Abstract
Peritoneal cells from Mycobacterium bovis BCG-infected C3H/HeN mice produced nitrite (NO2-, an oxidative end product of nitric oxide [NO] synthesis) and inhibited the growth of Francisella tularensis, a facultative intracellular bacterium. Both NO2- production and inhibition of bacterial growth were suppressed by NG-monomethyl-L-arginine, a substrate inhibitor of nitrogen oxidation of L-arginine, and monoclonal antibodies (MAbs) to gamma interferon (IFN-gamma) and tumor necrosis factor alpha (TNF-alpha). Intraperitoneal injection of mice with BCG increased urinary nitrate (NO3-) excretion coincident with development of activated macrophages capable of secreting nitrogen oxides and inhibiting F. tularensis growth in vitro. Eight days after BCG inoculation, mice survived a normally lethal intraperitoneal challenge with F. tularensis. Treatment of these BCG-infected mice with MAbs to IFN-gamma or TNF-alpha at the time of BCG inoculation reduced urinary NO3- levels to those found in normal uninfected mice for up to 14 days. The same anticytokine antibody treatment abolished BCG-mediated protection against F. tularensis: mice died within 4 to 6 days. Intraperitoneal administration of anti-IFN-gamma or anti-TNF-alpha antibody 8 days after BCG infection also reduced urinary NO3- and abolished protection against F. tularensis. Isotype control (immunoglobulin G) or anti-interleukin 4 MAbs had little effect on these parameters at any time of treatment. IFN-gamma and TNF-alpha were clearly involved in the regulation of macrophage activation by BCG in vivo. Protection against F. tularensis challenge by BCG depended upon the physiological generation of reactive nitrogen oxides induced by these cytokines.
Full text
PDF

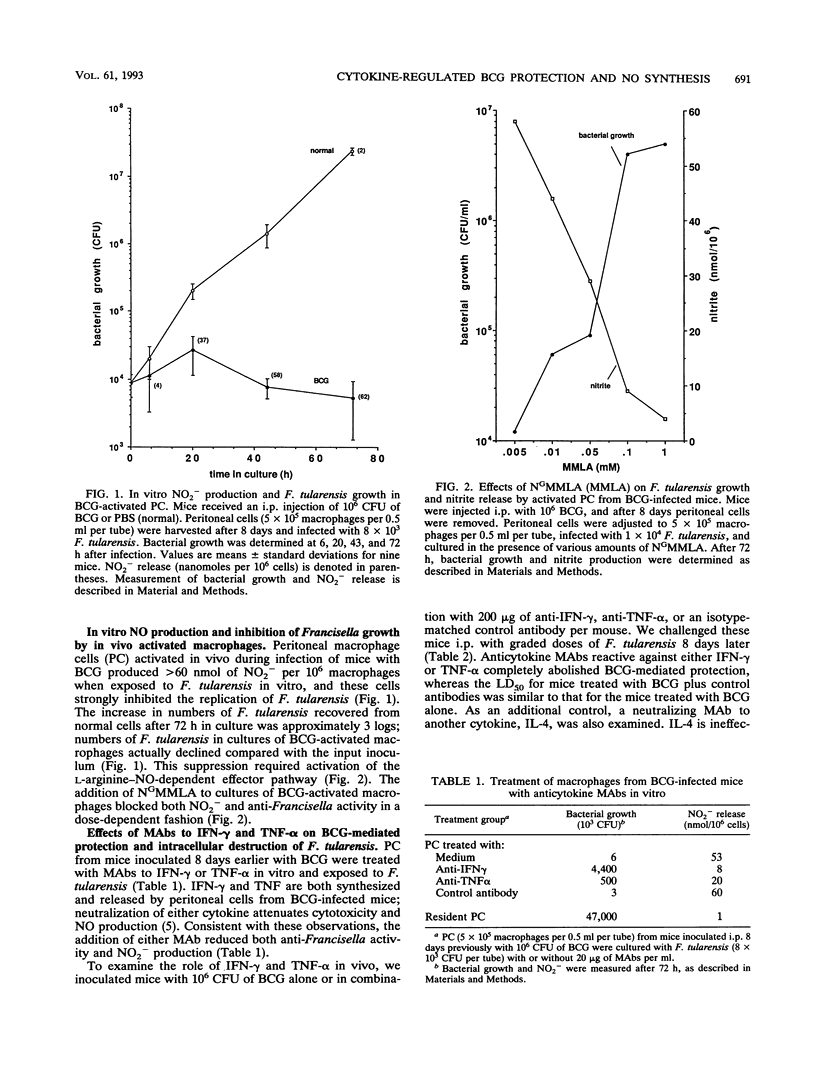
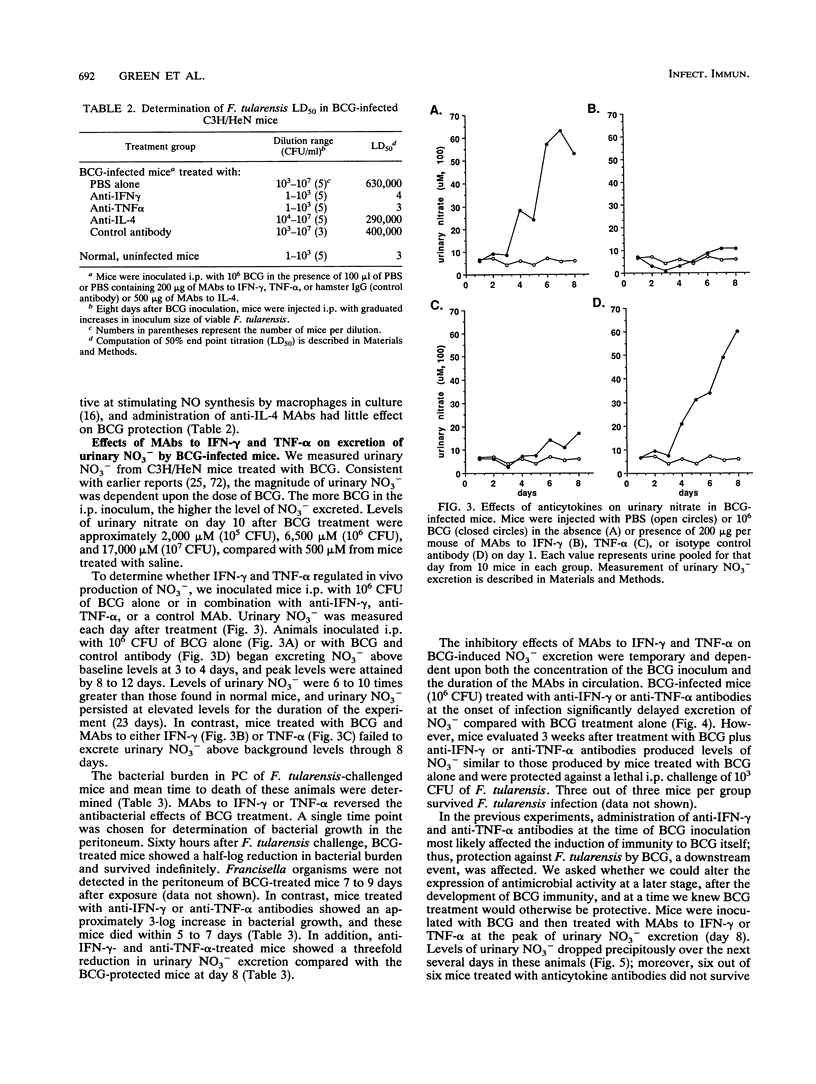




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Franzblau S. G., Vavrin Z., Hibbs J. B., Jr, Krahenbuhl J. L. L-arginine-dependent macrophage effector functions inhibit metabolic activity of Mycobacterium leprae. J Immunol. 1991 Sep 1;147(5):1642–1646. [PubMed] [Google Scholar]
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Albina J. E., Mills C. D., Henry W. L., Jr, Caldwell M. D. Regulation of macrophage physiology by L-arginine: role of the oxidative L-arginine deiminase pathway. J Immunol. 1989 Dec 1;143(11):3641–3646. [PubMed] [Google Scholar]
- Amber I. J., Hibbs J. B., Jr, Parker C. J., Johnson B. B., Taintor R. R., Vavrin Z. Activated macrophage conditioned medium: identification of the soluble factors inducing cytotoxicity and the L-arginine dependent effector mechanism. J Leukoc Biol. 1991 Jun;49(6):610–620. doi: 10.1002/jlb.49.6.610. [DOI] [PubMed] [Google Scholar]
- Amber I. J., Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Cytokines induce an L-arginine-dependent effector system in nonmacrophage cells. J Leukoc Biol. 1988 Jul;44(1):58–65. doi: 10.1002/jlb.44.1.58. [DOI] [PubMed] [Google Scholar]
- Anthony L. S., Morrissey P. J., Nano F. E. Growth inhibition of Francisella tularensis live vaccine strain by IFN-gamma-activated macrophages is mediated by reactive nitrogen intermediates derived from L-arginine metabolism. J Immunol. 1992 Mar 15;148(6):1829–1834. [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Bancroft G. J., Sheehan K. C., Schreiber R. D., Unanue E. R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989 Jul 1;143(1):127–130. [PubMed] [Google Scholar]
- Barnes P. F., Fong S. J., Brennan P. J., Twomey P. E., Mazumder A., Modlin R. L. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990 Jul 1;145(1):149–154. [PubMed] [Google Scholar]
- Billiar T. R., Curran R. D., Stuehr D. J., West M. A., Bentz B. G., Simmons R. L. An L-arginine-dependent mechanism mediates Kupffer cell inhibition of hepatocyte protein synthesis in vitro. J Exp Med. 1989 Apr 1;169(4):1467–1472. doi: 10.1084/jem.169.4.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. J., Lee S. H. Effects of interferon-gamma and tumor necrosis factor-alpha on the expression of an Ia antigen on a murine macrophage cell line. J Immunol. 1986 Nov 1;137(9):2853–2856. [PubMed] [Google Scholar]
- Corbett J. A., Lancaster J. R., Jr, Sweetland M. A., McDaniel M. L. Interleukin-1 beta-induced formation of EPR-detectable iron-nitrosyl complexes in islets of Langerhans. Role of nitric oxide in interleukin-1 beta-induced inhibition of insulin secretion. J Biol Chem. 1991 Nov 15;266(32):21351–21354. [PubMed] [Google Scholar]
- Corbett J. A., Lancaster J. R., Jr, Sweetland M. A., McDaniel M. L. Interleukin-1 beta-induced formation of EPR-detectable iron-nitrosyl complexes in islets of Langerhans. Role of nitric oxide in interleukin-1 beta-induced inhibition of insulin secretion. J Biol Chem. 1991 Nov 15;266(32):21351–21354. [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Hofmann K., Simmons R. L. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. J Exp Med. 1989 Nov 1;170(5):1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991 Jan;132(1):150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986 Sep;78(3):790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Wietzerbin J., Hibbs J. B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991 Sep;59(9):2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991 Sep;59(9):3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier A. H., Mock B. A., Meltzer M. S., Nacy C. A. Mycobacterium bovis BCG-induced protection against cutaneous and systemic Leishmania major infections of mice. Infect Immun. 1987 Jul;55(7):1707–1714. doi: 10.1128/iai.55.7.1707-1714.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier A. H., Polsinelli T., Green S. J., Nacy C. A. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect Immun. 1992 Mar;60(3):817–825. doi: 10.1128/iai.60.3.817-825.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier A. H., Slayter M. V., Ziemba R., Meltzer M. S., Nacy C. A. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991 Sep;59(9):2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Broadnax L. M. Urinary nitrate excretion in relation to murine macrophage activation. Influence of dietary L-arginine and oral NG-monomethyl-L-arginine. J Immunol. 1991 Feb 15;146(4):1294–1302. [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990 Jan;85(1):264–273. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Ruiz de Luzuriaga K., Wagner D. A., Rand W., Istfan N., Young V. R., Tannenbaum S. R. Nitrate biosynthesis in man. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Tannenbaum S. R., Goldman P. Nitrate synthesis in the germfree and conventional rat. Science. 1981 Apr 3;212(4490):56–58. doi: 10.1126/science.6451927. [DOI] [PubMed] [Google Scholar]
- Green S. J., Chen T. Y., Crawford R. M., Nacy C. A., Morrison D. C., Meltzer M. S. Cytotoxic activity and production of toxic nitrogen oxides by macrophages treated with IFN-gamma and monoclonal antibodies against the 73-kDa lipopolysaccharide receptor. J Immunol. 1992 Sep 15;149(6):2069–2075. [PubMed] [Google Scholar]
- Green S. J., Crawford R. M., Hockmeyer J. T., Meltzer M. S., Nacy C. A. Leishmania major amastigotes initiate the L-arginine-dependent killing mechanism in IFN-gamma-stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990 Dec 15;145(12):4290–4297. [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Havell E. A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989 Nov 1;143(9):2894–2899. [PubMed] [Google Scholar]
- Havell E. A. Production of tumor necrosis factor during murine listeriosis. J Immunol. 1987 Dec 15;139(12):4225–4231. [PubMed] [Google Scholar]
- Hibbs J. B., Jr Activated macrophages as cytotoxic effector cells. II. Requirement for local persistence of inducing antigen. Transplantation. 1975 Jan;19(1):81–87. doi: 10.1097/00007890-197501000-00016. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Iron depletion: possible cause of tumor cell cytotoxicity induced by activated macrophages. Biochem Biophys Res Commun. 1984 Sep 17;123(2):716–723. doi: 10.1016/0006-291x(84)90288-2. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Westenfelder C., Taintor R., Vavrin Z., Kablitz C., Baranowski R. L., Ward J. H., Menlove R. L., McMurry M. P., Kushner J. P. Evidence for cytokine-inducible nitric oxide synthesis from L-arginine in patients receiving interleukin-2 therapy. J Clin Invest. 1992 Mar;89(3):867–877. doi: 10.1172/JCI115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L., Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989 Dec 15;143(12):4208–4212. [PubMed] [Google Scholar]
- Kilbourn R. G., Belloni P. Endothelial cell production of nitrogen oxides in response to interferon gamma in combination with tumor necrosis factor, interleukin-1, or endotoxin. J Natl Cancer Inst. 1990 May 2;82(9):772–776. doi: 10.1093/jnci/82.9.772. [DOI] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Hibbs J. B., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langermans J. A., Van der Hulst M. E., Nibbering P. H., Hiemstra P. S., Fransen L., Van Furth R. IFN-gamma-induced L-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J Immunol. 1992 Jan 15;148(2):568–574. [PubMed] [Google Scholar]
- Leaf C. D., Wishnok J. S., Tannenbaum S. R. L-arginine is a precursor for nitrate biosynthesis in humans. Biochem Biophys Res Commun. 1989 Sep 15;163(2):1032–1037. doi: 10.1016/0006-291x(89)92325-5. [DOI] [PubMed] [Google Scholar]
- Lepoivre M., Chenais B., Yapo A., Lemaire G., Thelander L., Tenu J. P. Alterations of ribonucleotide reductase activity following induction of the nitrite-generating pathway in adenocarcinoma cells. J Biol Chem. 1990 Aug 25;265(24):14143–14149. [PubMed] [Google Scholar]
- Lepoivre M., Fieschi F., Coves J., Thelander L., Fontecave M. Inactivation of ribonucleotide reductase by nitric oxide. Biochem Biophys Res Commun. 1991 Aug 30;179(1):442–448. doi: 10.1016/0006-291x(91)91390-x. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Millott S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990 Dec 15;145(12):4306–4310. [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauël J., Ransijn A., Buchmüller-Rouiller Y. Killing of Leishmania parasites in activated murine macrophages is based on an L-arginine-dependent process that produces nitrogen derivatives. J Leukoc Biol. 1991 Jan;49(1):73–82. doi: 10.1002/jlb.49.1.73. [DOI] [PubMed] [Google Scholar]
- Meshnick S. R., Eaton J. W. Leishmanial superoxide dismutase: a possible target for chemotherapy. Biochem Biophys Res Commun. 1981 Oct 15;102(3):970–976. doi: 10.1016/0006-291x(81)91633-8. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kohanawa M., Chen Y., Sato H., Moriyama M., Tsuruoka N. Interactions between endogenous gamma interferon and tumor necrosis factor in host resistance against primary and secondary Listeria monocytogenes infections. Infect Immun. 1989 Nov;57(11):3331–3337. doi: 10.1128/iai.57.11.3331-3337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüssler A., Drapier J. C., Rénia L., Pied S., Miltgen F., Gentilini M., Mazier D. L-arginine-dependent destruction of intrahepatic malaria parasites in response to tumor necrosis factor and/or interleukin 6 stimulation. Eur J Immunol. 1991 Jan;21(1):227–230. doi: 10.1002/eji.1830210134. [DOI] [PubMed] [Google Scholar]
- Ochoa J. B., Udekwu A. O., Billiar T. R., Curran R. D., Cerra F. B., Simmons R. L., Peitzman A. B. Nitrogen oxide levels in patients after trauma and during sepsis. Ann Surg. 1991 Nov;214(5):621–626. doi: 10.1097/00000658-199111000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Pellat C., Henry Y., Drapier J. C. IFN-gamma-activated macrophages: detection by electron paramagnetic resonance of complexes between L-arginine-derived nitric oxide and non-heme iron proteins. Biochem Biophys Res Commun. 1990 Jan 15;166(1):119–125. doi: 10.1016/0006-291x(90)91919-j. [DOI] [PubMed] [Google Scholar]
- Poston R. M., Kurlander R. J. Analysis of the time course of IFN-gamma mRNA and protein production during primary murine listeriosis. The immune phase of bacterial elimination is not temporally linked to IFN production in vivo. J Immunol. 1991 Jun 15;146(12):4333–4337. [PubMed] [Google Scholar]
- Reddy D., Lancaster J. R., Jr, Cornforth D. P. Nitrite inhibition of Clostridium botulinum: electron spin resonance detection of iron-nitric oxide complexes. Science. 1983 Aug 19;221(4612):769–770. doi: 10.1126/science.6308761. [DOI] [PubMed] [Google Scholar]
- Roach T. I., Kiderlen A. F., Blackwell J. M. Role of inorganic nitrogen oxides and tumor necrosis factor alpha in killing Leishmania donovani amastigotes in gamma interferon-lipopolysaccharide-activated macrophages from Lshs and Lshr congenic mouse strains. Infect Immun. 1991 Nov;59(11):3935–3944. doi: 10.1128/iai.59.11.3935-3944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockett K. A., Awburn M. M., Cowden W. B., Clark I. A. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991 Sep;59(9):3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan K. C., Ruddle N. H., Schreiber R. D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989 Jun 1;142(11):3884–3893. [PubMed] [Google Scholar]
- Sibley L. D., Adams L. B., Fukutomi Y., Krahenbuhl J. L. Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J Immunol. 1991 Oct 1;147(7):2340–2345. [PubMed] [Google Scholar]
- Stadler J., Stefanovic-Racic M., Billiar T. R., Curran R. D., McIntyre L. A., Georgescu H. I., Simmons R. L., Evans C. H. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J Immunol. 1991 Dec 1;147(11):3915–3920. [PubMed] [Google Scholar]
- Stuehr D. J., Gross S. S., Sakuma I., Levi R., Nathan C. F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989 Mar 1;169(3):1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincendeau P., Daulouède S. Macrophage cytostatic effect on Trypanosoma musculi involves an L-arginine-dependent mechanism. J Immunol. 1991 Jun 15;146(12):4338–4343. [PubMed] [Google Scholar]
- Wagner D. A., Young V. R., Tannenbaum S. R. Mammalian nitrate biosynthesis: incorporation of 15NH3 into nitrate is enhanced by endotoxin treatment. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4518–4521. doi: 10.1073/pnas.80.14.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub J., Weinbaum F. I. The effect of BCG on experimental cutaneous leishmaniasis in mice. J Immunol. 1977 Jun;118(6):2288–2290. [PubMed] [Google Scholar]
- Welsh N., Sandler S. Interleukin-1 beta induces nitric oxide production and inhibits the activity of aconitase without decreasing glucose oxidation rates in isolated mouse pancreatic islets. Biochem Biophys Res Commun. 1992 Jan 15;182(1):333–340. doi: 10.1016/s0006-291x(05)80149-4. [DOI] [PubMed] [Google Scholar]
- Werner-Felmayer G., Werner E. R., Fuchs D., Hausen A., Reibnegger G., Wachter H. Tetrahydrobiopterin-dependent formation of nitrite and nitrate in murine fibroblasts. J Exp Med. 1990 Dec 1;172(6):1599–1607. doi: 10.1084/jem.172.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe S. A., Tracey D. E., Henney C. S. BCG-induced murine effector cells. II. Characterization of natural killer cells in peritoneal exudates. J Immunol. 1977 Sep;119(3):1152–1158. [PubMed] [Google Scholar]


