Abstract
An 80-year-old Caucasian woman had been diagnosed with right herpes zoster ophthalmicus 2 ½ weeks before presentation to our department. Ten days after stopping oral aciclovir, she presented with periorbital pain, visual loss, ptosis and complete ophthalmoplegia. On examination, visual acuity in her right eye was hand movements, with a relative afferent pupillary defect and 2 mm proptosis. MRI demonstrated contrast enhancement within the orbit extending into the apex, suggestive of an inflammatory process. Oral treatment was started with oral aciclovir and corticosteroids for 2 months, when she had resolution of the optic neuropathy and ophthalmoplegia. Vision recovered to 6/9 and repeat neuroimaging revealed regression of the inflammatory process.
Background
A better understanding of the immune and inflammatory responses taking place in orbital apex syndrome (OAS) associated with herpes zoster ophthalmicus (HZO) and how to modify them appropriately is critical to improve our management. Several questions remain unanswered:
Is there continuous viral replication or is it mainly an auto-immune process?
Is the severity of the symptoms related to the amount of viral shedding?
How long should the treatment of OAS be maintained for?
Can OAS secondary to HZO be prevented?
Case presentation
An 80-year-old Caucasian woman presented to the eye department in Maidstone Hospital, Kent, UK, with loss of vision in the right eye, complete ptosis and ophthalmoplegia with abrupt onset and progression over 5 days. She described 4 weeks of ongoing ‘excruciating’ pain behind the right eye, and 2 ½ weeks of skin rash around the eye and on the right side of the forehead, consistent with the diagnosis of HZO. Treatment with oral aciclovir (800 mg, five times a day) had been started within 24 h of skin rash onset and maintained for 1 week, with improvement of her symptoms. She denied any past ocular history of trauma, surgery or inflammatory disease. Her medical history was significant for migraine, essential hypertension and hypercholesterolaemia, which were well controlled on doxazosin, bendrofluazide and simvastatin. For 10 days prior to presentation, she had been off antiviral medication.
On examination, the patient appeared well, and there was complete ptosis on the right side. An erythematous pustular rash with no vesicles could be seen in the dermatomal distribution of the first (ophthalmic, V1) and second (maxillary, V2) divisions of the fifth (trigeminal, V) cranial nerve respecting the midline, with signs of cutaneous involvement of the nasociliary nerve (Hutchinson's sign). Visual acuity in her right eye was hand movements with an afferent pupillary defect and 2 mm proptosis. The anterior chamber showed signs of inflammation with associated elevation of the intraocular pressure (IOP) (28 mm Hg). The visual acuity in the left eye was 6/9, the anterior chamber quiet and the IOP 18 mm Hg. The irides in both eyes were normal. Both lenses showed nucleosclerotic changes. Dilated fundal examination demonstrated the right optic disc to be paler than the left. Both maculae and peripheral fundi were normal. In association with optic nerve dysfunction, there was complete ophthalmoplegia (figure 1A).
Figure 1.
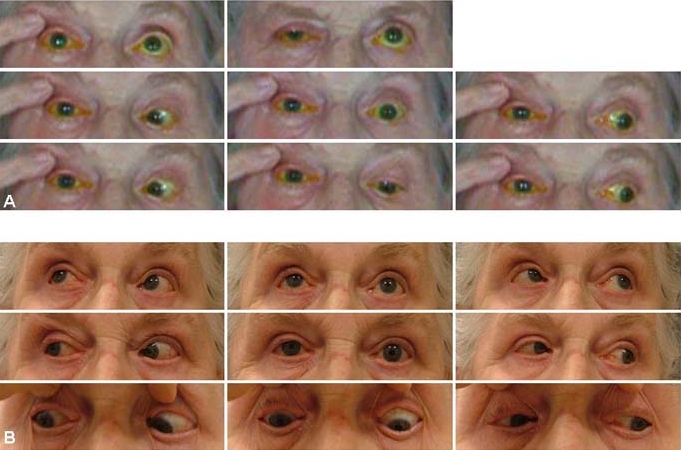
(A) Complete ophthalmoplegia accompanying complete ptosis on presentation. The patient experienced severe discomfort when asked to move her eyes and examination of some of the extreme positions of gaze was not possible. (B) Ocular movements nearly completely recovered 6 months later.
Investigations
Laboratory testing for inflammation and infection (including erythrocyte sedimentation rate (ESR), C reactive protein (CRP), full blood count with differential, angiotensin converting enzyme (ACE), perinuclear and cytoplasmic antineutrophil cytoplasmic antibody (ANCA)) and chest x-ray were normal. High resolution MRI with contrast and fat suppression sequences was also obtained (figure 2).
Figure 2.
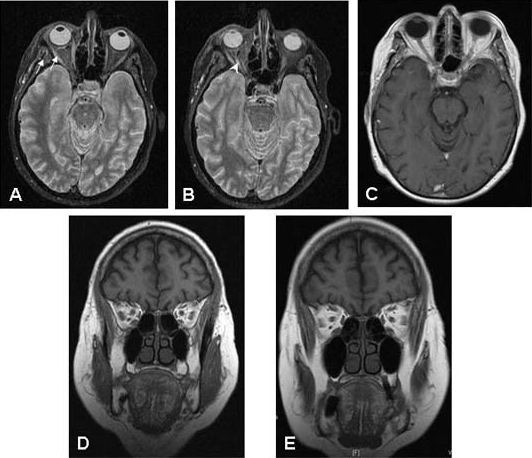
(A, B) Axial fat suppressed MRI showing high signal within the right lateral and medial extraocular muscles (A, arrows) and optic nerve (B, arrowhead) on presentation. (C) Six weeks after starting treatment with oral prednisolone and aciclovir, the signs of inflammation within the orbit, extraocular muscles and optic nerve had resolved. (D) Coronal T-2 weighted MRI on presentation. (E) Six weeks after starting on systemic treatment, coronal T-2 weighted MRI confirms that the inflammatory changes in the orbit, extraocular muscles and around the optic nerve have resolved.
Differential diagnosis
The diagnosis of right OAS secondary to ipsilateral HZO was made. Other potential causes (eg, idiopathic orbital inflammation, thyroid eye disease, lymphoproliferative disorders) were evaluated and ruled out. Due to the ipsilateral HZO preceding the ophthalmoplegia by 2 ½ weeks, the absence of similar episodes in the past and the patient's age, it was considered her orbital signs and symptoms were related to varicella zoster virus (VZV).
Treatment
The patient was admitted to hospital. Oral treatment with aciclovir 800 mg five times per day, prednisolone (60 mg daily for the first 3 days, then gradual reduction to 5 mg) and co-codamol was started, in combination with topical dexamethasone (0.3% four times daily), cyclopentolate (1% twice daily) and timolol (0.25% twice daily). She was discharged 1 week later as her symptoms were improving.
Outcome and follow-up
Oral treatment with steroids and antiviral medication was maintained for 2 months, when the patient had resolution of the optic neuropathy and improvement of eye movements. Ocular motility was nearly completely recovered 6 months later (figure 3). Vision recovered to 6/9 and repeat neuroimaging revealed regression of the inflammatory process (figure 2B,C,E). Topical treatment with steroids was continued for 6 months. The mechanisms involved in the development of OAS secondary to HZO are poorly understood. Treatment with systemic steroids and antivirals has been proven to be effective and with good long term prognosis. Patients with HZO should be prescribed oral antiviral drugs at the first sign of VZV reactivation, in order to try to prevent the development of secondary complications. Moreover, in older patients prolonged treatment for more than 10 days may have an effect on the incidence of complications.
Figure 3.
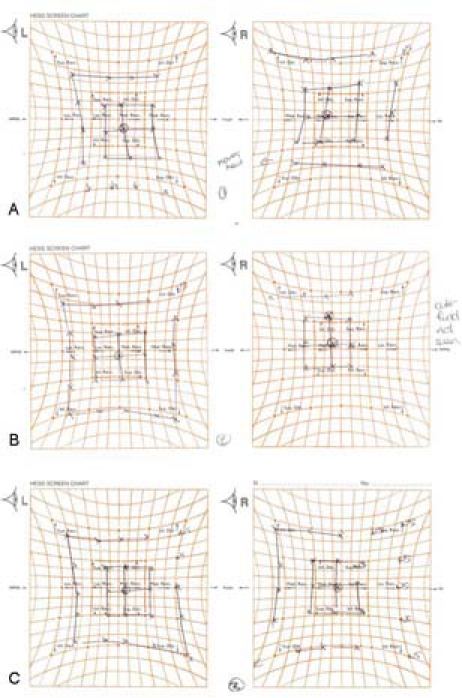
Serial Hess charts show improvement of eye movements 2 months after starting treatment (A) with oral steroids and antivirals and near complete recovery 6 months later (C).
Discussion
OAS has been described as a syndrome involving damage to the oculomotor nerve (III), trochlear nerve (IV), abducens nerve (VI) and V1 in association with optic nerve dysfunction. This syndrome may be caused by inflammatory, infectious, neoplastic, traumatic or vascular processes.1 OAS is very rarely associated with acute ophthalmic zoster, with less than 20 cases reported in the literature to date.2–7 We reviewed these cases to determine the spectrum of clinical presentation, disease, diagnostic features, treatments used and outcomes. All patients were over the age of 50. The typical signs and symptoms included reduced visual acuity, proptosis, ptosis, mydriasis and complete ophthalmoplegia. Myositis of extraocular muscles associated with HZO has been confirmed radiologically in three previous case reports.8–10 HZO preceded these symptoms by 2–60 days (mean 10 days) in most patients. Aciclovir and corticosteroids were administered orally or intravenously. Most patients had not received treatment before the onset of complete ophthalmoplegia. The prognosis of recovery was significant improvement typically seen within 2 months and complete or near resolution within 10 months. However, occasionally OAS can be associated with meningoencephalitis,11 a serious complication with considerable morbidity and mortality.
Evaluation of OAS
Neuroimaging with high resolution MRI with contrast and fat suppression sequences is the preferred imaging modality in OAS. CT scan also plays an important role in the setting of trauma or in patients with suspected magnetic foreign bodies, surgical clips or with other contraindications to MRI. If a vascular lesion is suspected, magnetic resonance angiography or CT angiography may be helpful.1 MRI revealed contrast enhancement within the orbit involving the superior, medial and inferior extraocular muscles and the optic nerve, extending into the apex. The appearance was suggestive of a diffuse inflammatory process, which led to the diagnosis of OAS secondary to HZO.
Laboratory testing for inflammation and infection should include ESR, CRP, full blood count with differential and ACE, as well as perinuclear and cytoplasmic ANCA levels. Blood cultures (for Mucormycosis, Aspergillogis, bacterial, Treponemal and/or HIV infection), lumbar puncture and neurosurgical or otorhinolaryingological consultation should be considered if the clinical findings are suggestive of a systemic or neurological process.
Pathogenesis of OAS associated with HZO
During an attack of zoster ophthalmicus, VZV (human herpes virus type 3) reactivates in the trigeminal ganglion due to a diminished virus-specific, cell-mediated immunity, which is related to age. The virus then migrates along the ophthalmic division (V1) of the trigeminal nerve and spreads to the corresponding dermatome. The V1 is purely sensory or afferent in function. It supplies branches to the ciliary body, the cornea and the iris; to the lacrimal gland and conjunctiva; to portions of the mucous membrane of the nasal cavity, sphenoidal sinus and frontal sinus; to the skin of the eyebrow, eyelids, forehead and nose; and to the tentorium cerebelli, dura mater and the posterior area of the falx cerebri. In addition, it supplies sensory and proprioceptive innervation to the extra-ocular muscles.
Following reactivation of VZV and spread along V1, direct tissue infection and an immune response may take place within the orbit. The immune response, the process for removing an offending stimulus, is both humoral and cellular. Neutralising antibody rises quickly, typically within 2 days of initiation of reactivation, reaching a peak at 2–3 weeks and declining to very low levels at a year.
The sequence of molecular and cellular events triggered by the immune response results in an inflammatory response with five clinical manifestations (ie, pain, hyperaemia, oedema, heat and loss of function). These clinical signs of inflammation reflect two main physiological changes: cellular recruitment (mediated by and resulting in the release of cytokines and lipid mediators) and altered vascular permeability.
In cases were HZO is associated with an inflammatory response in the orbit, this may involve the orbital apex (OAS), extraocular muscles (myositis), blood vessels (vasculitis) and/or nerve sheaths (perineuritis).11 The involvement of orbital tissue ipsilateral to the original cutaneous lesions supports the view that the pathogenesis is likely to be due to direct viral infection of orbital cells and consequent immune cell attack. Passive leakage of antibody followed by immune complex formation with tissue bound antigen, rather than deposition of circulating immune response complexed with bloodborne antigens, may play a role. Circulating immuno-complex mediated mechanisms could potentially involve contralateral orbital tissues.
If free antibodies passively leak out from the serum into the orbital tissue, they could combine with tissue bound VZV antigens trapped in the extracellular matrix or with cell-associated antigens such as viral protein expressed on the surface of infected cells. Tissue bound antigen-antibody complexes are capable of activating complement with release of chemotactic factors for neutrophils. The neutrophils then can infiltrate the area and release lysosomal enzymes that destroy the basement membrane of blood vessels resulting in vascular inflammation and injury (ie, vasculitis).
Endothelial cells have been suggested to play a pivotal role in the pathogenesis of vasculitis. Endothelial cells have significant pro-inflammatory activities, amplifying and perpetuating the inflammatory process and contributing to tissue injury. Further studies are necessary to determine whether VZV reactivation may potentiate endothelial cell activation and vessel damage in the orbit.
Vitamin D deficiency has been suggested to play a role in postherpetic neuralgia12 and it also has the theoretical potential of being involved in the pathogenesis of OAS secondary to HZO by increasing inflammation. Circulating 25 hydroxyvitamin D (25 (OH)D), an accurate measure of vitamin D status, is inversely related to tumour necrosis factor α (an inflammatory marker) concentrations in healthy women,13 suggesting a role of this vitamin in the prevention and/or treatment of inflammatory diseases. Further studies are necessary to establish whether vitamin D deficiency is a risk factor for OAS in these patients and whether vitamin D therapy could have a beneficial effect.
Uncertain issues
A better understanding of the immune and inflammatory responses taking place in OAS associated with HZO and how to modify them appropriately is critical to improve our management. Several questions remain unanswered:
Is there continuous viral replication or is it mainly an auto-immune process?
Is the severity of the symptoms related to the amount of viral shedding?
How long should the treatment of OAS be maintained for?
Can OAS secondary to HZO be prevented?
Management of OAS secondary to HZO
The approach to management is twofold. Systemic treatment with corticosteroids is based on their anti-inflammatory action. Their use should be adjuvant to systemic treatment with antivirals; aciclovir, a virustatic agent which interferes with viral thymidine kinase and DNA polymerase, is one of the most widely used. A new generation of more potent antivirals, famcliclovir and valaciclovir, is also available at a considerably higher cost. Oral antiviral therapy is recommended for 7–10 days.
Can OAS complication be prevented in acute HZO?
The severity of herpes zoster ophthalmicus and risk of complications could depend on the virulence of the virus, quantity of the virus and host resistance. Viral replication is short-lived and confined to the very onset of the disease. Therefore, antivirals have to be delivered very early to have any effect. Treatment started within 72 h after rash onset has been suggested to reduce the risk of ocular complications14 and postherpetic neuralgia.15 It seems reasonable to believe that early treatment might also have an effect on the incidence of OAS. The difficulty is early diagnosis of the disease. In some cases, even at the onset of the rash, virus reactivation might have been going on for days and much neuronal and tissue damage may have occurred, so antivirals may not achieved much benefit. This could have been the case with our patient. Reactivation of VZV could have started 1 week prior to the skin rash and antiviral treatment onset, when the severe pain behind the right eye was first noticed.
The recommended approach to the management of patients with acute HZO is to maintain systemic treatment for 7–10 days. Little is known about the efficacy of antiviral medication for more than 10 days on the incidence of OAS. Longer treatments might need to be considered in older patients, because their immune response is usually delayed, and consequently, viral shedding may last longer. Longer term therapy, hypothetically, could reduce the risk of OAS in susceptible patients (ie, older age, more severe pain at presentation, greater degree of skin surface involvement). Further investigations are necessary to determine whether long term therapy does prevent this serious complication. It should also be kept in mind that long term treatment with antiviral drugs can potentially contribute to the development of drug-resistant VZV strains, and there is a risk of toxicity.
The patient presented here was admitted to hospital. Oral treatment with aciclovir 800 mg five times a day, prednisolone (60 mg daily for the first 3 days, then gradual reduction to 5 mg) and co-codamol was started, in combination with topical dexamethasone (0.3% four times daily), cyclopentolate (1% twice daily) and timolol (0.25% twice daily). She was discharged 1 week later as her symptoms were improving. Oral treatment with steroids and antiviral medication was maintained for 2 months, when she had resolution of the optic neuropathy and improvement of eye movements. Ocular motility was nearly completely recovered 6 months later (figure 1B). Vision recovered to 6/9 and repeat neuroimaging revealed regression of the inflammatory process (figure 2B,C,E). Topical treatment with steroids was continued for 6 months. The mechanisms involved in the development of OAS secondary to HZO are poorly understood. Treatment with systemic steroids and antivirals has been proven to be effective and with good long term prognosis. Patients with HZO should be prescribed oral antiviral drugs at the first sign of VZV reactivation, in order to try to prevent the development of secondary complications. Moreover, in older patients prolonged treatment for more than 10 days may have an effect on the incidence of complications. It was suggested by Marsh11 more than 10 years ago that in the long term, optimal results might be achieved with VZV vaccination.
A VZV vaccine, Zostavax, is now available. This is a one-dose, high-potency, live, attenuated VZV vaccine that boosts VZV-specific cell-mediated immunity. Zoster vaccine is registered in the EU for use in adults over the age of 50 for the prevention of herpes zoster and herpes zoster-related complications (ie, postherpetic neuralgia). In the Shingles Prevention Study,16 a placebo-controlled trial in adults aged 60 years or over (n=38 546), zoster vaccine led to a sustained boost of VZV-specific cell-mediated immunity. Over a mean period of 3.1 years, zoster vaccine reduced the herpes zoster-related burden of illness by 61%, reduced the incidence of herpes zoster by 51% and reduced the incidence of postherpetic neuralgia by 67%. Further studies evaluating the role of vaccination in the incidence and/or severity of OAS secondary to HZO are necessary.
Learning points.
-
▶
Orbital apex syndrome (OAS) (damage to the optic nerve, III, IV, ophthalmic division of V and VI) can develop in association with herpes zoster ophthalmicus (HZO).
-
▶
It occurs mostly in patients over the age of 50, usually within 2 weeks of skin rash development.
-
▶
The pathogenesis of OAS associated with HZO is thought to be related to immune complexes, direct tissue infection with varicella zoster virus (VZV) and/or a secondary vasculitis.
-
▶
Treatment of OAS associated with HZO includes systemic treatment with corticosteroids (to control the inflammatory response) together with systemic antivirals.
-
▶
The prognosis is usually good with complete recovery within 10 months.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Yeh S, Foroozan R. Orbital apex syndrome. Curr Opin Ophthalmol 2004;15:490–8 [DOI] [PubMed] [Google Scholar]
- 2.Kattah JC, Kennerdell JS. Orbital apex syndrome secondary to herpes zoster ophthalmicus. Am J Ophthalmol 1978;85:378–82 [DOI] [PubMed] [Google Scholar]
- 3.Bourke RD, Pyle J. Herpes zoster ophthalmicus and the orbital apex syndrome. Aust N Z J Ophthalmol 1994;22:77–80 [DOI] [PubMed] [Google Scholar]
- 4.Vardy SJ, Rose GE. Orbital disease in herpes zoster ophthalmicus. Eye (Lond) 1994;8 (Pt 5):577–9 [DOI] [PubMed] [Google Scholar]
- 5.Shin HM, Lew H, Yun YS. A case of complete ophthalmoplegia in herpes zoster ophthalmicus. Korean J Ophthalmol 2005;19:302–4 [DOI] [PubMed] [Google Scholar]
- 6.Papeix C, Dumurgier J, Miléa D, et al. Complete ophthalmoplegia complicating ophthalmic herpes zoster. Rev Neurol (Paris) 2005;161:590–2 [DOI] [PubMed] [Google Scholar]
- 7.Im M, Kim BJ, Seo YJ, et al. Complete ophthalmoplegia after herpes zoster. Clin Exp Dermatol 2007;32:162–4 [DOI] [PubMed] [Google Scholar]
- 8.Liesegang TJ. Herpes zoster virus infection. Curr Opin Ophthalmol 2004;15:531–6 [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki A, Borruat FX. An unusual presentation of herpes zoster ophthalmicus: orbital myositis preceding vesicular eruption. Am J Ophthalmol 2003;136:574–5 [DOI] [PubMed] [Google Scholar]
- 10.Krasnianski M, Sievert M, Bau V, et al. External ophthalmoplegia due to ocular myositis in a patient with ophthalmic herpes zoster. Neuromuscul Disord 2004;14:438–41 [DOI] [PubMed] [Google Scholar]
- 11.Marsh RJ. Herpes zoster ophthalmicus. J R Soc Med 1997;90:670–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartley J. Post herpetic neuralgia, schwann cell activation and vitamin D. Med Hypotheses 2009;73:927–9 [DOI] [PubMed] [Google Scholar]
- 13.Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 2008;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cobo LM, Foulks GN, Liesegang T, et al. Oral acyclovir in the treatment of acute herpes zoster ophthalmicus. Ophthalmology 1986;93:763–70 [DOI] [PubMed] [Google Scholar]
- 15.Harding SP, Porter SM. Oral acyclovir in herpes zoster ophthalmicus. Curr Eye Res 1991;10 Suppl:177–82 [DOI] [PubMed] [Google Scholar]
- 16.Oxman MN, Levin MJ, Johnson GR, et al. ; Shingles Prevention Study Group A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005;352:2271–84 [DOI] [PubMed] [Google Scholar]


