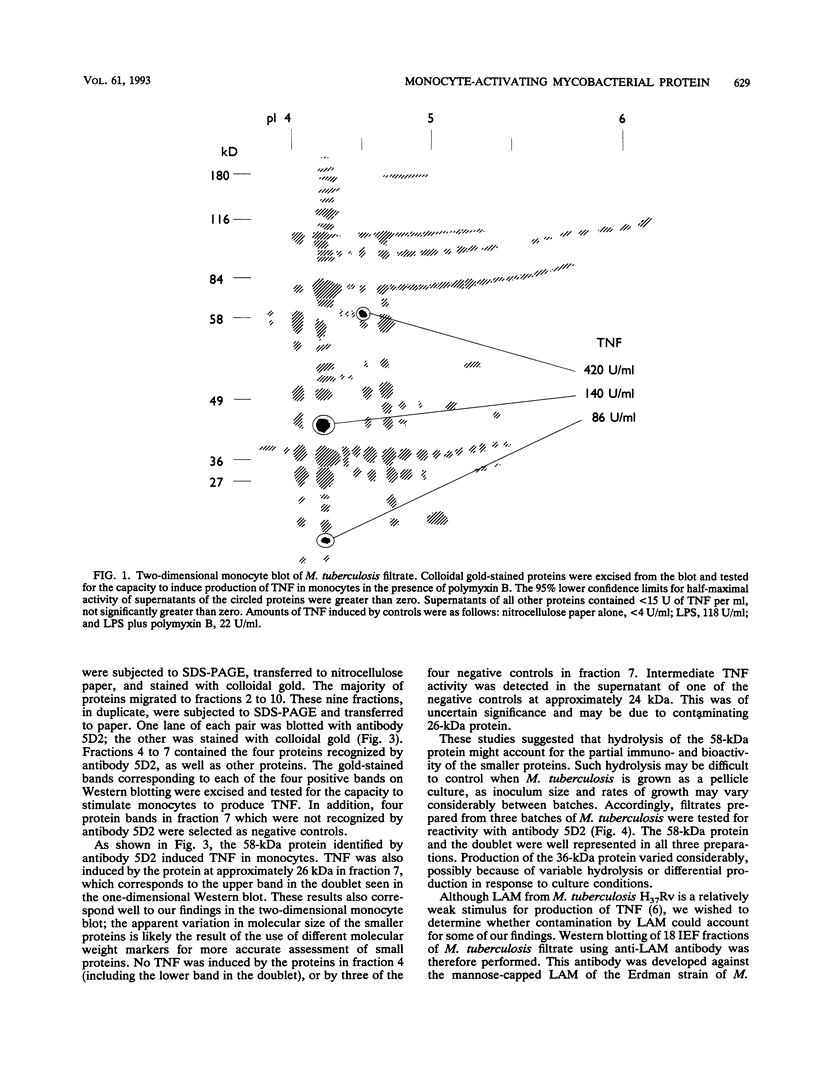
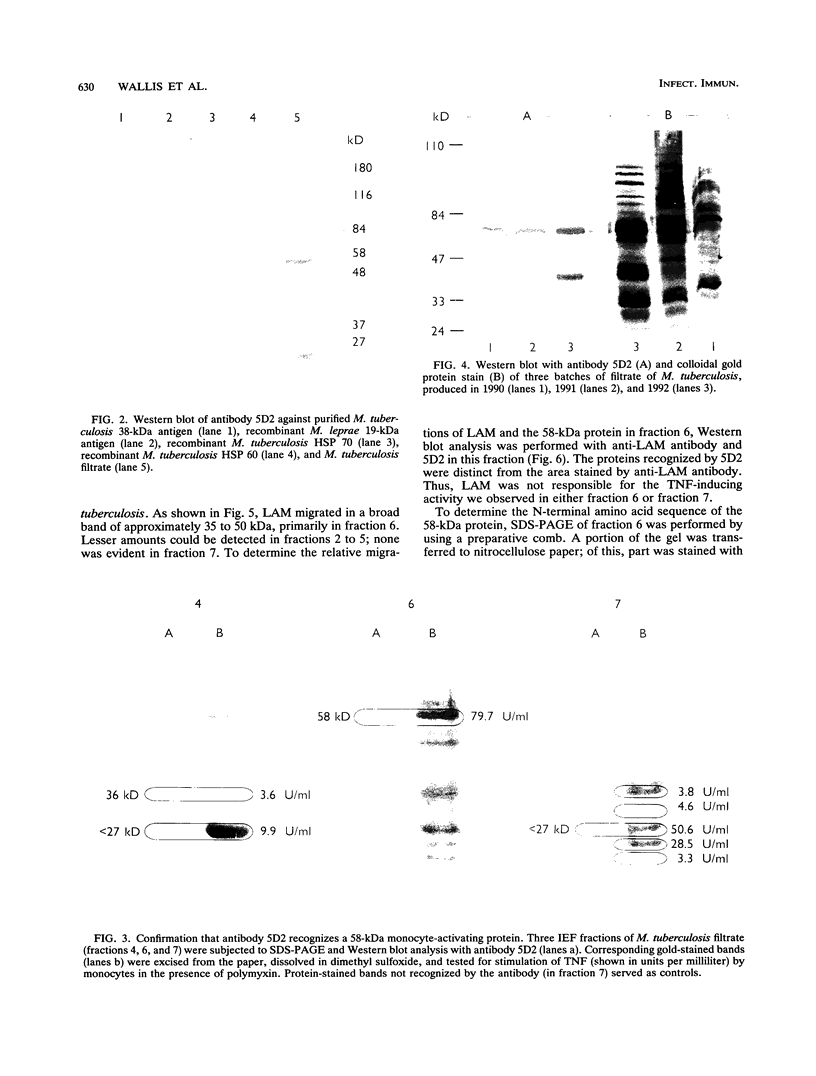
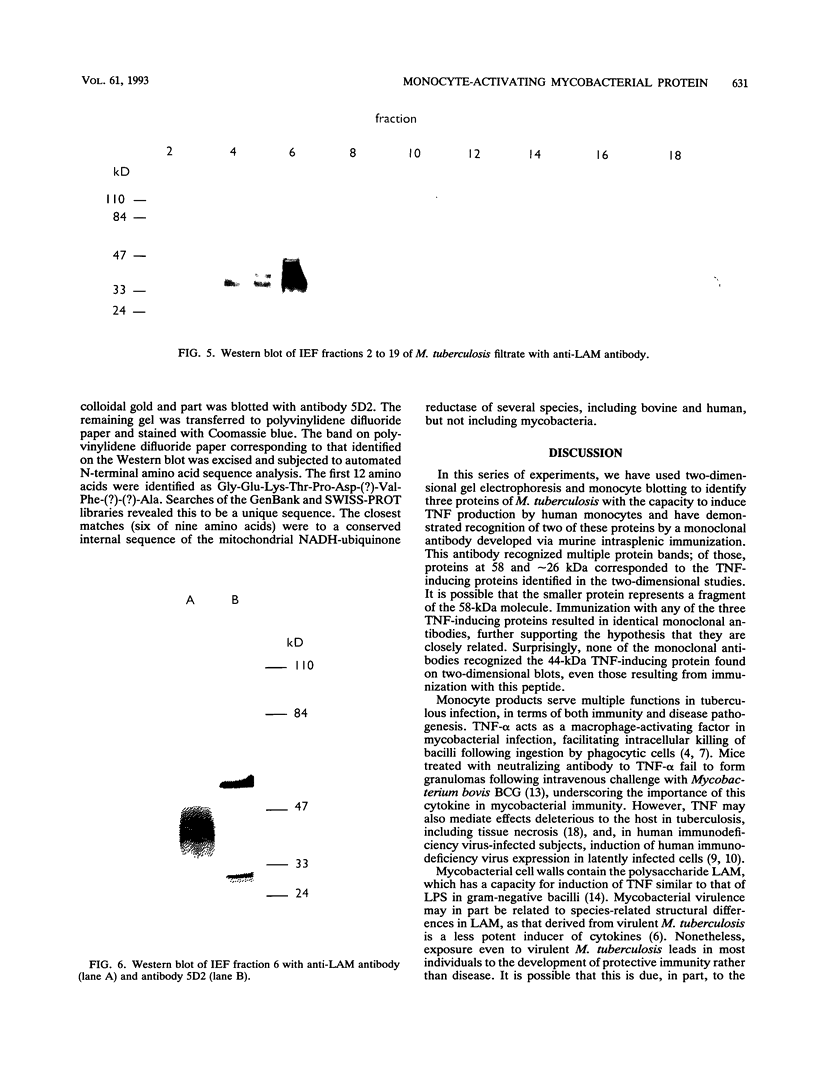
We have previously identified proteins in fractions of culture filtrate of Mycobacterium tuberculosis with the capacity to induce cytokine production in monocytes, by using a technique we defined as "monocyte Western blotting" (immunoblotting). In this series of experiments, we have extended this technique to two-dimensional gel electrophoresis and have identified a novel 58-kDa protein of M. tuberculosis which induces production of tumor necrosis factor by human monocytes. Nitrocellulose particles bearing this protein were used to develop murine monoclonal antibodies by the technique of intrasplenic immunization. The protein was purified by preparative isoelectric focusing and gel electrophoresis and subjected to N-terminal amino acid sequence analysis. As tumor necrosis factor is a mediator of both pulmonary necrosis and macrophage activation for intracellular killing, this 58-kDa protein may play an important role both in the immunopathogenesis of tuberculosis and in mycobacterial immunity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Fong S. J., Brennan P. J., Twomey P. E., Mazumder A., Modlin R. L. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990 Jul 1;145(1):149–154. [PubMed] [Google Scholar]
- Bermudez L. E., Kolonoski P., Young L. S. Natural killer cell activity and macrophage-dependent inhibition of growth or killing of Mycobacterium avium complex in a mouse model. J Leukoc Biol. 1990 Feb;47(2):135–141. doi: 10.1002/jlb.47.2.135. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Activities of amikacin, roxithromycin, and azithromycin alone or in combination with tumor necrosis factor against Mycobacterium avium complex. Antimicrob Agents Chemother. 1988 Aug;32(8):1149–1153. doi: 10.1128/aac.32.8.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S., Gupta S. 1,25 Dihydroxyvitamin D3-dependent inhibition of growth or killing of Mycobacterium avium complex in human macrophages is mediated by TNF and GM-CSF. Cell Immunol. 1990 May;127(2):432–441. doi: 10.1016/0008-8749(90)90144-g. [DOI] [PubMed] [Google Scholar]
- Chatterjee D., Roberts A. D., Lowell K., Brennan P. J., Orme I. M. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect Immun. 1992 Mar;60(3):1249–1253. doi: 10.1128/iai.60.3.1249-1253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Involvement of cytokines in determining resistance and acquired immunity in murine tuberculosis. J Leukoc Biol. 1991 Nov;50(5):495–501. doi: 10.1002/jlb.50.5.495. [DOI] [PubMed] [Google Scholar]
- Duff G. W., Atkins E. The inhibitory effect of polymyxin B on endotoxin-induced endogenous pyrogen production. J Immunol Methods. 1982 Aug 13;52(3):333–340. doi: 10.1016/0022-1759(82)90005-9. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Schnittman S. M., Poli G., Koenig S., Pantaleo G. NIH conference. Immunopathogenic mechanisms in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1991 Apr 15;114(8):678–693. doi: 10.7326/0003-4819-114-8-678. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Dinarello C. A., Fauci A. S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987 Nov 6;238(4828):800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Jacobs D. M., Morrison D. C. Inhibition of the mitogenic response to lipopolysaccharide (LPS) in mouse spleen cells by polymyxin B. J Immunol. 1977 Jan;118(1):21–27. [PubMed] [Google Scholar]
- Kim S. J., Kim H. I., Lee Y. H., Kim S. K. Production of tumor necrosis factor-alpha by alveolar macrophages from patients with pulmonary tuberculosis. J Korean Med Sci. 1991 Mar;6(1):45–53. doi: 10.3346/jkms.1991.6.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Moreno C., Taverne J., Mehlert A., Bate C. A., Brealey R. J., Meager A., Rook G. A., Playfair J. H. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumour necrosis factor from human and murine macrophages. Clin Exp Immunol. 1989 May;76(2):240–245. [PMC free article] [PubMed] [Google Scholar]
- Nilsson B. O., Svalander P. C., Larsson A. Immunization of mice and rabbits by intrasplenic deposition of nanogram quantities of protein attached to Sepharose beads or nitrocellulose paper strips. J Immunol Methods. 1987 May 4;99(1):67–75. doi: 10.1016/0022-1759(87)90033-0. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Uchida H., Kusumoto Y., Mori Y., Yamamura Y., Hamada S. Increase in tumor necrosis factor alpha- and interleukin-6-secreting cells in peripheral blood mononuclear cells from subjects infected with Mycobacterium tuberculosis. Infect Immun. 1991 Sep;59(9):3021–3025. doi: 10.1128/iai.59.9.3021-3025.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein J. L., Schreiber H. Synergy between tumor necrosis factor and bacterial products causes hemorrhagic necrosis and lethal shock in normal mice. Proc Natl Acad Sci U S A. 1988 Jan;85(2):607–611. doi: 10.1073/pnas.85.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima T., Ueta C., Tsuyuguchi I., Kishimoto S. Production of tumor necrosis factor alpha by monocytes from patients with pulmonary tuberculosis. Infect Immun. 1990 Oct;58(10):3286–3292. doi: 10.1128/iai.58.10.3286-3292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valone S. E., Rich E. A., Wallis R. S., Ellner J. J. Expression of tumor necrosis factor in vitro by human mononuclear phagocytes stimulated with whole Mycobacterium bovis BCG and mycobacterial antigens. Infect Immun. 1988 Dec;56(12):3313–3315. doi: 10.1128/iai.56.12.3313-3315.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S., Alde S. L., Havlir D. V., Amir-Tahmasseb M. H., Daniel T. M., Ellner J. J. Identification of antigens of Mycobacterium tuberculosis using human monoclonal antibodies. J Clin Invest. 1989 Jul;84(1):214–219. doi: 10.1172/JCI114143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S., Amir-Tahmasseb M., Ellner J. J. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte western blot. Proc Natl Acad Sci U S A. 1990 May;87(9):3348–3352. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S., Fujiwara H., Ellner J. J. Direct stimulation of monocyte release of interleukin 1 by mycobacterial protein antigens. J Immunol. 1986 Jan;136(1):193–196. [PubMed] [Google Scholar]
- Wallis R. S. PROBIT: a computer program analysis. J Immunol Methods. 1991 Dec 15;145(1-2):267–268. doi: 10.1016/0022-1759(91)90338-g. [DOI] [PubMed] [Google Scholar]