Abstract
This paper investigates the effector mechanisms of immune clearance in the lungs of rats immunized against mucoid Pseudomonas aeruginosa. After the gut-associated lymphoid tissue was primed and after a subsequent pulmonary challenge with live bacteria, significantly accelerated bacterial clearances from the lung and raised levels of anti-P. aeruginosa antibodies in sera (immunoglobulin G [IgG], IgA, and IgM) and bronchoalveolar lavages (IgG and IgA) were observed for all immune animals. These changes were associated with enhanced recruitment, chemotaxis, chemokinesis, phagocytic indices, and chemiluminescence of pulmonary polymorphonuclear neutrophils (PMN). In the alveolar spaces of immune animals, an increase in the level of PMN recruitment was not associated with higher levels of leukotriene B4 (LTB4). In contrast, in nonimmune animals that were intratracheally infected with P. aeruginosa, the levels of recruitment and activity of alveolar PMN were lower than those in immune rats but PMN infiltration correlated with a significant increase in the synthesis of LTB4 in the alveolar space. In pulmonary tissue, LTB4 synthesis for both groups was elevated. These findings suggest that accelerated clearance of mucoid P. aeruginosa from the lungs of intestinally immunized rats is due at least in part to factors that induce the enhancement of PMN recruitment and activity in the alveolar space. The mediators that regulate this enhanced response remain unknown but do not seem to include LTB4. The high levels of LTB4 measured in the bronchoalveolar lavages and pulmonary tissues from nonimmune animals infected with live bacteria implicate LTB4 as an important amplifier of the inflammatory response during acute pulmonary infections with mucoid P. aeruginosa in unimmunized hosts.
Full text
PDF


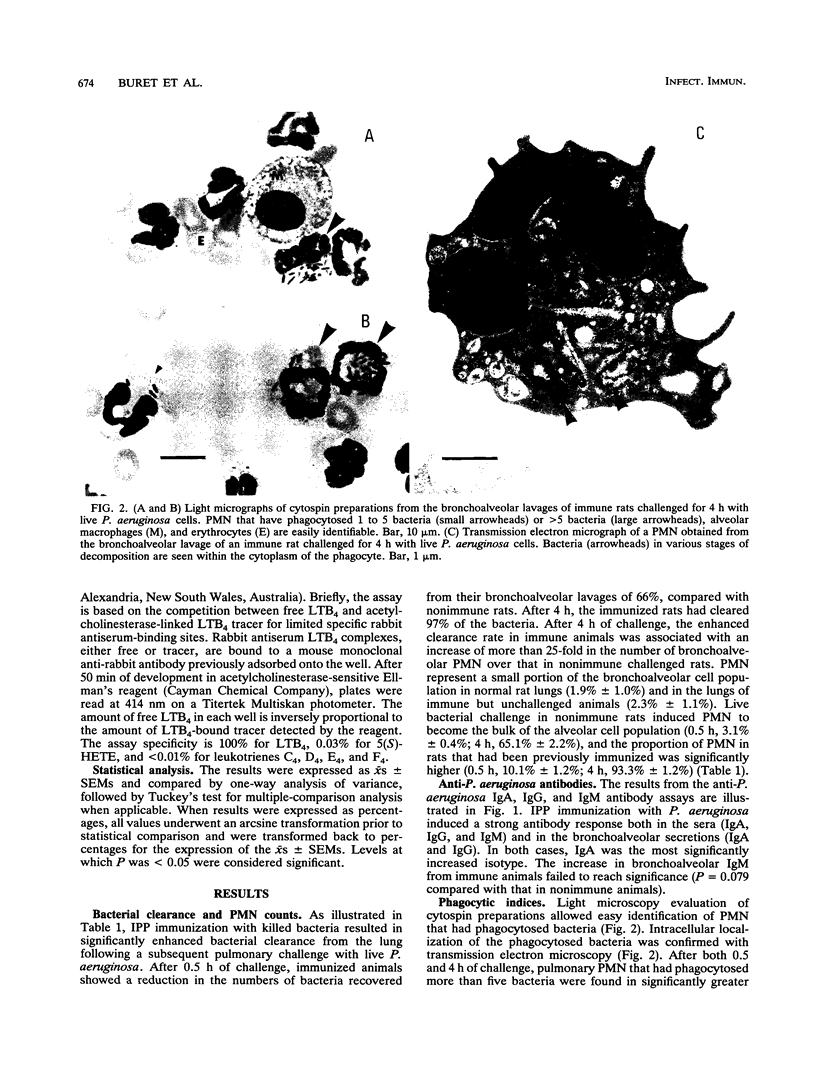
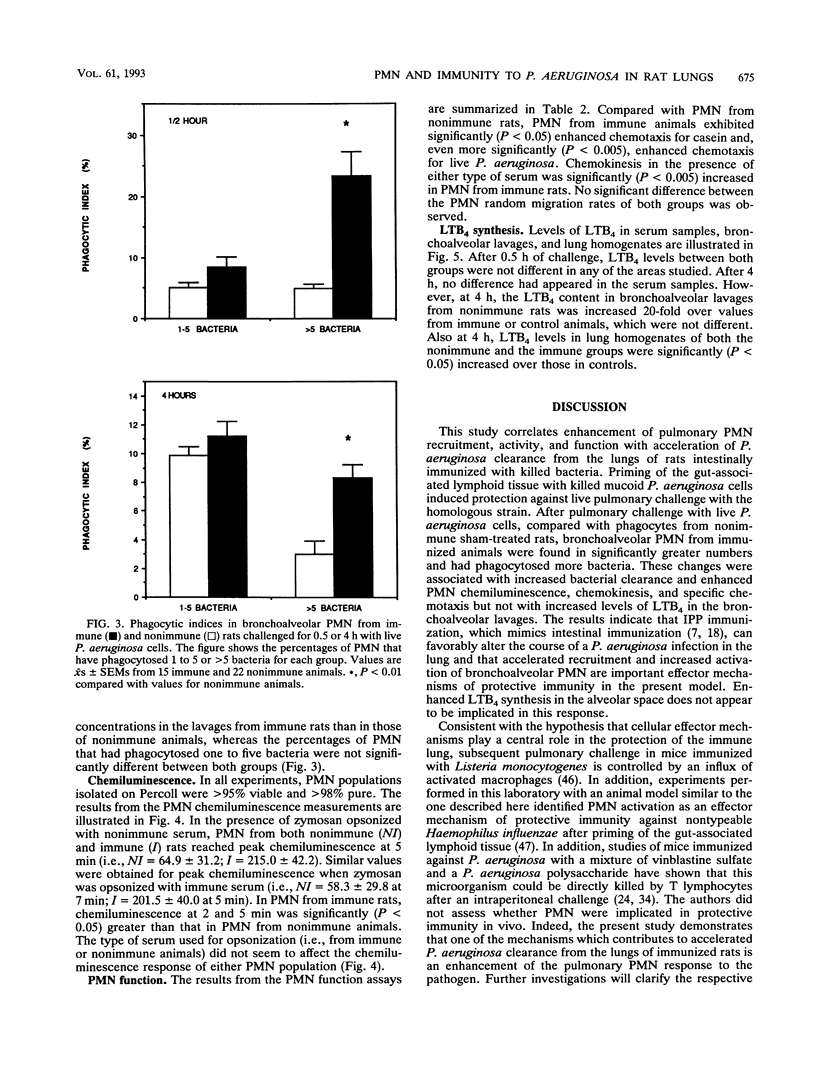
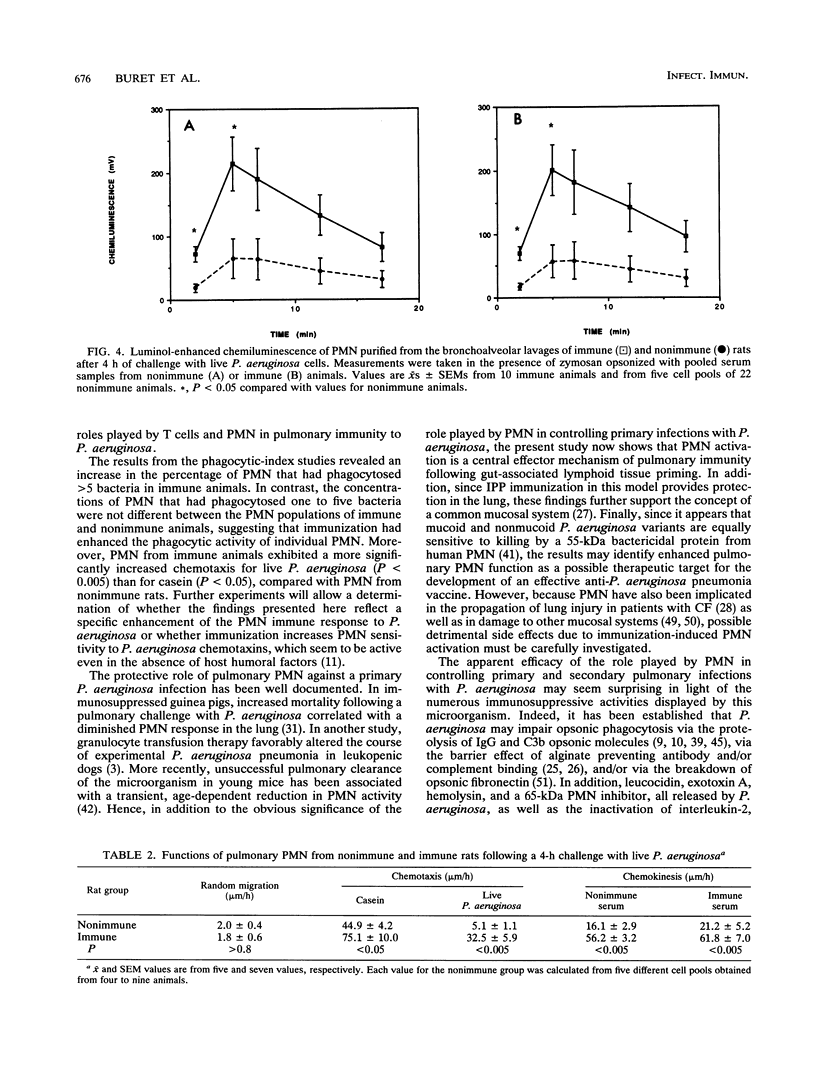
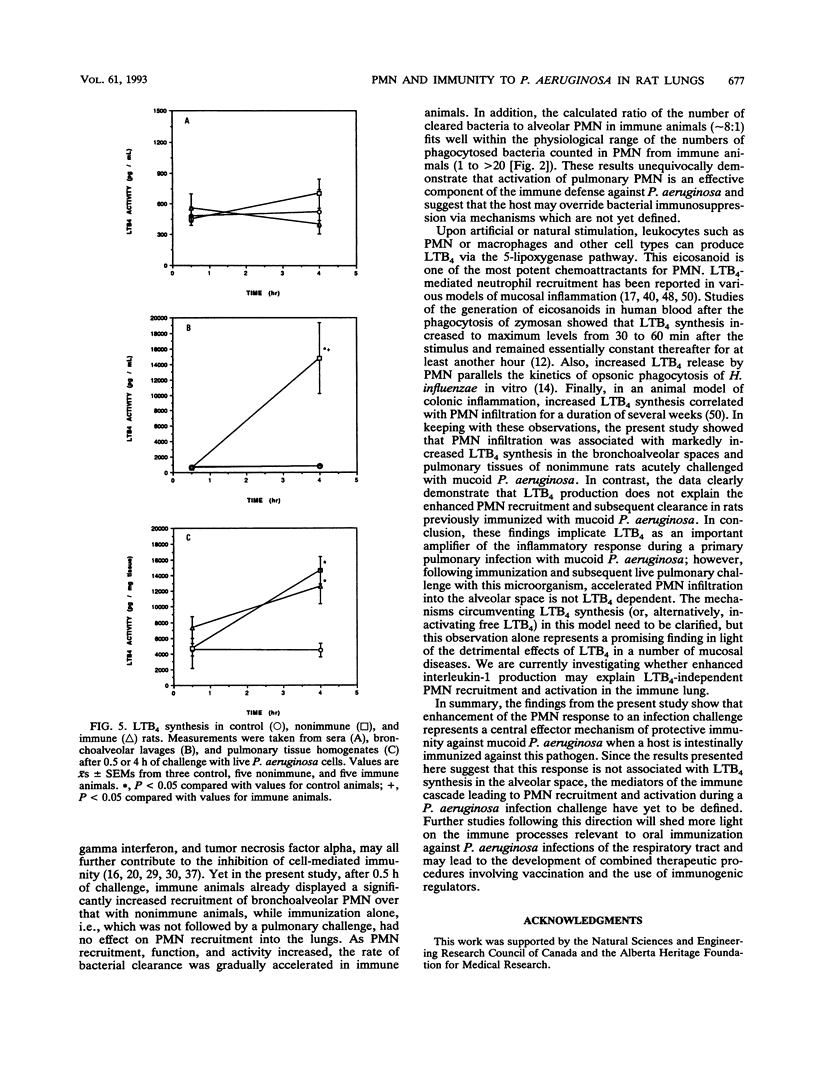


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred C. D., Margetts J., Hill H. R. Luminol-induced neutrophil chemiluminescence. Biochim Biophys Acta. 1980 Aug 13;631(2):380–385. doi: 10.1016/0304-4165(80)90311-6. [DOI] [PubMed] [Google Scholar]
- Dale D. C., Reynolds H. Y., Pennington J. E., Elin R. J., Pitts T. W., Graw R. G., Jr Granulocyte transfusion therapy of experimental Pseudomonas pneumonia. J Clin Invest. 1974 Sep;54(3):664–671. doi: 10.1172/JCI107804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley M. L., Husband A. J. Routes of priming and challenge for IgA antibody-containing cell responses in the intestine. Immunol Lett. 1990 Nov;26(2):165–170. doi: 10.1016/0165-2478(90)90140-l. [DOI] [PubMed] [Google Scholar]
- Döring G., Dalhoff A., Vogel O., Brunner H., Dröge U., Botzenhart K. In vivo activity of proteases of Pseudomonas aeruginosa in a rat model. J Infect Dis. 1984 Apr;149(4):532–537. doi: 10.1093/infdis/149.4.532. [DOI] [PubMed] [Google Scholar]
- Döring G., Høiby N. Longitudinal study of immune response to Pseudomonas aeruginosa antigens in cystic fibrosis. Infect Immun. 1983 Oct;42(1):197–201. doi: 10.1128/iai.42.1.197-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring G., Obernesser H. J., Botzenhart K., Flehmig B., Høiby N., Hofmann A. Proteases of Pseudomonas aeruginosa in patients with cystic fibrosis. J Infect Dis. 1983 Apr;147(4):744–750. doi: 10.1093/infdis/147.4.744. [DOI] [PubMed] [Google Scholar]
- Fick R. B., Jr, Baltimore R. S., Squier S. U., Reynolds H. Y. IgG proteolytic activity of Pseudomonas aeruginosa in cystic fibrosis. J Infect Dis. 1985 Apr;151(4):589–598. doi: 10.1093/infdis/151.4.589. [DOI] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Matthay R. A., Reynolds H. Y. Cystic fibrosis pseudomonas opsonins. Inhibitory nature in an in vitro phagocytic assay. J Clin Invest. 1981 Oct;68(4):899–914. doi: 10.1172/JCI110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Squier S. U., Wood R. E., Gee J. B., Reynolds H. Y. Proteins of the cystic fibrosis respiratory tract. Fragmented immunoglobulin G opsonic antibody causing defective opsonophagocytosis. J Clin Invest. 1984 Jul;74(1):236–248. doi: 10.1172/JCI111407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontán P. A., Amura C. R., García V. E., Cerquetti M. C., Sordelli D. O. Preliminary characterization of Pseudomonas aeruginosa peptide chemotactins for polymorphonuclear leukocytes. Infect Immun. 1992 Jun;60(6):2465–2469. doi: 10.1128/iai.60.6.2465-2469.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin A., Zirrolli J. A., Maclouf J., Vausbinder L., Henson P. M., Murphy R. C. Platelet-activating factor and leukotriene biosynthesis in whole blood. A model for the study of transcellular arachidonate metabolism. J Immunol. 1989 Dec 1;143(11):3680–3685. [PubMed] [Google Scholar]
- Freihorst J., Merrick J. M., Ogra P. L. Effect of oral immunization with Pseudomonas aeruginosa on the development of specific antibacterial immunity in the lungs. Infect Immun. 1989 Jan;57(1):235–238. doi: 10.1128/iai.57.1.235-238.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo R., Faden H., Sharma S., Ogra P. L. Release of leukotriene B4 from human neutrophils after interaction with nontypeable Haemophilus influenzae. Infect Immun. 1991 Nov;59(11):4221–4226. doi: 10.1128/iai.59.11.4221-4226.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan P. H. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991 Jan;4(1):35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt P. S., Misfeldt M. L. Variables which affect suppression of the immune response induced by Pseudomonas aeruginosa exotoxin A. Infect Immun. 1986 Apr;52(1):96–100. doi: 10.1128/iai.52.1.96-100.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R. C., Fells G., Gadek J., Pacholok S., Humes J., Crystal R. G. Neutrophil accumulation in the lung in alpha 1-antitrypsin deficiency. Spontaneous release of leukotriene B4 by alveolar macrophages. J Clin Invest. 1991 Sep;88(3):891–897. doi: 10.1172/JCI115391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Dunkley M. L. Lack of site of origin effects on distribution of IgA antibody-containing cells. Immunology. 1985 Feb;54(2):215–221. [PMC free article] [PubMed] [Google Scholar]
- Johansen H. K., Høiby N. Local IgA and IgG response to intratracheal immunization with Pseudomonas aeruginosa antigens. APMIS. 1992 Jan;100(1):87–90. [PubMed] [Google Scholar]
- Kharazmi A. Mechanisms involved in the evasion of the host defence by Pseudomonas aeruginosa. Immunol Lett. 1991 Oct;30(2):201–205. doi: 10.1016/0165-2478(91)90026-7. [DOI] [PubMed] [Google Scholar]
- Klinger J. D., Straus D. C., Hilton C. B., Bass J. A. Antibodies to proteases and exotoxin A of Pseudomonas aeruginosa in patients with cystic fibrosis: Demonstration by radioimmunoassay. J Infect Dis. 1978 Jul;138(1):49–48. doi: 10.1093/infdis/138.1.49. [DOI] [PubMed] [Google Scholar]
- Lam J. S., Mutharia L. M., Hancock R. E., Høiby N., Lam K., Baek L., Costerton J. W. Immunogenicity of Pseudomonas aeruginosa outer membrane antigens examined by crossed immunoelectrophoresis. Infect Immun. 1983 Oct;42(1):88–98. doi: 10.1128/iai.42.1.88-98.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D. T., Hiller J. Prospective, controlled study of a polyvalent pseudomonas vaccine in cystic fibrosis--three year results. Arch Dis Child. 1984 Dec;59(12):1131–1134. doi: 10.1136/adc.59.12.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham R. B., Pier G. B., Goellner J. J., Mizel S. B. In vitro T cell-mediated killing of Pseudomonas aeruginosa. II. The role of macrophages and T cell subsets in T cell killing. J Immunol. 1985 Jun;134(6):4112–4117. [PubMed] [Google Scholar]
- Marrie T. J., Harding G. K., Ronald A. R., Dikkema J., Lam J., Hoban S., Costerton J. W. Influence of mucoidy on antibody coating of Pseudomonas aeruginosa. J Infect Dis. 1979 Mar;139(3):357–361. doi: 10.1093/infdis/139.3.357. [DOI] [PubMed] [Google Scholar]
- May T. B., Shinabarger D., Maharaj R., Kato J., Chu L., DeVault J. D., Roychoudhury S., Zielinski N. A., Berry A., Rothmel R. K. Alginate synthesis by Pseudomonas aeruginosa: a key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin Microbiol Rev. 1991 Apr;4(2):191–206. doi: 10.1128/cmr.4.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M. R., Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979 May;122(5):1892–1898. [PubMed] [Google Scholar]
- McElvaney N. G., Hubbard R. C., Birrer P., Chernick M. S., Caplan D. B., Frank M. M., Crystal R. G. Aerosol alpha 1-antitrypsin treatment for cystic fibrosis. Lancet. 1991 Feb 16;337(8738):392–394. doi: 10.1016/0140-6736(91)91167-s. [DOI] [PubMed] [Google Scholar]
- Nonoyama S., Kojo H., Mine Y., Nishida M., Goto S., Kuwahara S. Inhibitory effect of Pseudomonas aeruginosa on the phagocytic and killing activity of rabbit polymorphonuclear leukocytes: mechanisms of action of a polymorphonuclear leukocyte inhibitor. Infect Immun. 1979 May;24(2):399–403. doi: 10.1128/iai.24.2.399-403.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmely M., Gale A., Clabaugh M., Horvat R., Zhou W. W. Proteolytic inactivation of cytokines by Pseudomonas aeruginosa. Infect Immun. 1990 Sep;58(9):3009–3014. doi: 10.1128/iai.58.9.3009-3014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Ehrie M. G. Pathogenesis of Pseudomonas aeruginosa pneumonia during immunosuppression. J Infect Dis. 1978 Jun;137(6):764–774. doi: 10.1093/infdis/137.6.764. [DOI] [PubMed] [Google Scholar]
- Pennington J. E., Reynolds H. Y., Wood R. E., Robinson R. A., Levine A. S. Use of a Pseudomonas Aeruginosa vaccine in pateints with acute leukemia and cystic fibrosis. Am J Med. 1975 May;58(5):629–636. doi: 10.1016/0002-9343(75)90498-2. [DOI] [PubMed] [Google Scholar]
- Piedra P., Ogra P. L. Immunologic aspects of surface infections in the lung. J Pediatr. 1986 May;108(5 Pt 2):817–823. doi: 10.1016/s0022-3476(86)80751-x. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Markham R. B. Induction in mice of cell-mediated immunity to Pseudomonas aeruginosa by high molecular weight polysaccharide and vinblastine. J Immunol. 1982 May;128(5):2121–2125. [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Sadoff J. C. Protective immunity induced in mice by immunization with high-molecular-weight polysaccharide from Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):919–925. doi: 10.1128/iai.22.3.919-925.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pseudomonas aeruginosa infections: persisting problems and current research to find new therapies. Ann Intern Med. 1975 Jun;82(6):819–831. doi: 10.7326/0003-4819-82-6-819. [DOI] [PubMed] [Google Scholar]
- Scharmann W., Jacob F., Porstendörfer J. The cytotoxic action of leucocidan from Pseudomonas aeruginosa on human polymorphonuclear leucocytes. J Gen Microbiol. 1976 Apr;93(2):303–308. doi: 10.1099/00221287-93-2-303. [DOI] [PubMed] [Google Scholar]
- Schreiber J. R., Pier G. B., Grout M., Nixon K., Patawaran M. Induction of opsonic antibodies to Pseudomonas aeruginosa mucoid exopolysaccharide by an anti-idiotypic monoclonal antibody. J Infect Dis. 1991 Sep;164(3):507–514. doi: 10.1093/infdis/164.3.507. [DOI] [PubMed] [Google Scholar]
- Sibille Y., Reynolds H. Y. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990 Feb;141(2):471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- Siefferman C. M., Regelmann W. E., Gray B. H. Pseudomonas aeruginosa variants isolated from patients with cystic fibrosis are killed by a bactericidal protein from human polymorphonuclear leukocytes. Infect Immun. 1991 Jun;59(6):2152–2157. doi: 10.1128/iai.59.6.2152-2157.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordelli D. O., Djafari M., García V. E., Fontán P. A., Döring G. Age-dependent pulmonary clearance of Pseudomonas aeruginosa in a mouse model: diminished migration of polymorphonuclear leukocytes to N-formyl-methionyl-leucyl-phenylalanine. Infect Immun. 1992 Apr;60(4):1724–1727. doi: 10.1128/iai.60.4.1724-1727.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speert D. P. Host defenses in patients with cystic fibrosis: modulation by Pseudomonas aeruginosa. Surv Synth Pathol Res. 1985;4(1):14–33. doi: 10.1159/000156962. [DOI] [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]
- Wallace F. J., Clancy R. L., Cripps A. W. An animal model demonstration of enhanced clearance of nontypable Haemophilus influenzae from the respiratory tract after antigen stimulation of gut-associated lymphoid tissue. Am Rev Respir Dis. 1989 Aug;140(2):311–316. doi: 10.1164/ajrccm/140.2.311. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Arfors K. E., McKnight G. W. A monoclonal antibody against the CD18 leukocyte adhesion molecule prevents indomethacin-induced gastric damage in the rabbit. Gastroenterology. 1991 Apr;100(4):878–883. doi: 10.1016/0016-5085(91)90259-n. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Bass J. A., Johanson W. G., Jr, Straus D. C. Role of adherence in the pathogenesis of Pseudomonas aeruginosa lung infection in cystic fibrosis patients. Infect Immun. 1980 Dec;30(3):694–699. doi: 10.1128/iai.30.3.694-699.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]




