Abstract
We describe the case of a 71-year-old man with idiopathic pulmonary fibrosis (usual interstitial pneumonia (UIP) pattern) diagnosed on clinical, radiological and lung function criteria, in accordance with the American Thoracic Society/European Respiratory Society consensus criteria (2000), who had been in close proximity to three atmospheric nuclear bomb blasts during military service in 1957. He does not have clubbing and clinically and radiologically his lung disease is stable. He also has bladder carcinoma and carotid arteriosclerosis, both recognised consequences of radiation injury. This is the first reported case of UIP in a nuclear test veteran. Awareness of this potential association is important given the current attempts of the British Nuclear Test Veterans Association to gain compensation for claimed injuries.
Background
Radiation injury to the thorax is a well-recognised cause of acute lung injury and, in the context of radiotherapy causes an early local pneumonitis with scarring developing up to several months or years later. Prior to this report, however, there have been no accounts of pulmonary fibrosis, with characteristic radiological features of usual interstitial pneumonia (UIP), occurring in military personnel stationed at World War II nuclear test sites.1 This case adds to the list of potential health problems encountered by nuclear test site veterans.
Case presentation
We report the case of a 71-year-old retired Royal Navy engineer who presented with a dry cough, mild exertional breathlessness and an intercurrent lower respiratory tract infection. He had stopped smoking 30 years earlier, with a 10 pack-year history. He had no recognised exposure to asbestos, organic particles or drugs known to cause pulmonary fibrosis. He had no features of connective tissue disease, in particular no gastro-oesophageal reflux, Raynaud's, arthralgia or photosensitivity. There was no family history of auto-immune disease or pulmonary fibrosis. His history included hypertension, gout, carotid endarterectomy and bladder carcinoma that had been treated over many years with intravesicle diathermy and BCG. Of interest, prior to his multiple cystoscopies, which had been conducted at regular intervals over the previous 20 years, he had always been noted to have bibasal coarse inspiratory crepitations.
In 1957 our patient was a Royal Naval serviceman stationed near Malden Island, Kiribati in the Pacific Ocean and witnessed on open deck three nuclear test explosions, test names ‘Short Granite’, ‘Orange Herald’ and ‘Purple Granite’, all part of the ‘Grapple’ series of tests. The ship, HMS Warrior, was located 20 nautical miles from the test site; the servicemen were provided with dosimeters but no personal protection and witnessed the blast on open deck (figure 1). The largest bomb was 720 kiloton in size and all detonations occurred between 2200 and 2400 metres above sea level. The initial blast was sufficiently intense to knock the servicemen off their feet.
Figure 1.

A photograph of naval servicemen witnessing an atomic bomb test while on open deck, wearing dosimeters but no personal shielding.
Examination revealed neck scars consistent with his previous carotid surgery and marked mid and lower zone inspiratory ‘Velcro-like’ crepitations only. There was no digital clubbing. The remainder of the examination was normal.
Investigations
Thoracic high resolution CT at the upper, mid and lower zones (figures 2A–C, respectively) demonstrated mild centrilobular emphysema, but in addition subpleural reticulation (arrowhead) with traction bronchiolectasis (arrow) and honeycombing most marked at the lung bases (curved arrow) was seen. These findings are in keeping with a UIP pattern of pulmonary fibrosis.
Figure 2.
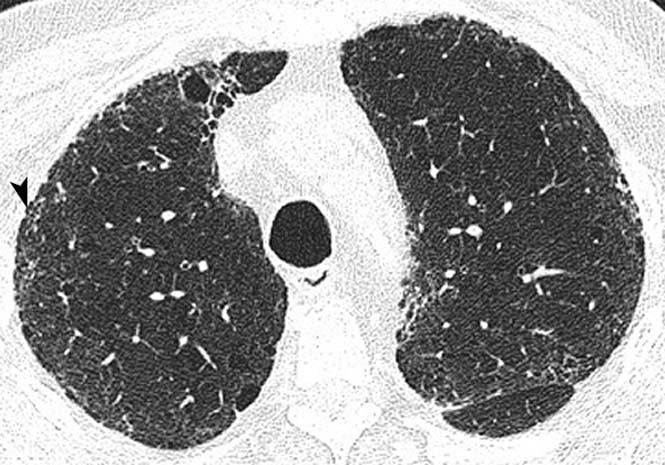
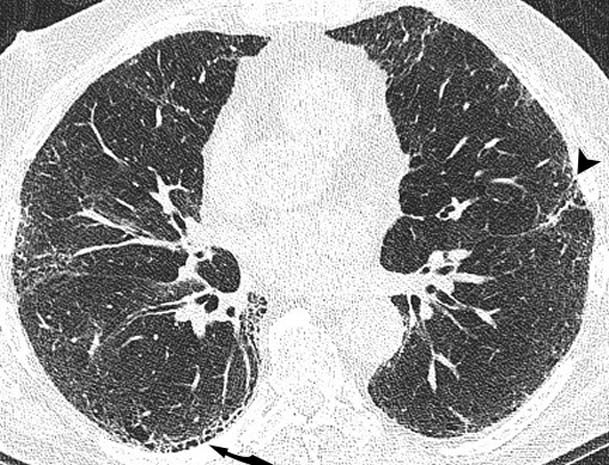
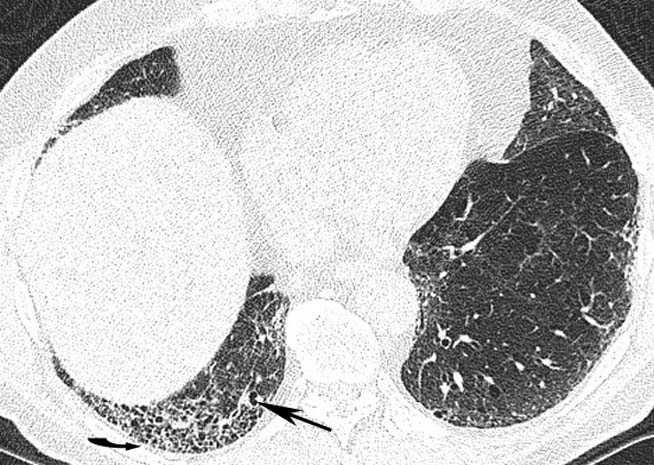
Thoracic high-resolution CT scan. The upper (A), mid (B) and lower (C) lung zones demonstrate mild centrilobular emphysema, and in addition subpleural reticulation (arrow head) with traction bronchiolectasis (arrow) and honeycombing (curved arrow).
Lung function testing showed a mild restrictive ventilatory defect with a total lung capacity of 84% predicted, residual volume of 76% predicted and marked impairment of gas transfer with a TLCO and KCO of 50% and 71% predicted, respectively. The FEV1/VC and flow-volume loop were normal.
Erythrocyte sedimentation rate was normal and auto-antibodies including anti-nuclear antibodies, rheumatoid factor, anti-cyclic citrullinated peptide antibodies and anti-neutrophil cytoplasmic antibodies were negative. There was no evidence of pulmonary hypertension on echocardiogram.
Differential diagnosis
The differential diagnosis included idiopathic pulmonary fibrosis, radiation induced pulmonary fibrosis, combined emphysema and pulmonary fibrosis and fibrotic non-specific interstitial pneumonia.
Treatment
Long term monitoring was instituted. Treatment was not indicated given the stability of his disease over 12 months.
Outcome and follow-up
There has been no interval change in this patient's symptoms or lung function over a 12-month follow-up period. He is currently being re-assessed for a war pension, which was previously denied to him.
Discussion
The effects of acute high level radiation exposure are well documented as are the longer term effects of radiation on the incidence of lung, breast, salivary gland, thyroid and haematological malignancy.2 Keloid scar formation (secondary to the flashburn effects of nuclear bombs), cataract, infertility and cardiovascular disease are also highly prevalent in Hiroshima (Ur-235) and Nagasaki (Pl-239) survivors.3 4
Ascertaining the long term effects of whole body radiation on the lung is more difficult. Radiation-induced mutations in the conserved region of the p53 tumour suppressor gene are well recognised and likely to be implicated in the greater incidence of cancer.5 Likewise, use of multicolour fluorescent in situ hybridisation (mFISH) to investigate chromosome abnormalities in New Zealand nuclear test veterans (compared to carefully matched non-exposed individuals) has shown significantly higher (p<0.0001) frequencies of chromosomal abnormalities (275 translocations and 12 dicentrics in 9360 cells versus 96 translocations and 1 dicentric in 9548 cells in the controls), as well as a significant excess of complex chromosomal rearrangements in the veterans.6 Others however have argued that a single non-fatal total body irradiation dose does not produce permanent or chronic somatic damage.7
The incidence of UIP is less certain, although poorly validated reports of ‘lung fibrosis’ in the Hiroshima bomb survivors have suggested an incidence of 0.8%, equating to an OR of 2.01 over the non-exposed population.8 Plutonium (Pu-239) inhalation at an annual lung dose of >10 mSv is more firmly associated with lung fibrosis (‘plutonium pneumosclerosis’) and there are similar reports for workers in the USA involved in underground uranium mining during 1942–1971. Interestingly, intrapleural administration of radioisotopes (eg, Au-198) produces a pleural reaction with reportedly little collateral lung damage.9 Despite the above data, the Radiation Exposure Compensation Act passed by the US Congress in 1990 does not list lung fibrosis as a compensable injury in ‘onsite’ observers of the Nevada, Pacific, Trinity or South Atlantic atmospheric nuclear test sites.
It is also recognised that the damage produced in the lung is less of a function of the total dose of radiation, more the rate at which the dose is delivered. Hence fractionation of a dose appears to reduce the severity of lung injury. The extent of lung damage at least in the acute setting can also vary widely between individuals. In patients receiving radiotherapy for breast or lung cancer or lymphoma, where normal tissue in unavoidably captured, three phases of lung injury are recognised, including an acute phase, radiation pneumonitis and late radiation fibrosis.10 In such cases pulmonary fibrosis is usually well established by 12 months. Radiation therapy to the lung has also been reported to cause reactivation of pulmonary tuberculosis, pleural effusion, secondary pneumothorax, rib fractures and tracheoesophageal fistula.10
Estimating the precise radiation exposure in our patient is difficult. Data derived from the British Nuclear Test Veterans Association (http://www.bntva.com/history/history.htm) indicate that our patient was 20 miles away from three airburst (2200–2400 m) detonations ranging in size from 200 to 720 kiloton. Support for this claim and the lack of personal shielding comes from contemporary photographs (figure 1). A moratorium on such atmospheric nuclear tests was announced in 1958. We speculate that the close proximity of our patient to three large nuclear detonations and the fact that he has two other conditions well recognised to be associated with radiation injury (bladder carcinoma and atherosclerosis of a major artery), together with the chronicity of his pulmonary fibrosis all support a causal link. The Ministry of Defence have reported that on-board dosimetry showed background levels of radiation only and, in contrast to the USA (1990 Radiation Exposure Compensation Act), have denied compensation for British Servicemen (see 1998 McGinley and Egan v The United Kingdom, European Court of Human Rights, http://www.hrcr.org/safrica/access_information/ECHR/mcginley_egan.html). Such dosimetry however may have grossly underestimated the exposure to α-particles and the later exposure to contaminated dust, rain and sea water.
Learning points.
-
▶
Pulmonary fibrosis may result from acute radiation injury and should be suspected in individuals exposed to high level whole body radiation.
-
▶
Pulmonary fibrosis, especially if occurring secondary to a defined insult, can be associated with stable symptoms and preserved lung function.
-
▶
Careful history taking in patients with idiopathic pulmonary fibrosis/usual interstitial pneumonia is important to identify patients with secondary causes.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS) and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161(2 Pt 1):646–64 [DOI] [PubMed] [Google Scholar]
- 2.Little MP. Cancer and non-cancer effects in Japanese atomic bomb survivors. J Radiol Prot 2009;29(2A):A43–59 [DOI] [PubMed] [Google Scholar]
- 3.Yamada M, Wong FL, Fujiwara S, et al. Noncancer disease incidence in atomic bomb survivors, 1958-1998. Radiat Res 2004;161:622–32 [DOI] [PubMed] [Google Scholar]
- 4.Shimizu Y, Kodama K, Nishi N, et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950-2003. BMJ 2010;340:b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeshima Y, Seyama T, Bennett WP, et al. p53 mutations in lung cancers from non-smoking atomic-bomb survivors. Lancet 1993;342:1520–1 [DOI] [PubMed] [Google Scholar]
- 6.Wahab MA, Nickless EM, Najar-M'kacher R, et al. Elevated chromosome translocation frequencies in New Zealand nuclear test veterans. Cytogenet Genome Res 2008;121:79–87 [DOI] [PubMed] [Google Scholar]
- 7.Vodopick H, Andrews GA. Accidental radiation exposure. Arch Environ Health 1974;28:53–6 [DOI] [PubMed] [Google Scholar]
- 8.Masaaki K, Ken O, Hiromi K, et al. Examination of pulmonary fibrosis in atomic bomb victims. Genshi Bakudango Shogai Kenkyukai Tokushugo 2002;43:210–14 [Google Scholar]
- 9.Kniseley RM, Andrews GA. Pathological changes following intracavitary therapy with colloidal Au198. Cancer 1953;6:303–12 [DOI] [PubMed] [Google Scholar]
- 10.Gross NJ. Pulmonary effects of radiation therapy. Ann Intern Med 1977;86:81–92 [DOI] [PubMed] [Google Scholar]


