Abstract
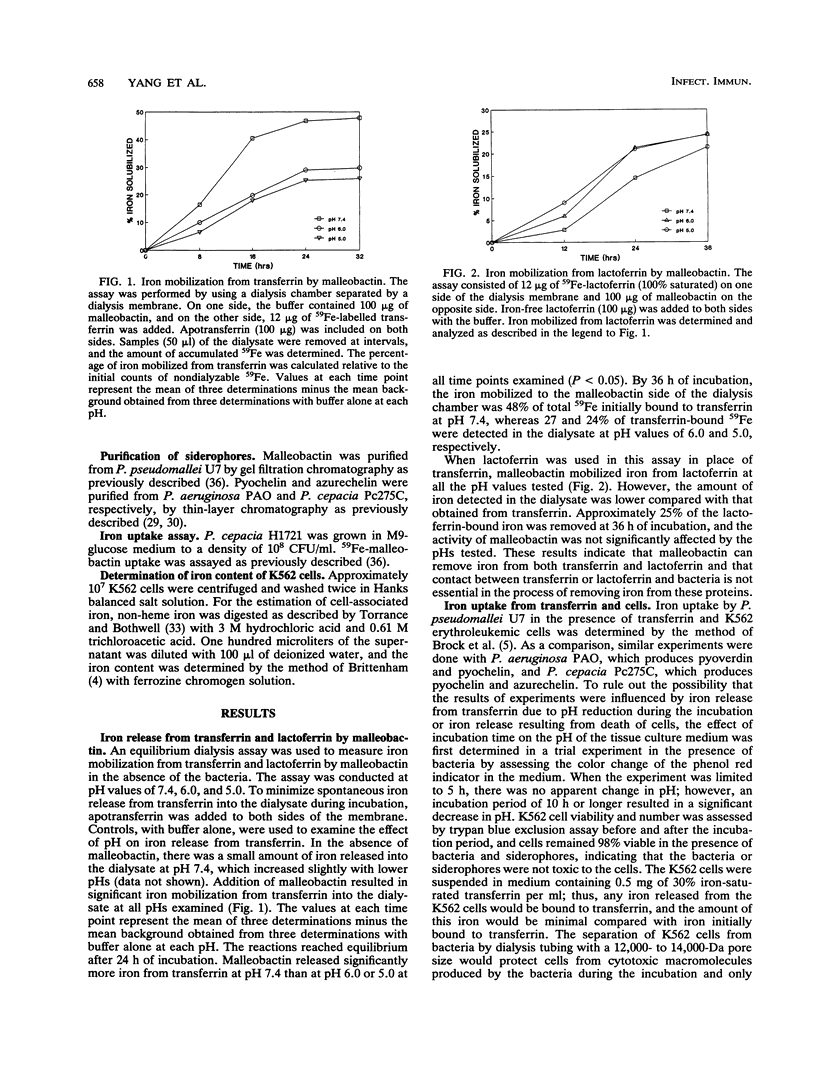
The ability of malleobactin to mobilize iron from transferrin and lactoferrin was examined in an equilibrium dialysis assay in the absence of bacteria. Malleobactin was capable of removing iron from both transferrin and lactoferrin at pH values of 7.4, 6.0, and 5.0. However, the levels of iron mobilization were greater for transferrin than for lactoferrin at all the pH values used in the assay. The ability of Pseudomonas pseudomallei to acquire iron from 30% iron-saturated transferrin and K562 human erythroleukemic cells was compared in parallel cultures as described previously (J. H. Brock, P. H. Williams, J. Liceaga, and K. G. Woldridge, Infect. Immun. 59:3185-3190, 1991). P. pseudomallei U7 tended to acquire iron from transferrin. In contrast, P. aeruginosa PAO and P. cepacia Pc275C acquired iron from both sources. P. cepacia H1721, which does not produce detectable siderophores, but can utilize malleobactin, pyochelin, and azurechelin as iron sources, was used in a similar experiment. Addition of malleobactin resulted in iron uptake only from transferrin, whereas pyochelin and azurechelin promoted iron uptake from both sources. When the siderophores were incubated with K562 cells alone, malleobactin was less efficient at removing iron from cells than pyochelin and azurechelin. It was also determined that malleobactin was less effective in binding to or entering cells than pyochelin and azurechelin. These results suggest that malleobactin can acquire iron more effectively from host proteins than from cellular sources. Pyochelin and azurechelin can acquire cell-derived iron in addition to iron bound to host proteins.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford C. E., King T. E., Jr, Campbell P. A. Role of transferrin, transferrin receptors, and iron in macrophage listericidal activity. J Exp Med. 1991 Aug 1;174(2):459–466. doi: 10.1084/jem.174.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay R. The role of iron in infection. Med Lab Sci. 1985 Apr;42(2):166–177. [PubMed] [Google Scholar]
- Brittenham G. Spectrophotometric plasma iron determination from fingerpuncture specimens. Clin Chim Acta. 1979 Jan 15;91(2):203–211. doi: 10.1016/0009-8981(79)90458-3. [DOI] [PubMed] [Google Scholar]
- Brock J. H., Williams P. H., Licéaga J., Wooldridge K. G. Relative availability of transferrin-bound iron and cell-derived iron to aerobactin-producing and enterochelin-producing strains of Escherichia coli and to other microorganisms. Infect Immun. 1991 Sep;59(9):3185–3190. doi: 10.1128/iai.59.9.3185-3190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Armstrong J. A. The role of lactoferrin in the bactericidal function of polymorphonuclear leucocytes. Immunology. 1979 Apr;36(4):781–791. [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Graham R. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):357–364. doi: 10.1128/jb.137.1.357-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton R. R., Charloteaux-Wauters M. Iron transport and storage. Eur J Biochem. 1987 May 4;164(3):485–506. doi: 10.1111/j.1432-1033.1987.tb11155.x. [DOI] [PubMed] [Google Scholar]
- Fibach E., Konijn A. M., Bauminger R. E., Ofer S., Rachmilewitz E. A. Effect of extracellular hemin on hemoglobin and ferritin content of erythroleukemia cells. J Cell Physiol. 1987 Mar;130(3):460–465. doi: 10.1002/jcp.1041300321. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Sciortino C. V., McIntosh M. A. Role of iron in microbe-host interactions. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S759–S777. doi: 10.1093/clinids/5.supplement_4.s759. [DOI] [PubMed] [Google Scholar]
- Holloway B. W., Krishnapillai V., Morgan A. F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979 Mar;43(1):73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe C., Sampath A., Spotnitz M. The pseudomallei group: a review. J Infect Dis. 1971 Dec;124(6):598–606. doi: 10.1093/infdis/124.6.598. [DOI] [PubMed] [Google Scholar]
- Ismail G., Razak N., Mohamed R., Embi N., Omar O. Resistance of Pseudomonas pseudomallei to normal human serum bactericidal action. Microbiol Immunol. 1988;32(7):645–652. doi: 10.1111/j.1348-0421.1988.tb01426.x. [DOI] [PubMed] [Google Scholar]
- Jacobson S. H., Hammarlind M., Lidefeldt K. J., Osterberg E., Tullus K., Brauner A. Incidence of aerobactin-positive Escherichia coli strains in patients with symptomatic urinary tract infection. Eur J Clin Microbiol Infect Dis. 1988 Oct;7(5):630–634. doi: 10.1007/BF01964240. [DOI] [PubMed] [Google Scholar]
- Konopka K., Bindereif A., Neilands J. B. Aerobactin-mediated utilization of transferrin iron. Biochemistry. 1982 Dec 7;21(25):6503–6508. doi: 10.1021/bi00268a028. [DOI] [PubMed] [Google Scholar]
- Konopka K., Neilands J. B. Effect of serum albumin on siderophore-mediated utilization of transferrin iron. Biochemistry. 1984 May 8;23(10):2122–2127. doi: 10.1021/bi00305a003. [DOI] [PubMed] [Google Scholar]
- Leelarasamee A., Bovornkitti S. Melioidosis: review and update. Rev Infect Dis. 1989 May-Jun;11(3):413–425. doi: 10.1093/clinids/11.3.413. [DOI] [PubMed] [Google Scholar]
- Lytton S. D., Cabantchik Z. I., Libman J., Shanzer A. Reversed siderophores as antimalarial agents. II. Selective scavenging of Fe(III) from parasitized erythrocytes by a fluorescent derivative of desferal. Mol Pharmacol. 1991 Oct;40(4):584–590. [PubMed] [Google Scholar]
- Molloy A. L., Winterbourn C. C. Release of iron from phagocytosed Escherichia coli and uptake by neutrophil lactoferrin. Blood. 1990 Feb 15;75(4):984–989. [PubMed] [Google Scholar]
- Montgomerie J. Z., Bindereif A., Neilands J. B., Kalmanson G. M., Guze L. B. Association of hydroxamate siderophore (aerobactin) with Escherichia coli isolated from patients with bacteremia. Infect Immun. 1984 Dec;46(3):835–838. doi: 10.1128/iai.46.3.835-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. G., Yancey R. J., Lankford C. E., Earhart C. F. Bacteriostatic enterochelin-specific immunoglobulin from normal human serum. Infect Immun. 1980 Feb;27(2):418–423. doi: 10.1128/iai.27.2.418-423.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack S. Malaria and iron. Br J Haematol. 1983 Feb;53(2):181–183. doi: 10.1111/j.1365-2141.1983.tb02009.x. [DOI] [PubMed] [Google Scholar]
- Pruksachartvuthi S., Aswapokee N., Thankerngpol K. Survival of Pseudomonas pseudomallei in human phagocytes. J Med Microbiol. 1990 Feb;31(2):109–114. doi: 10.1099/00222615-31-2-109. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Iron uptake in colicin B-resistant mutants of Escherichia coli K-12. J Bacteriol. 1976 Jun;126(3):1052–1062. doi: 10.1128/jb.126.3.1052-1062.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanzer A., Libman J., Lytton S. D., Glickstein H., Cabantchik Z. I. Reversed siderophores act as antimalarial agents. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6585–6589. doi: 10.1073/pnas.88.15.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson C., Brener D., DeVoe I. W. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitidis. Infect Immun. 1982 Apr;36(1):107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Lewis C. J., Dennis J. J. Isolation of a novel siderophore from Pseudomonas cepacia. J Med Microbiol. 1992 Mar;36(3):184–189. doi: 10.1099/00222615-36-3-184. [DOI] [PubMed] [Google Scholar]
- Sokol P. A. Production and utilization of pyochelin by clinical isolates of Pseudomonas cepacia. J Clin Microbiol. 1986 Mar;23(3):560–562. doi: 10.1128/jcm.23.3.560-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- Sriyosachati S., Cox C. D. Siderophore-mediated iron acquisition from transferrin by Pseudomonas aeruginosa. Infect Immun. 1986 Jun;52(3):885–891. doi: 10.1128/iai.52.3.885-891.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Carbonetti N. H. Iron, siderophores, and the pursuit of virulence: independence of the aerobactin and enterochelin iron uptake systems in Escherichia coli. Infect Immun. 1986 Mar;51(3):942–947. doi: 10.1128/iai.51.3.942-947.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. M., Chaowagul W., Sokol P. A. Siderophore production by Pseudomonas pseudomallei. Infect Immun. 1991 Mar;59(3):776–780. doi: 10.1128/iai.59.3.776-780.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


