Abstract
Severe pulmonary arterial hypertension (PAH) is characterized by clustered proliferation of endothelial cells in the lumina of small size pulmonary arteries resulting in concentric obliteration of the lumina and formation of complex vascular structures known as plexiform lesions. This debilitating disease occurs more frequently in women, yet both animal studies in classical models of PAH and limited clinical data suggest protective effects of estrogens: the estrogen paradox in pulmonary hypertension. Little is known about the role of estrogens in PAH, but one line of evidence strongly suggests that the vascular protective effects of 17β-estradiol (estradiol; E2) are mediated largely by its downstream metabolites. Estradiol is metabolized to 2-hydroxyestradiol (2HE) by CYP1A1/CYP1B1, and 2HE is converted to 2-methoxyestradiol (2ME) by catechol-O-methyl transferase. 2ME is extensively metabolized to 2-methoxyestrone, a metabolite that lacks biologic activity but which may be converted back to 2ME. 2ME has no estrogenic activity and its effects are mediated by estrogen receptors-independent mechanism(s). Notably, in systemic and pulmonary vascular endothelial cells, smooth muscle cells, and fibroblasts 2ME exerts stronger anti-mitotic effects than E2 itself. E2 and 2ME, despite having similar effects on other cardiovascular cells, have opposing effects on endothelial cells; that is, in endothelial cells, E2 is pro-mitogenic, pro-angiogenic and anti- apoptotic, whereas 2ME is antimitogenic, anti-angiogenic and pro-apoptotic. This may have significant ramifications in severe PAH that involves uncontrolled proliferation of monoclonal, apoptosis resistant endothelial cells. Based on its cellular effects, 2ME should be expected to attenuate the progression of disease and provide protection in severe PAH. In contrast, E2, due to its mitogenic, angiogenic, and anti-apoptotic effects (otherwise desirable in normal, quiescent endothelial cells), may even adversely affect endothelial remodeling in PAH and this may be even more significant if the E2’s effects on injured endothelium are not opposed by 2ME (e.g., in the event of reduced E2 conversion to 2ME due to hypoxia, inflammation, drugs, environmental factors, or genetic polymorphism of metabolizing enzymes). This review focuses on the effects of estrogens and their metabolites on pulmonary vascular pathobiology and the development of experimental PAH, and offers potential explanation for the estrogen paradox in PAH. Furthermore, we propose that unbalanced estradiol metabolism may lead to the development of PAH. Recent animal data and studies in patients with PAH support this concept.
Keywords: Estradiol, estradiol metabolism, 2-methoxyestradiol, 2-methoxyestrone, pulmonary arterial hypertension, endothelial cells
Pulmonary hypertension is not a disease per se but rather a heterogeneous group of clinical entities with broad spectrum of histopathological changes, defined by a single hemodynamic parameter, a mean pulmonary artery pressure exceeding the upper normal limit of 25mmHg at rest (1). The current classification of pulmonary hypertension subtypes (2) includes six large groups (Table 1). Among these groups, pulmonary arterial hypertension (PAH) is a group of diverse diseases with several clinical, histopathological and prognostic features in common. PAH is characterized by severe and progressive increase in pulmonary artery pressure that frequently leads to right ventricular failure and premature death (3). In addition to media hypertrophy and appearance of smooth muscle cells in normally non-muscular arteries (neomuscularization), pulmonary vascular disease in severe PAH is characterized by clustered proliferation of endothelial cells in the lumina of small size pulmonary arteries resulting in concentric obliteration of the lumina and formation of complex vascular structures known as plexiform lesions (4).
Table 1.
Updated Clinical Classification of Pulmonary Hypertension (Dana Point 2008) 2
|
|
This debilitating disease occurs worldwide more frequently in women (5-7), yet animal studies suggest that estrogens have favorable effects in experimental PAH; this is the estrogen paradox in PAH. Little is known about the role of estrogens in PAH, and the role of estrogens with respect to vascular homeostasis in cardiovascular disease, including PAH, remains unclear. The effects of E2 on the structure and function of pulmonary vasculature are complex and still not completely defined, and may vary depending on the targeted vascular layer (media vs. intima) and the condition of the endothelium (intact/quiescent vs. dysfunctional/proliferative) . The plausible effect of E2 on phenotypically modified endothelial cells (the highly proliferative, angiogenic, apoptosis resistant ECs that are seen in severe PAH), would be to adversely affect the progression of disease. This contrasts with the recently identified effects of the estradiol metabolite 2-methoxyestradiol (infra vide).
The goal of this review is to provide insight into the effects of estradiol and its metabolites in PAH. The apparent contradictions posed by the overall effects of estrogens in experimental and human PAH are discussed in the light of the complexity of estradiol metabolism, limitations of the experimental models used and potentially significant influence that the delicate balance between estradiol and its metabolites may exert on pulmonary vascular homeostasis.
Estrogens and Pulmonary Arterial Hypertension
Estrogens exhibit a multitude of cardiovascular effects through genomic pathway by transcriptional activation of their cytosolic or membrane-bound receptors (ER) α and β. However, building evidence indicates an alternative rapid nongenomic pathway that involves membrane G-protein-coupled ER (8, 9). The effects of estrogens on pulmonary vasculature are well defined and are mediated through both non-genomic and genomic mechanisms. Thus estradiol, via rapid, non-genomic mechanisms, increases prostacyclin release and production of nitric oxide (10, 11), and through estrogen receptor-dependent mechanisms increases endothelial cells’ eNOS mRNA levels and eNOS activity (12). Furthermore, ovariectomy augments hypoxia-induced increase in endothelin-1 (ET-1) and prepro-ET1 mRNA levels, and E2 replacement reduces hypoxia-induced increase in preproET-1 mRNA and ET-1 peptide expression (13). The later effect of E2 is mediated via inhibition of hypoxia-induced increases in ET-1 promoter and estrogen response element-mediated reporter gene activity and involves a functional interaction between ER and HIF-dependent pathways. Notably, several reports suggest that 2ME (a major non-estrogenic metabolite of E2 with little or no affinity for estradiol receptors) is more potent that E2 itself in increasing prostacyclin and NO synthesis and in inhibiting endothelin synthesis (infra vide). In vitro, estradiol causes vasorelaxation of pulmonary artery under normoxic conditions (14) and inhibits hypoxic pulmonary vasoconstriction (15). Importantly, exogenous E2 rapidly attenuates pulmonary artery vasoreactivity and acute hypoxic vasoconstriction, suggesting non-genomic mechanisms of E2-induced pulmonary vasorelaxation (16).
In experimental PAH induced by pneumotoxin monocrotaline (MCT) or by exposure to chronic hypoxia, 17β-estradiol (E2) has protective effects. When exposed to chronic hypoxia, female rats, mice and swine develop less severe PAH than male animals and female rats with intact ovaries develop more severe PAH than ovariectomized (OVX) rats, with estradiol attenuating the severity of disease in OVX rats (17-20). Also, estradiol improves pulmonary hemodynamics and vascular remodeling in perinatal PAH in lambs (21), suggesting that estradiol may be protective in PAH. Similarly, in rats with monocrotaline-induced PAH females develop less severe disease than males (22, 23); pretreatment of male rats with E2 attenuates development of PAH and prevents against pulmonary vascular remodeling and right ventricular hypertrophy (24); and ovariectomy (OVX) exacerbates disease, whereas treatment of OVX rats with E2 attenuates it (25). Finally, in female and OVX rats with MCT-induced PAH, both selective estrogen receptor modulator raloxifene and phytoestrogens attenuate PAH (26, 27).
The apparent beneficial effects of estrogens in MCT- and hypoxia-induced PAH paradoxically oppose the female preponderance of the disease. In both chronic hypoxia and MCT models, the hallmark vascular change is medial thickening with little or no alteration of endothelium (Table 2). This opposes the marked endothelial alterations seen in severe PAH in humans. As described below, in pulmonary artery for E2 to inhibit medial remodeling may require intact and quiescent endothelium, and therefore hypoxia- and MCT-induced PAH may not be appropriate model for studying the effects of estrogens in PAH. Our recent findings support this notion. In a rat model of occlusive/angioproliferative PAH which shares many features of the human form of the disease including endothelial disruption (28, 29; Table 2), female rats develop more severe PAH than males, ovariectomy delays the development of PAH, and E2 has no preventive or therapeutic effects on elevated pulmonary artery pressure in OVX rats (30). However, in this model (similarly to humans) females develop less severe right ventricular hypertrophy than males. Finally, in recently developed models of PAH in mice in which disease is induced by consumption of dexfenfluramine (31) or over-expression of serotonin transporter (32) female gender does not provide protection, but rather is permissive in the development of PAH. These recent findings (30-32) contradict earlier studies of protective effects of female gender and estrogens in classical models of experimental PAH (17-27), and suggest that the classical models of PAH (i.e., chronic hypoxia and monocrotaline) may not be appropriate for evaluation of the effects of estrogens in PAH.
Table 2.
Rodent Models of Pulmonary Arterial Hypertension
| Experimental Model | Species | Clinical and histopathological features |
|---|---|---|
| Classical models of PAH | ||
| Monocrotaline (MCT) 141-143 | Rats (Mice) |
In rats, initial mild endothelial injury and early influx of mononuclear inflammatory cells and vascular inflammation (Day 3-7), significant media remodeling and adventitia widening (Week 1-2) and low survival rate (Week 5 post MCT). No neointimal occlusive lesions. In mice less predictable effects. |
| Chronic Hypoxia 143-146 | Rat Mice |
In mice minimal vascular remodeling with mild neomuscularization and media thickening. In rats, more pronounced media remodeling but no intimal injury; younger animals more sensitive; distinct difference in gene expression induced by hypoxia between mice and rats. Animals partially or fully recover after re-exposure to normoxia. |
| New models of “human-like” PAH | ||
| VEGF antagonist SU5416 + Chronic Hypoxia 28,29,140 |
Rat | Development of severe and persistent PAH. Disease continues to progress after removing animals from hypoxic environment; precapillary occlusive endothelial proliferation and formation of plexiform lesions. |
| Monocrotalin + Unilateral Pneumonectomy 147-149 |
Rat | Media hypertrophy and formation of occlusive neointimal lesions. Younger rats develop perivasular proliferative lesions positive for VEGF receptor 2 and α–SM actin. |
| Genetically modified models of PAH | ||
| - Vasoactive intestinal peptide KO (VIP −/−) mouse150 - Overexpression of IL6 151 - Overexpression of S100A4 /Mits1 153,152 - Heterozygous BMPR2-mutant mice154 |
Mice | Develop PAH spontaneously or under hypoxia; not fully characterized models; neointimal injury and/or plexiform lesions present in some but not all models. |
|
Endothelin B receptor deficient rat +MCT155 |
Rats | Occlusive neointimal lesions develop in distal pulmonary arteries |
Human studies, like animal studies, are inconclusive and even contradictory. That is, PAH mainly develops in young women and females are at higher risk of developing PAH (33, 34), yet observational studies suggest estradiol may be protective against development of PAH in high altitude natives (35) and among patients with systemic sclerosis (36, 37). In contrast, a recent study suggests that higher penetration of disease in women with a familial form of PAH is due to altered E2 metabolism (38). Thus, women diagnosed with familial PAH who have a bone morphogenic protein receptor type 2 mutation (BMPR2) have lower 2-hydroxyestradiol /16α–hydroxyestrone urine level ratios than healthy women with the BMPR2 mutation and never diagnosed with familial PAH (39). Furthermore, female gender is associated with increased risk of portopulmonary hypertension in patients with advanced liver disease (39). Also, independently of gender, in patients with advanced liver disease, genetic variation in aromatase (the rate-limiting enzyme in the conversion of androgens to estrogens), which leads to elevated plasma estradiol levels, is associated with increased risk of portopulmonary hypertension (40). Finally, the high prevalence of prolonged exposure to exogenous estrogens in both premenopausal and postmenopausal women with PAH suggests that estrogens may contribute to disease pathogenesis or susceptibility in PAH (41). The above discussion underscores the need for further investigation of the role of estradiol metabolism in PAH.
Estradiol Metabolism and Pulmonary Arterial Hypertension
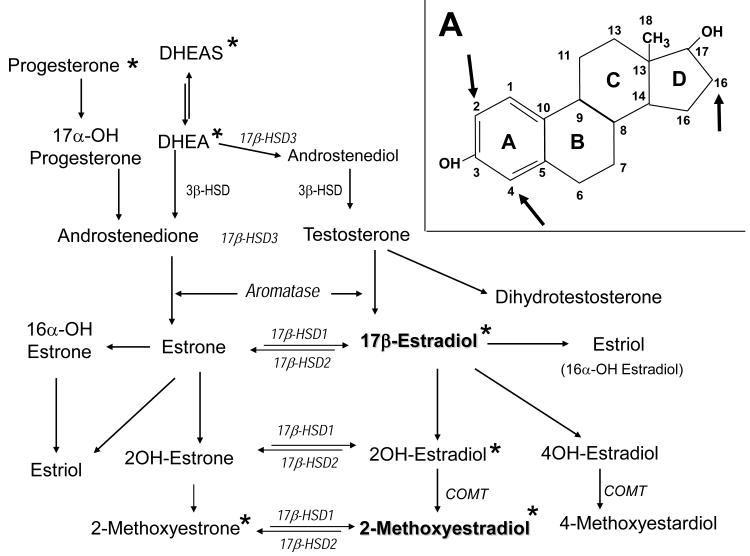
The human body produces three estrogenic steroids: 17-β estradiol (estradiol), estrone and estriol, with estradiol being the most important and estrone and estriol contributing only marginally to the total estrogenic activity in premenopausal women. Biosynthetically, estrogens arise from androstenedione or testosterone by aromatization of the A ring (Figure 1). Androstenedione is produced by both ovaries and adrenal glands in premenopausal women and primarily from adrenal gland in postmenopausal women. Androstenedione and its metabolic precursor dehydroepiandrosterone (DHEA), the most abundant circulating steroids, can be converted to estrogens in a number of extragonadal tissues. Circulating androstenedione is converted to estrone in peripheral tissues. Aromatase in adipose tissue and skin fibroblasts is chiefly responsible for peripheral aromatization of androstenedione to estrone, a weak estrogen. Although only small quantities of estrogens are produced by individual adipocyte or skin fibroblasts, because of their relative abundance, these cells contribute to circulating estradiol levels. Within the same tissue, estrogenically weak estrone is further converted to 17β-estradiol (E2) by type-1 17β-hydroxysteroid dehydrogenase (17β-HSD-1). Both enzymes are present not only in ovaries, but also in many other tissues. Thus, E2, the biologically most active estrogen, in addition to direct secretion from the ovary in reproductive-age women, is also produced in estrogen targeted physiologic tissues (breast, brain, skin, adipocytes, vascular wall) or pathologic tissues (breast cancer, endometriosis). Enzymes responsible for E2 synthesis are also present in cardiovascular cells, i.e., endothelial and vascular smooth muscle cells (VSMCs) and cardiac fibroblasts and myocytes (42, 43); suggesting that local synthesis of estradiol may be important in the cardiovascular system. Significant quantities of circulating androstenedione can also be converted to testosterone. In peripheral tissues, testosterone behaves as a pro-hormone and is converted into its active metabolites, 17β-estradiol and dihydrotestosterone (DHT), the most potent estrogenic and androgenic hormone, respectively.
Figure 1.
Estradiol metabolism and metabolic inter-connections of estrogens, progestins and androgens, and their gonadal and/or adrenal precursors (* - Studied in experimental PAH; See Table 1); Figure 1A - Oxidative metabolism of E2 mainly occurs at the C2, C4 and C16 positions and leads to formation of metabolites with different and often contrary biological effects.
In both in men and women, E2 metabolism occurs primarily by oxidation and this oxidative metabolism of E2 largely determines the nature of its biological effects (44), with dozen of E2 metabolites found to be biologically active. The first step is the conversion of E2 to less estrogenic estrone (E1) by oxidation at C17 position by 17 β -hydroxysteroid-dehydrogenase (17β-HSD), a process that is reversible and favors formation of E1. The reverse reduction may occur, albeit more slowly. Further metabolism includes the metabolic pathways, i.e., oxidation at C16, C4 and C2 position, resulting in production of biologically active “bad” and “good” metabolites. In this regard, oxidative hydroxylation of the D ring at C16 is a major metabolic pathway, the 16-hydroxylation pathway, which leads to the production of estriol (16α–hydroxyestradiol) and 16α-hydroxyestrone. Estriol has less estrogenic activity than E2. However, 16α-hydroxyestrone has estrogenic effects comparable to those of to E2, with significant pro-inflammatory, pro-mitogenic and pro-angiogenic properties. Furthermore, in contrast to E2, 16α-hydroxyestrone exhibits low binding affinity for sex hormone binding globulin, and can covalently bind to estrogen receptors, causing prolonged hyper-estrogenic stimulus in targeted tissue, including proliferation (45,46). The C4 hydroxylation, although a minor metabolic pathway, leads to formation of 4-hydroxyestradiol, a catechol estrogen with estrogenic and carcinogenic activity.
The other major metabolic pathway includes hydroxylation at C2 position. This 2-hydroxylation pathway is the dominant pathway for E2 hydroxylation (47). In contrast to C4 and C16 hydroxylation, C2 hydroxylation is a direct process that involves no unstable intermediates (48) and leads to formation of non-estrogenic metabolites. E2 is metabolized to 2-hydroxyestradiol (2HE) by CYP1A1/CYP1B1. 2HE has little estrogenic activity and is quickly cleared from the plasma (t/2=90′; 49) by prompt conversion (i.e., O-methylation by catechol-O-methyltransferase) to 2-methoxyestradiol (2ME), a metabolite with no estrogenic activity (50, 51). Importantly, the CYP-450 isoforms largely responsible for 2-hydroxylation of E2 (i.e., CYP1A1/2 CYP1B1) are expressed in cardiovascular cells, and conversion of 2HE to 2ME takes place in both endothelial cells and VSMCs (44, 52-54).
2-Methoxyestradiol is extensively metabolized by type-2 17β-hydroxysteroid dehydrogenase (17βHSD-2) to 2-methoxyestrone (2ME1), a metabolite largely lacking antiproliferative activity. Importantly, over-expression and increased activity of 17βHSD-2 abolish the anti-mitogenic effects of 2ME in tumor cells (55). 2ME1 has little or no antimitogenic effect in cardiovascular cells, but may be converted back to 2ME by 17βHSD type-1 and thereby exhibit cardiovascular effects. All-trans retinoic acid (atRA) increases 17βHSD type-1 expression and activity (56, 57), and would thereby be expected to increase the conversion of 2ME1 to 2ME. Our preliminary studies confirm the importance of this metabolic conversion in experimental PAH. In monocrotaline pulmonary hypertensive rats, preventive treatment with 2ME1 attenuates development of PAH, right ventricular hypertrophy, and pulmonary vascular remodeling (58; Table 3), suggesting that in vivo significant conversion of 2ME1 back to 2ME takes place in PAH (56). Furthermore, in male rats with MCT-induced PAH treated with atRA, 2ME or their combination, 2ME and atRA have synergistic effects in ameliorating PAH and vascular remodeling (59). These studies strongly suggest that 2ME-2ME1 inter-conversion takes place in vivo and that the 17β-hydroxysteroid dehydrogenase pathway may play a significant role in the development of PAH.
Table 3.
The Effects of Gender and Sex Steroid Hormones and Their Metabolites and Analogs in Experimental Pulmonary Hypertension
| Animal Model | Intervention Treatment |
Effects | Refernce |
|---|---|---|---|
| Monocrotaline | Gender effects | Female rats develop less severe PAH |
Kiyamate et al 199222 Kiyamate et al 199423 |
| Monocrotaline | Pretreatment with E2 in male rats |
Protection against pulmonary vascular remodeling and right ventricular hypertrophy |
Fargan et al. 199324 |
| Monocrotaline | E2 treatment in OVX rats |
Ovariectomy exacerbates and estradiol attenuates RV hypertrophy |
Ahn et al. 200325 |
| Monocrotaline | Raloxifen in intact females (F) and OVX rats |
Prevents development of PAH in F and OVX rats. More effectively inhibits RV hypertrophy and vascular remodeling in OVX than F rats. |
Nishida et al. 200926 |
| Monocrotaline | Phytoestrogens | Attenuates development of PAH | Homma et al. 200627 |
| Monocrotaline | 2-methoxyestradiol | Preventive and therapeutic effects in male rat |
Tofovic et al. 2005115 Tofovic et al. 2010118 |
| Monocrotaline | 2-hydroxyestradiol | Preventive effects in male rats | Tofovic et al. 2005115 |
| Monocrotaline | 2-methoxyestradiol in ovariectomized rats |
Ovariectomy exacerbates PAH, whereas 2ME attenuate PAH in OVX rats |
Tofovic et al. 2006120 |
| Monocrotaline | 2-ethoxyestradiol | Attenuates development of PAH and vascular remodeling |
Tofovic et al. 200889 |
| Monocrotaline | 2Methoxyestradiol +/− retinoic acid |
Synergistic effects of 2ME with retinoic acid on amelioration of PAH |
Tofovic et al. 200859 |
| Monocrotaline | 2-methoxyestrone | Attenuates development of PAH | Tofovic et al. 200958 |
| Monocrotaline | 2-methoxyestradiol +/− sildenafil or bosentan |
Synergistic effects of 2ME with bosentan or sildenafil on amelioration of PAH and vascular remodeling |
Tofovic et al. 2010a119 |
| Hypoxia | Gender, Age | Female rats develop less severe RV hypertrophy and vascular remodeling; ovariectomy exacerbates PAH; younger animals more sensitive to hypoxia |
Smith et al 197417 Rabinovitch et al 1981182 Resta et al 200119 |
| Hypoxia | Preventive and therapeutic effects of 2- methoxyestradiol in male rats |
Reduces PAH, RV hypetrophy and vascular remodeling |
Tofovic et al. 2006116 |
| Hypoxia | DHEA or DHEAS in male rats |
Inhibits and reverses PAH | Hampl et al. 2003134 Bonett et al. 2003136 Oka 2007136 |
| Bleomyci- ninduced PAH and pulmonary Fibrosis |
OVX, 2ME | Ovariectomy exacerbates disease, whereas 2ME attenuate pulmonary fibrosis and PAH in OVX rats |
Tofovic et al. 200988 |
| Perinatal PAH in lamb |
In utero estradiol treatment |
Reduces pulmonary vascular resistance to normoxia and hypoxia and inhibits vascular remodeling |
Parker et al. 200021 |
| α-naphthyl- thiourea-induced PAH |
2ME | Decreases pleural effusion, reduces acute mortality and attenuates PAH in male rats |
Tofovic et al. 2005117 |
| SERT Mice | Over-expression of serotonin transporter |
Only female SERT mice develop PAH Exposed to hypoxia, wild type females develop less severe PAH than wild type male mice whereas hypoxic females SERT mice develop more severe PAH than male SERT mice. |
Dempsie et al. 2009a32 |
| Dexfenfluramine induced PAH |
Gender effects | Only female mice develop PAH | Dempsie te al. 2009b31 |
| Monocrotaline | Progesterone in OVX rats |
Attenuates the development of PAH | Tofovic et al 2009129 |
| Monocrotaline | Medroxyprogesterone in OVX rats |
Attenuates the development of PAH | Jones et al. 2006130 |
| Monocrotaline | Tibolone | Prevents against development of PAH |
Jones et al. 2006130 |
| Monocrotaline and unilateral pneumectomy |
DHEA | Reverses the development of PAH | Homma et al 2008137 |
| Occlusive-Angio- proliferative PAH in rats |
DHEA | Attenuates established severe PAH | Alzoubi et al. 2010138 |
| Occlusive-Angio- proliferative PAH in rats |
Gender effects | Females protected against RV hypertrophy; Variable effects of female gender on PAH |
Sweeney et al. 2009139 |
| Occlusive angio- proliferative PAH in rats |
Gender effects Ovariectomy Estradiol 2-Methoxyestradiol |
Females develop more severe PAH, but less RV hypertrophy than males; OVX does not exacerbate PAH; E2 and 2ME have similar effects in attenuating RV hypertrophy; 2ME, but not E2, has preventive/therapeutic effects |
Tofovic et al. 200930 |
Limited data exists regarding plasma levels of estradiol and its metabolites in PAH. Importantly, in patients with liver disease, variation in the genes encoding for high activity of aromatase (the rate-limiting enzyme in the conversion of androgens to estrogens) and increased E2 plasma levels are associated with increased risk of portopulmonary hypertension (40). Typical plasma 2ME levels in PAH patients are unknown, but in healthy pre-menopausal women, we recently detected plasma concentrations of 2ME 2-3 fold higher (60) than average menstrual cycle E2 plasma levels (61). A recent study suggests reduced activity of the 2-hydroxylation pathway and reduced 2-hydroxyestradiol/16α-hydroxyestrone ratios in urine in women with familial PAH (38), and it is plausible to expect reduced 2ME plasma levels in PAH.
Hypoxia, and oxidative stress in general, play significant roles in experimental and human PAH (62), and both hypoxia and oxidative stress down-regulate expression of CYP1A1/2 (63, 64), key enzymes controlling the 2-hydroxylation pathway. Hypoxia not only affects E2 metabolism, but also has significant influence on pulmonary vascular remodeling, stimulating proliferation of pulmonary ECs, VSMCs, and adventitial fibroblasts (65), and hypoxia and E2 have synergistic mitogenic effects in endothelial cells (66).
Inflammation is also considered to be an important component in the pathobiology of various types of human PAH because the mediators of inflammation cause vasoconstriction and inflammatory factors/cells may contribute to vascular remodeling (67, 68). Both elevated levels of pro-inflammatory cytokines (69) and the presence of inflammatory cells around plexiform lesions, including macrophages and T and B lymphocytes, have been reported in patients with PAH (70). Inflammatory cells such as macrophages and lymphocytes and pro-inflammatory cytokines such as TNFα, and IL-6 strongly induce aromatase activity (71-73) and peripheral E2 synthesis (74). Notably, estriol (16α-hydroxyestradiol; E3) strongly stimulates the expression of TNFα and IL-6 (75), and thus the shift toward the 16α-hydroxylation pathway should be expected to increase peripheral E2 synthesis. In contrast, 2ME inhibits both basal and TNFα stimulated aromatase activity (76) and thereby may reduce extragonadal synthesis of E2; elevated aromatase activity and plasma E2 levels are associated with increased risk of PAH in patient with portopulmonary PAH (40). Recent studies indicate that in two common inflammatory autoimmune diseases, rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE; a disease with female preponderance), inflamed tissue in both female and male patients hosts significant peripheral conversion of upstream androgenic precursors (androstenedione and testosterone) to estrogens (estradiol and estrone; 71,77,78). Furthermore, a shift in E2 metabolism toward the 16-hydroxylation pathway and production of mitogenic, angiogenic and pro-inflammatory metabolites estriol and 16α-estrone have been reported in patients with RA or SLE (77, 78). The fact that the changes are detected in both genders and in two different immunologic diseases (TH1 vs. TH2) suggests that increased peripheral E2 production and altered E2 metabolism is not gender or disease specific, but rather occurs in a pro-inflammatory environment. The effects of inflammation on E2 metabolism in PAH are unknown. Nonetheless, similarly to the case in other autoimmune inflammatory diseases with female preponderance (RA and SLE), it is plausible that in the pro-inflammatory environment observed in PAH, alterations in E2 metabolism toward increased production of estrogenic, pro-inflammatory, angiogenic and mitogenic metabolites may take place, and that this may adversely affect the pathobiology of severe PAH. Based on the cellular effects of E2 and 2ME discussed below, we propose that conditions leading to unbalanced E2 metabolism (hypoxia, oxidative stress, inflammation, drugs, genetic polymorphism of metabolizing enzymes), i.e., increased E2 and decreased 2ME, may have adverse effects on pulmonary vascular remodeling and progression of disease in severe PAH.
Estradiol Metabolites and Vascular Pathobiology in PAH
A severe form of PAH in humans is characterized by clustered proliferation of endothelial cells (ECs) in the lumina of small size pulmonary arteries resulting in concentric obliteration of the lumina and the formation of complex vascular structures known as plexiform lesions (79). Compared with normal ECs, the endothelial cells in affected vessels show reduced prostacyclin and nitric oxide synthesis (80, 81) and over-expression of endothelin-1 (82). Three-dimensional analysis of vascular lesions reveals the existence of two EC phenotypes in severe PAH: (i) normal quiescent, apoptosis-sensitive ECs located in the peripheral areas that are negative for phosphorylated MAPK and have a high expression of cell cycle inhibitory protein p27kip1 (a marker of low growth potential), and (ii) highly proliferative, apoptosis-resistant cells in the central core of the vascular lesion that have elevated MAPK activity and increased expression of HIF1-α, VEGF protein and VEGF-2 receptor and low expression of p27kip1 (4,83). Thus, the tumor-like proliferation of ECs may be the underlying process in severe PAH (84). Therefore, it is conceivable that agents that reverse the above alterations and have strong anti-proliferative, anti-angiogenic, and pro-apoptotic effects would be effective in the treatment of severe PAH. The published data suggest that this may be the case with 2-methoxyestradiol.
Cumulating evidence suggests that E2 and its downstream metabolites may differ significantly in regard to their antimitogenic effects in cell lines involved in pulmonary vascular remodeling and fibrosis. Both the antimitogenic effects of E2 on proliferation of various types of animal and human vascular smooth muscle cells (VSMCs) and the beneficial effects of E2 on vascular remodeling in systemic circulation are well documented (85,86). However, data on the effects of E2 on pulmonary artery vascular smooth muscle cells (PASMCs) are limited, and it seems that the effects of E2 on PASMCs may differ from those in systemic VSMCs. In this regard, Farhat et al. (87) have reported that E2 dose-dependently stimulates thymidine incorporation in rat PASMCs; furthermore, in canine pulmonary arterial segments, E2 tends to inhibit PASMCs proliferation in segments with intact endothelium, but significantly stimulates thymidine incorporation in segments stripped of endothelium (87). It is possible that E2 may have different anti-remodeling effects on systemic and pulmonary blood vessels of different phylogenetic origin. Additionally, our own recent study in human PASMCs indicates that 2ME exhibits concentration-dependent antigrowth effects whereas E2 has only mild antimitogenic effects and only at micro molar (pharmacological) concentrations (88). Therefore, it is plausible that, in contrast to the well described inhibitory effects of E2 on pulmonary vascular remodeling in classical models of PAH (infra vide; Table 3), in PAH patients with severely altered endothelium, E2 may have no effect on media remodeling or, as described below, may even adversely affect endothelial remodeling.
In human pulmonary artery endothelial cells (hPAECs), E2 stimulates proliferation at physiological concentrations (1–10 nM), has no effects at high physiological concentrations (100 nM), and inhibits EC growth at pharmacological concentrations (1 μM and 10 μM; 89). This is consistent with the previous report of biphasic effects of E2 on growth of human umbilical vein ECs (90). E2, via estrogen receptors, promotes the phosphorylation of p42/44 and p38 MAPK and downregulates cell cycle inhibitor p27Kip1, stimulates migration and proliferation of ECs, induces the synthesis of VEGF and HIF-1α expression, and protects ECs against apoptosis (91-94). The antigrowth effects of E2 observed at higher concentrations may be explained by E2 conversion to 2ME, as both enzymes critical to this conversion (CYP1A1 and COMT) are present in ECs. Further studies should test this possibility. In contrast to E2, 2ME at physiological concentrations (1– 10 nM) had no effects on hPAEC growth (88, 110), but significant though mild stimulatory effects on human umbilical vein ECs (90). It is not clear whether this discrepancy is due to different experimental conditions or to the different EC type examined. In this regard, at 100 nM concentrations, 2ME stimulates the growth of porcine vascular ECs, but has antimitogenic effects in rabbit vascular endothelial cells (95).
Previous studies of cellular growth in fibroblasts from various origins reveal both stimulatory and inhibitory properties of E2 (96, 97). The variable effects of E2 may reflect the phenotypic heterogeneity among fibroblasts, including their differentiation into myofibroblasts. In fetal lung fibroblasts, E2 produces an antimitogenic effect only at micro molar concentrations (98), whereas in our recent study in human lung fibroblasts (hLFs), E2 had no effects on hLFs growth in concentrations up to 10 μM, while 2ME concentration-dependently (10nM-10uM) inhibited proliferation (88). Importantly, in all three types of cells involved in pulmonary vascular remodeling (hPAECs, hPASMCs and hLFs), the synthetic estradiol analog 2-ethoxyestradiol (2EE) is ten times more potent than 2ME in inhibiting growth (89). 2EE also inhibits vascular remodeling in MCT-induced PAH (89), suggesting that anti-proliferative agents, including synthetic analogs of estradiol metabolites, may be protective in PAH.
2ME is extensively metabolized by 17βHSD-2 to 2ME1, a metabolite largely considered to be biologically inactive. Indeed, recently we confirmed that in hPASMCs and human lung fibroblast only at high pharmacological concentration (10μM) 2ME1 has mild (−10%) anti-mitogenic effects (58). However, in the presence of retinoic acid, a type-2 17βHSD-2 inducer, 2ME1 strongly and concentration-dependently (10nM-10μM) inhibits growth in both types of cells (58), indicating that 2ME– 2ME1 inter-conversion takes place in cells involved in vascular remodeling in PAH. The fact that very fast cellular uptake and high (micromolar) intracellular plasma concentrations of 2ME have been reported (99), suggests that 2ME disposition, i.e., 2ME2-2ME1 inter-conversion may play critical role in the biological and pharmacological effects of 2ME in PAH.
The first important aspect of the cellular effects of 2ME is that this major non-estrogenic metabolite of estradiol may modify many of the previously described alterations seen in humans with severe PAH, including levels of prostacyclin, endothelin and nitric oxide, three targets for currently approved therapies for PAH. In this regard, 2ME is markedly more potent, and 2HE somewhat more potent, than E2 itself in increasing prostacyclin synthesis and release (100,101) and in inhibiting endothelin synthesis and MAPK activity in endothelial cells (102), and 2ME is more potent than E2 in stimulating both basal and ionophore induced-NO release from aortic endothelium (103). The order of potency (2ME>2HE>E2) for inhibition of endothelin synthesis and MAPK activity is opposite the order of potency for binding to or activation of estrogen receptors, suggesting the involvement of estrogen receptor-independent mechanisms. At the present in is not clear whether stimulatory effects of E2 on prostacyclin and NO synthesis/release and inhibitory effects of E2 on endothelin system are mediated in part by its downstream metabolite 2ME. Importantly, 2ME inhibits cell growth and induces apoptosis in actively growing, but not in quiescent, endothelial cells (104,105). Furthermore, in a rat model of endothelial injury, 2-ME up-regulates p27Kip1, a key cell cycle inhibitor and a marker of low growth potential (106). 2ME also inhibits the synthesis of hypoxia–inducible factor-1α (HIF-1α 107,108), a transcription factor which regulates more than forty genes and their respective protein products, including those that play a key role in vascular reactivity and remodeling, angiogenesis, and cell proliferation (109). The significance of HIF-1α is evident (i) in severe PAH in humans where HIF-1α is over-expressed in obliterative endothelial lesions (4); (ii) in MCT- and chronic hypoxia-induced PAH, where a similar time dependent increase in HIF-1α levels correlates with the development of PAH and vascular remodeling (110; and (iii) in mice where heterozygous deficiency of HIF-1α and HIF-2α protects against development of PAH (111,112).
The second important aspect of the cellular effects of 2ME is that at the endothelium level, 2ME may behave as a biological antagonist to E2. First, contrary to the case in other cardiovascular cells, E2 and 2ME have opposing effects on endothelial cells, a pivotal cell type in the pathobiology of PAH. Second, at physiological concentrations, 2ME interferes with non-genomic, ERα-mediated action of E2. Thus, in human leiomyoma cells, over-expression of COMT inhibits E2-induced proliferation, ERα signaling and HIF-1α expression (113), and importantly, the same effects are produced by 2ME. Similarly, in breast cancer cells, physiological concentrations (10-50nM) of 2ME inhibit the E2-induced growth and prevent E2-induced Akt phosphorylation (114). Therefore, it is plausible that in endothelium 2ME antagonizes the effects of E2.
Based on its cellular effects, as a unique endogenous molecule, 2ME may be capable of correcting key alterations in pulmonary vasculature seen in severe PAH, and therefore should be expected to provide protection in severe PAH. Indeed, our previous studies unequivocally confirm that 2ME and its metabolic precursors and analogs are effective in treatment and prevention of experimental PAH in rats. Thus, in male rats, 2ME, its metabolic precursor 2HE, and/or its synthetic analog 2-ethoxyestradiol attenuate MCT-, hypoxia-, and α-naphthylthiourea-induced PAH (89,115-119). Furthermore, in studies in ovariectomized female rats with MCT-induced PH and bleomycin-induced pulmonary fibrosis and PAH, 2ME mediates the protective effects of E2 (89,120). Also, in a model of occlusive angioproliferative PAH, 2ME, but not E2, has preventive and therapeutic effects in intact and OVX female rats (30).
The effects of progestins and androgens in experimental PAH
The effects of progestins in experimental PAH have been poorly studied and the effects of progesterone in patients with PAH are unknown. This is surprising, as progesterone receptors are expressed in intact human endothelial cells (ECs) and in modified ECs within plexiform lesions from patients with PAH (121,122); natural progesterone inhibits proliferation of ECs and VSMCs (123-126); and progesterone exerts vasodilatatory properties in various vascular beds (127,128). With this mind, we recently examined the effects of natural progesterone and synthetic progestins medroxyprogesterone and tibolone on the development of MCT-induced PAH in ovariectomized female rats. Progesterone attenuated development of PAH and right ventricular hypertrophy and inhibited pulmonary vascular remodeling (129). In a separate study, medroxyprogesterone showed effects similar to progesterone, and notably, tibolone, a combined progestin/estrogen compound, prevented development of PAH and RV hypertrophy and eliminated MCT-induced late (35 days post-administration) mortality (130). These studies are limited by the fact that they were conducted in estradiol (E2) deficient animals, and therefore, it is unclear whether progesterone would demonstrate the same effects on PAH development in the presence of E2, given the significant and complex receptor-level interaction between the two compounds. Further studies are warranted to investigate more fully the role of progesterone in PAH and how its interaction with E2 influences its effects.
There are no data on the effects of testosterone in PAH and limited data concerning its effects on pulmonary circulation. Testosterone induces vasodilation in the isolated rat pulmonary vasculature most likely via calcium antagonistic effects (131), and it has been confirmed that in isolated human pulmonary arteries, these vasodilatory effects are independent of gender (132). In rodents, testosterone is an even more potent pulmonary vasodilator than estradiol (133). Interestingly, recent studies suggest that dehydroepiandrostendione (DHEA), which serves as a circulating precursor for both estradiol and testosterone, has significant effects in experimental PAH. DHEA protects against development of PAH and reverses PAH associated with chronic hypoxia (134-136), and these effects are associated with increased expression and function of pulmonary artery Ca++ activated K+ channels (135) and upregulated soluble guanylate cyclase (136). Furthermore, DHEA reverses PAH in male rats with PAH induced by MCT and unilateral pneumectomy (which exhibits neointimal proliferation) and also in a model of occlusive/angioproliferative PAH (SU5416+hypoxia; 137,138); treatment with DHEA is associated with increased plasma levels of both E2 and testosterone. The fact that DHEA and its sulfated ester DHEAS have the highest level of all circulating steroids indicates that interconversion of circulating steroid hormones (Figure 1) may serve as the mediator of the female predominance of human PAH, but data regarding the effects of DHEA in female animal models and patients with PAH are lacking.
Hypothesis
We previously proposed (115) that the apparent contradictions posed by the overall effects of estrogens in both experimental and human PAH—the estrogen paradox in PAH—may be explained by the complexity of estradiol metabolism and the influential balance between estradiol and its metabolites on pulmonary vascular homeostasis (Figure 1). We further propose that in PAH, 2ME present in injured endothelium acts as biological antagonist of E2, and inhibits the mitogenic, angiogenic, and anti-apoptotic effects of E2 ( effects otherwise desirable in normal, quiescent endothelial cells). The adverse vascular effects of E2 in PAH may be even more significant if they are not opposed by 2ME (e.g., in the event of reduced E2 conversion to 2ME due to hypoxia, inflammation, drugs, environmental factors or genetic polymorphism of metabolizing enzymes). Both local conversion of estradiol to 2-methoxyestradiol and in situ 2ME disposition (i.e., 2ME -2ME1 inter-conversion by 17β-HSD) may have a beneficial impact on vascular pathobiology in PAH. Therefore, we hypothesize that unbalanced estradiol metabolism (i.e., a shift toward the 16-hydroxylation pathway and/or reduced activity of the 2-hydroxylation pathway, corresponding to elevated E2 and decreased 2ME levels) increases the risk of PAH and/or exacerbates the progression of disease.
Future directions
The female preponderance of PAH is well documented, yet our understanding of the role of estrogens in development and progression of PAH are quite limited. Further studies of estradiol metabolism and in-depth investigation of the vascular effects of the more than dozen biologically active metabolites of E2 in PAH are required. Preclinical studies should include newer animal models of PAH with significant pulmonary endothelial injury and with genetically or pharmacologically altered E2 metabolism. The highly selective and sensitive liquid chromatography-mass spectrometry methods rapidly becoming available for simultaneous quantification of multiple estradiol metabolites should foster further investigation and elucidation of the roles of E2 and its metabolites in PAH.
The effects of 2ME in patients with PAH are unknown. However, in several clinical studies investigating the potential antitumor effects of 2ME, high doses of 2ME were well tolerated in patients with solid malignancies (156). Likewise, in a phase I clinical trial in healthy volunteers, we recently detected no estrogenic or other adverse effects from a subcutaneously injected long-acting formulation of 2ME in doses up to 10 mg/kg (157). Both agents with strong anti-proliferative, anti-angiogenic and pro-apoptotic effects, such as 2-methoxyestradiol, and interventions that increase the bioavailability of endogenous 2ME potentially could be effective in the treatment of severe PAH. In this regard, a dozen 2ME analogs and inhibitors of 17β-HSD (which may improve 2ME disposition) have been synthesized and are available for pharmacological evaluation, including investigation of potential therapeutic effects in PAH.
Finally, our knowledge about the effects of progestins and androgens in PAH are scant at best. Progestins and androgens may also have significant influence on vascular pathobiology; because of their significant multilevel interactions with estrogens, further studies in both animals and humans are needed to fully understand the effects of sex steroid interplay on the development and progression of PAH.
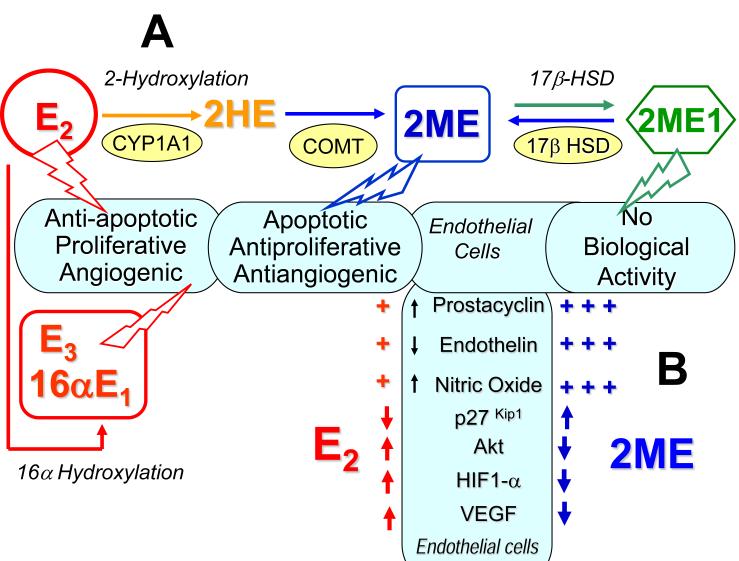
Figure 2. The potential role of estradiol metabolism in PAH.
(A) Activities of 2-hydroxylation, 16α-hydroxylation and 17β–HSD pathways determine the overall biological effects of E2 in PAH. (B) In endothelial cells, 2-methoxyestradiol (2ME) is a more potent modulator of prostacyclin, endothelin and nitric oxide synthesis/release than estradiol (E2); 2ME and E2 have opposite effects on endothelial pathobiology in PAH (2HE, 2-Hydroxyestradiol; 2ME1, 2-Methoxyestrone; E3, Estriol; 16αE1 , 16α–Estrone).
Acknowledgment
The author thanks Victor Bilan, BS, for assistance in editing the article.
This work was supported in part by an award (#0455778U) from the American Heart Association and NIH grant # HL080560. The author is co-inventor on U.S. Patent: Estradiol Metabolites for the Treatment of Pulmonary Hypertension.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Badesh BD, Champion HC, Gomez-Sanchez MA, Hoper M, Loyd J, Manes A, McGoon MD, Naeije R, Olshewski H, Oudiz R, Torbicki A. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–S56. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine S, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: report of a National Heart, Lung and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 4.Cool CD, Stewart JS, Werahera P, Miller GJ, Williams RL, Voelkel NF, Tuder RM. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Am J Pathol. 1999;155:411–419. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thenappan T, Shah SJ, Rich S, Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension: 1982-2006. Eur Res J. 2007;30:1103–1110. doi: 10.1183/09031936.00042107. [DOI] [PubMed] [Google Scholar]
- 6.Jing ZC, Xu XQ, Han ZY, Wu Y, Deng KW, Wang H, Wang ZW, Cheng XS, Xu B, Hu SS, Hui RT, Yang YJ. Registry and survival study in Chinese patients with idiopathic and familial pulmonary hypertension. Chest. 2007;132:373–379. doi: 10.1378/chest.06-2913. [DOI] [PubMed] [Google Scholar]
- 7.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. Pulmonary Arterial Hypertension in France: Results from a National Registry. Am J Res Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 8.Mendelson ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol. 2002;90(1A):3F–6F. doi: 10.1016/s0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- 9.Ling S, Komesaroff P, Sudhir K. Cellular mechanism underlying the cardiocvascular actions of oestrogens. Clin Sci (Lond) 2006;111:107–118. doi: 10.1042/CS20050084. [DOI] [PubMed] [Google Scholar]
- 10.Lantin-Hermoso RL, Rosenfeld CR, Yuhanna IS, German Z, Chen Z, Shaul PW. Estrogen acutely stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. Am J Physiol. 1997;273:L119–126. doi: 10.1152/ajplung.1997.273.1.L119. [DOI] [PubMed] [Google Scholar]
- 11.Sherman TS, Chambliss KL, Gibson LL, Pace MC, Mendelsohn ME, Pfister SL, Shaul PW. Estrogen acutely activates prostacyclin synthesis in bovine fetal pulmonary artery endothelium. Am J Respir Cell Mol Biol. 2002;26:610–616. doi: 10.1165/ajrcmb.26.5.4528. [DOI] [PubMed] [Google Scholar]
- 12.MacRitchie AN, Jun SS, Chen Z, German Z, Yuhanna IS, Sherman TS, Shaul PW. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res. 1997;81:355–362. doi: 10.1161/01.res.81.3.355. [DOI] [PubMed] [Google Scholar]
- 13.Earley S, Resta TC. Estradiol attenuates hypoxia-induced pulmonary endothelin-1 gene expression. Am J Physiol Lung Cell Mol Physiol. 2002;283:L86–93. doi: 10.1152/ajplung.00476.2001. [DOI] [PubMed] [Google Scholar]
- 14.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Horm Metab Res. 2001;33:645–652. doi: 10.1055/s-2001-18689. [DOI] [PubMed] [Google Scholar]
- 15.Gordon JB, Wetzel RC, McGeady ML, Adkinson NF, Jr, Sylvester JT. Effects of indomethacin on estradiol-induced attenuation of hypoxic vasoconstriction in lamb lungs. J Appl Physiol. 1986;61:2116–2121. doi: 10.1152/jappl.1986.61.6.2116. [DOI] [PubMed] [Google Scholar]
- 16.Lahm T, Crisostomo PR, Markel TA, Wang M, Weil BR, Novotny NM, Meldrum DR. The effects of estrogen on pulmonary artery vasoreactivity and hypoxic pulmonary vasoconstriction: potential new clinical implications for an old hormone. Crit Care Med. 2008;36:2174–2183. doi: 10.1097/CCM.0b013e31817d1a92. [DOI] [PubMed] [Google Scholar]
- 17.Smith PH, Moosavi M, Wilson M, Heath D. The influence of age and sex on the response to right ventricle, pulmonary vasculature, and carotid bodies to hypoxia in rats. J Pathol. 1974;112:11–18. doi: 10.1002/path.1711120104. [DOI] [PubMed] [Google Scholar]
- 18.Rabinovitch M, Gamble WJ, Miettinen OS, Reid L. Age and sex influence on pulmonary hypertension of chronic hypoxia and recovery. Am J Physiol Heart Circ Physiol. 1981;240:H62–H72. doi: 10.1152/ajpheart.1981.240.1.H62. [DOI] [PubMed] [Google Scholar]
- 19.Resta TC, Knaggy NL, Walker BR. Estradiol-induced attenuation of pulmonary hypertension is not associated with altered eNOS expression. Am J Physiol Lung Cell Moll Physiol. 2001;280:L88–L97. doi: 10.1152/ajplung.2001.280.1.L88. [DOI] [PubMed] [Google Scholar]
- 20.Stupfel M, Pesce VH, Gourlet V, Bouley G, Elabed A, Lemercerre C. Sex-related factors in acute hypoxia survival in one strain of mice. Aviat Space Environ Med. 1984;55:136–140. [PubMed] [Google Scholar]
- 21.Parker TA, Ivy DD, Galan HL, Grover R, Kinsella JP, Abmam SH. Estradiol improves pulmonary hemodynamics and vascular remodeling in perinatal pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2000;278:L374–381. doi: 10.1152/ajplung.2000.278.2.L374. [DOI] [PubMed] [Google Scholar]
- 22.Kiyatake K, Kaneko N, Okada O, Kakusaka I, Nagao K, Kuriyama T. Role of liver microsome for sexual difference in monocrotaline-treated rats. Am Rev Respir Dis. 1992;145:A650. [Google Scholar]
- 23.Kiyatake K, Kakusaka I, Kasahara Y, Qi TM, Suzuki A, Nakano K, Kaneko N, Kitada M, Kuriyama T. Relationship between the converting ability of liver microsomes and monocrotaline-induced pulmonary hypertension in male, female and castrated male rats. Nihon Kyobu Shikkan Gakkai Zasshi. 1994;32:125–129. [PubMed] [Google Scholar]
- 24.Farhat MY, Chen MF, Bhatti T, Iqbal A, Cathapermal S, Ramwell PW. Protection by oestradiol against the development of cardiovascular changes associated with monocrotaline pulmonary hypertension in rats. Br J Pharmacol. 1993;110:719–723. doi: 10.1111/j.1476-5381.1993.tb13871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn BH, Park HK, Cho HG, Lee HA, Lee YM, Yang EK, Lee WJ. Estrogen and enalapril attenuate the development of right ventricular hypertrophy induced by monocrotaline in ovariectomized rats. J Korean Med Sci. 2003;18:641–648. doi: 10.3346/jkms.2003.18.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishida M, Hasegawa Y, Tanida I, Nakagawa E, Injai H, Ohkita M, Matsumura Y. Preventive effects of raloxifene, a selective estrogen receptor modulator, on monocrotaline-induced pulmonary hypertension in intact and ovariectomized female rats. Eur J Pharmacol. 2009;614:70–76. doi: 10.1016/j.ejphar.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Homma N, Morio Y, Takahashi H, Yamamoto A, Suzuki T, Sato K, Muramatsu M, Fukuchi Y. Genistein, a phytoestrogen, attenuates monocrotaline-induced pulmonary hypertension. Respiration. 2006;73:105–112. doi: 10.1159/000088946. [DOI] [PubMed] [Google Scholar]
- 28.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Waltenberger J McMahon, Voelkel NF, Tuder RM. inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 29.Taraseviciene-Stewart L, Gera L, Hirth P, Voelkel NF, Tuder RM, Stewart JM. Bradykinin antagonist and a caspase inhibitor prevent severe pulmonary hypertension in a rat model. Can J Physiol Pharmacol. 2002;80:269–274. doi: 10.1139/y02-047. [DOI] [PubMed] [Google Scholar]
- 30.Tofovic SP, Rafikova O. Preventive and Therapeutic Effects of 2-Methoxyestradiol, but not Estradiol, in Severe Occlusive Pulmonary Arterial Hypertension in Female Rats. Am J Res Crit Care Med. 2009;179:A1802. [Google Scholar]
- 31.Dempsie Y, MacRitchie NA, Morecroft I, Loughlin L, MacLean MR. The effects of gender on the development of dexfenfluramine-induced pulmonary hypertension in mice. Am J Res Crit Care Med. 2009;179:A1808. [Google Scholar]
- 32.Dempsie Y, Nilsen M, Loughlin L, MacLean MR. The effects of gender on the development of pulmonary arterial hypertension in mice over-expressing the serotonin transporter. Am J Res Crit Care Med. 2009;179:A1809. [Google Scholar]
- 33.Wood P. Pulmonary hypertension. Br Med Bull. 1952;8:348–53. doi: 10.1093/oxfordjournals.bmb.a074201. [DOI] [PubMed] [Google Scholar]
- 34.Rubin L. Primary Pulmonary Hypertension. N Engl J Med. 1997;33:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 35.Turini P, Allemann Y, Sartori C, Hutter D, Thalmann S, Salinas C, Villena M, Scherrer U. Protective effects of female sex hormones against pulmonary hypertension in Bolivian high altitude natives. Circulation. 2003;111:B54. [Google Scholar]
- 36.Scorza R, Caronni M, Bazzi S, Nador F, Beretta L, Antonioli R, Origgi L, Ponti A, Marchini M, Vanoli M. Post-menopause is the main risk factor for developing isolated pulmonary hypertension in systemic sclerosis. Ann N Y Acad Sci. 2002;966:238–246. doi: 10.1111/j.1749-6632.2002.tb04221.x. [DOI] [PubMed] [Google Scholar]
- 37.Arlett CM, Rasheed M, Russo-Stieglitz KE, Sawaya HHB, Jimenez SA. Influence of prior pregnancies on disease course and cause of death in systemic sclerosis. Ann Rheum Dis. 2002;61:346–350. doi: 10.1136/ard.61.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austin ED, Cogan JD, West JD, Hedges LK, Hamid R, Dawson EP, Wheeler LA, Parl FF, Loyd JE, Phillips JA., III Alterations in oestrogen metabolism: implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur Respir J. 2009;34:1093–1099. doi: 10.1183/09031936.00010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawut S, Krowka MJ, Trotter JF, Roberts KE, Benza RL, Badesch DB, Taichman DB, Horn EM, Zacks S, Kaplowitz N, Brown RS, Jr, Fallon MB. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008;48:196–203. doi: 10.1002/hep.22275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts KE, Fallon MB, Krowka MJ, Brown RS, Trotter JF, Peter I, Tighiouart H, Knowles JA, Rabinowitz D, Benza RL, Badesch DB, Taichman DB, Horn EM, Zacks S, Kaplowitz N, Kawut SM. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Respir Crit Care Med. 2009;179:835–842. doi: 10.1164/rccm.200809-1472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sweeney LB, Voelkel NF. Estrogen exposure in women with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2009;179:A4870. [Google Scholar]
- 42.Harda N, Sasano H, Mukamari H, Ohkuma T, Nagura H, Takagi Y. Localized expression of aromatase in human vascular tissues. Cir Res. 1999;84:1285–1291. doi: 10.1161/01.res.84.11.1285. [DOI] [PubMed] [Google Scholar]
- 43.Park BK. Cytochrome P450 enzymes in the heart. Lancet. 2000;355:945–946. doi: 10.1016/S0140-6736(00)90008-4. [DOI] [PubMed] [Google Scholar]
- 44.Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17β estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003;144:3382–3398. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- 45.Fishman J, Martucci C. Biological properties of 16a-hydroxyestrone: implications in estrogen physiology and pathophysiology. J Clin Endocr Metab. 1980;51:611–615. doi: 10.1210/jcem-51-3-611. [DOI] [PubMed] [Google Scholar]
- 46.Swanek GE, Fishman J. Covalent binding of the endogenous estrogen 16α-hydroxyestrone to estradiol receptor in human breast cancer cells: Characterization and intranuclear localization. Proc Natl Acad Sci USA. 1988;85:7831–7835. doi: 10.1073/pnas.85.21.7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jellinck PH, Hahn EF, Fishman J. Absence of reactive intermediates in the formation of catechol estrogens by liver microsomes. J Biol Chem. 1986;261:7729–7732. [PubMed] [Google Scholar]
- 48.Jellinck PH, Fishman J. P-450-catalyzed oxidations. Biochemistry. 1988;27:6111–6116. doi: 10.1021/bi00416a042. [DOI] [PubMed] [Google Scholar]
- 49.Ball P, Emons G, Kayser H, Teichmann J. Metabolic clearance rates of catechol estrogens in rats. Endocrinology. 1983;113:1781–1783. doi: 10.1210/endo-113-5-1781. [DOI] [PubMed] [Google Scholar]
- 50.Liu D, Bachmann KA. An investigation of the relationship between estrogen, estrogen metabolites and blood cholesterol levels in ovariectomized rats. J Pharmacol Exp Ther. 1988;286:561–568. [PubMed] [Google Scholar]
- 51.Lakham NJ, Sarkar MA, Venitz J, Figg WD. 2-methoxyestradiol, a promising anticancer drug. Pharmacotherapy. 2003;23:165–172. doi: 10.1592/phco.23.2.165.32088. [DOI] [PubMed] [Google Scholar]
- 52.Farin FM, Pohlman TH, Omiecinski CJ. Expression of cytochrome P450s and microsomal epoxide hydrolase in primary cultures of human umbilical vein endothelial cells. Toxicol Appl Pharmacol. 1994;124:1–9. doi: 10.1006/taap.1994.1001. [DOI] [PubMed] [Google Scholar]
- 53.Zacharia LC, Jackson EK, Gillespie DG, Dubey RK. Increased 2-methoxyestradiol production in human coronary versus aortic vascular cells. Hypertension. 2001;37:658–662. doi: 10.1161/01.hyp.37.2.658. [DOI] [PubMed] [Google Scholar]
- 54.Dubey RK, Gillespie DG, Zacharia LC, Roselli M, Imthurn B, Jackson EK. 2-Methoxyestradiol mediates the antimitogenic effects of locally applied estradiol on cardiac fibroblast growth. Hypertension. 2002;39:412–417. doi: 10.1161/hy0202.102837. [DOI] [PubMed] [Google Scholar]
- 55.Newman S, Ireson CR, Tutill HJ, Day JM, Parsons MFC, Leese MP, Potter BVL, Reed MJ, Purohit A. The role of 17β-hydroxysteroid dehydrogenases in modulating the activity of 2-methoxyestradiol in breast cancer cells. Cancer Res. 2006;66:324–330. doi: 10.1158/0008-5472.CAN-05-2391. [DOI] [PubMed] [Google Scholar]
- 56.Bayard F, Clamens S, Meggetto F, Blaes N, Delsol D, Faye JC. Estrogen synthesis, estrogen metabolism, and functional estrogen receptors in rat arterial smooth muscle cells in culture. Endocrinology. 1995;136:1523–1529. doi: 10.1210/endo.136.4.7895662. [DOI] [PubMed] [Google Scholar]
- 57.Piao Y-S, Peltoketo H, Jouppila A, Vihko R. Retinoic Acids increase 17ß-Hydroxysteroid Dehydrogenase Type 1 Expression in JEG-3 and T47D Cells, but the Stimulation Is Potentiated by Epidermal Growth Factor, 12-O- Tetradecanoylphorbol-13-Acetate, and Cyclic Adenosine 3′,5′-Monophosphate Only in JEG-3 Cells. Endocrinology. 1997;138:898–904. doi: 10.1210/endo.138.3.5008. [DOI] [PubMed] [Google Scholar]
- 58.Tofovic SP, Zhu H, Jackson EK, Rafikova O. 2-Methoxyestrone Inhibits Vascular Remodeling and Attenuates Monocrotaline-Induced Pulmonary Hypertension. Eur Respir J. 2008;32(Suppl. 52):164S. doi: 10.1016/j.vph.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Tofovic SP, Jackson EK, Rafikova O. Synergistic Effects of 2-Methoxyestradiol and Retinoic Acid on Amelioration of Monocrotaline-Induced Pulmonary Hypertension. Am J Res Crit Care Med. 2008;177:A592. [Google Scholar]
- 60.Tofovic SP, Jackson EK, Tofoski GJ. Dysregulated estradiol metabolism in preeclampsia. Physiologist. 2007;50:21. [Google Scholar]
- 61.Thorneycroft IH, Mishell DR, Jr, Stone SC, Kharma KM, Nakamura RM. The relation of serum 17-hydroxyprogesterone and estradiol-17-beta levels during the human menstrual cycle. Am J Obstet Gynecol. 1971;111:947–951. doi: 10.1016/0002-9378(71)90951-3. [DOI] [PubMed] [Google Scholar]
- 62.Hoshikawa Y, Ono S, Suzuki S, Tanita T, Chida M, Song C, Noda M, Tabats T, Voelkel NF, Fujimura S. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J Appl Physiol. 2001;90:1299–1306. doi: 10.1152/jappl.2001.90.4.1299. [DOI] [PubMed] [Google Scholar]
- 63.Fradette C, Batonga J, Teng S, Piquette-Miller M, Souich P. Animal models of acute moderate hypoxia are associated with a down-regulation of CYP1A1, 1A2, 2B4, 2C5, and 2C16 and up-regulation of CYP3A6 and P-glycoprotein in liver. Drug Metabolism Disposition. 2007;35:765–771. doi: 10.1124/dmd.106.013508. [DOI] [PubMed] [Google Scholar]
- 64.Taherzadeh M, Fradette C, Bleau A-M, Jomphe C, Trudeau L-E, du Souich P. The 21-aminosteroid U74389G prevents the down-regulation and decrease in activity of CYP1A1, 1A2 and 3A6 induced by an inflammatory reaction. Biochem Pharmacol. 2006;71:366–376. doi: 10.1016/j.bcp.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 65.Pak O, Aldashev A, Welsh D, Peacock A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J. 2007;30:364–372. doi: 10.1183/09031936.00128706. [DOI] [PubMed] [Google Scholar]
- 66.Kleinman ME, Greives MR, Churgin SS, Blechman KM, Chang EI, Ceradinin DJ, Tepper OM, Gurtner GC. Hypoxia-induced mediators of stem/progenitor cell trafficking are increased in children with hemangioma. Arterioscler Thromb Vasc Biol. 2007;27:2664–2670. doi: 10.1161/ATVBAHA.107.150284. [DOI] [PubMed] [Google Scholar]
- 67.Tuder RM, Voelkel NF. Pulmonary hypertension and inflammation. J Lab Clin Med. 1998;132:16–24. doi: 10.1016/s0022-2143(98)90020-8. [DOI] [PubMed] [Google Scholar]
- 68.Hassoun PM, Mouthon L, Barberà JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(Suppl 1):S10–19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151:1628–1631. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 70.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J. 2005;26:1110–1118. doi: 10.1183/09031936.05.00045705. [DOI] [PubMed] [Google Scholar]
- 71.Folomeev M, Dougados M, Beanue J, Kouyoumdjian JC, Nahoul K, Amor B, Alekberova Z. Plasma sex hormones and aromatase activity in tissues of patients with systemic lupus erythematous. Lupus. 1992;1:191–195. doi: 10.1177/096120339200100312. [DOI] [PubMed] [Google Scholar]
- 72.Reed MJ, Coldham NG, Patel SR, Ghilchik MW, James VHT. Interleukin-1 and interleukin-6 in breast cyst fluid: their role in regulating aromatase activity in breast cancer cells. J Endocrinol. 1992;132:R5–8. doi: 10.1677/joe.0.132r005. [DOI] [PubMed] [Google Scholar]
- 73.Macdiarmid F, Wang D, Duncan LJ, Purohit A, Ghilchik MW, Reed MJ. Stimulation of aromatase activity in breast fibroblasts by tumor necrosis factor. Mol Cell Endocrinol. 1994;106:17–21. doi: 10.1016/0303-7207(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 74.Seriolo B, Accardo S, Garnero A, Fasciolo D, Cutolo M. Association between anticardiolipin antibody positivity and increased 17-beta-estradiol levels in premenopausal women with rheumatoid arthritis. Ann N Y Acad Sci. 1999;876:159–163. doi: 10.1111/j.1749-6632.1999.tb07635.x. [DOI] [PubMed] [Google Scholar]
- 75.Zuckerman SH, Ahmari SE, Bryan-Poole N, Evans GF, Short L, Glasebrook AL. Estriol: a potent regulator of TNF and IL-6 expression in a murine model of endotoxemia. Inflammation. 1996;20:581–97. doi: 10.1007/BF01488797. [DOI] [PubMed] [Google Scholar]
- 76.Purohit A, Singh A, Ghlchik MW, Reed MJ. Inhibition of tumor necrosis factor a-stimulated aromatase activity by microtubule-stabilizing agents, paclitaxel and 2-methoxyestradiol. Biochem Biophys Res Commun. 1999;261:214–217. doi: 10.1006/bbrc.1999.1010. [DOI] [PubMed] [Google Scholar]
- 77.Castagnetta LA, Carruba G, Granata OM, Stefano R, Miele M, Schmidt M, Cutolo M, Straub RH. Increased estrogen formation and estrogen to androgen ratio in the synovial fluid of patients with rheumatoid arthritis. J Rheumatol. 2003;30:2597–2605. [PubMed] [Google Scholar]
- 78.Cutolo M, Capellino S, Sulli A, Serioli B, Secchi ME, Villaggio B, Straub RH. Estrogens and autoimmune diseases. Ann N Y Acad Sci. 2006;1089:538–47. doi: 10.1196/annals.1386.043. [DOI] [PubMed] [Google Scholar]
- 79.Tuder RM, Voelkel NF. Angiogenesis and pulmonary hypertension. A unique process in a unique disease. Antioxid Redox Signal. 2002;4:833–843. doi: 10.1089/152308602760598990. [DOI] [PubMed] [Google Scholar]
- 80.Tuder RM, Cool CD, Geracu MW, Wang J, Abman SH, Wright L, Badesch DB, Voelkel NF. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 81.Glaid A, Salah D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 82.Glaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shnnib H, Kimura S, Masaki T, Duguid WP, Steward DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 83.Tuder RM, Chacon M, Alger L, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci M, Semenza GL, Yacoub M, Polak JM, Voelkel NF. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathology. 2001;195:367–74. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 84.Loscalzo J. Endothelial dysfunction in pulmonary hypertension. N Engl J Med. 1992;327:70–75. doi: 10.1056/NEJM199207093270209. [DOI] [PubMed] [Google Scholar]
- 85.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 86.Klouche M. Estrogens in human vascular diseases. Ann N Y Acad Sci. 2006;1089:431–443. doi: 10.1196/annals.1386.032. [DOI] [PubMed] [Google Scholar]
- 87.Farhat MY, Vargas R, Dingaan B, Ramwell PW. In vitro effect of oestradiol on thymidine uptake in pulmonary vascular smooth muscle cell: role of the endothelium. Br J Pharmacol. 1992;107:679–683. doi: 10.1111/j.1476-5381.1992.tb14506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tofovic SP, Zhang X, Jackson EK, Zhu H, Petrusevska G. 2-Methoxyestradiol Attenuates Bleomycin-Induced Pulmonary Hypertension and Fibrosis in Estrogen-Deficient Rats. Vasc Pharmacol. 2009;51:190–197. doi: 10.1016/j.vph.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tofovic SP, Zhang X, Zhu H, Jackson EK, Rafikova O, Petrusevska G. 2-Ethoxyestradiol is antimitogenic and attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling. Vasc Pharmacol. 2008;48:174–183. doi: 10.1016/j.vph.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 90.Lippert C, Seeger H, Mueck AO, Lippert TH. The effects of A-ring and D-ring metabolites of estradiol on the proliferation of vascular endothelial cells. Life Sci. 2000;67:1653–1658. doi: 10.1016/s0024-3205(00)00747-5. [DOI] [PubMed] [Google Scholar]
- 91.Dupont J, Karas M, LeRoith D. The potentiation of estrogen of insulin-like growth factor I action in MCF-7 human cancer cells includes cell cycle components. J Bio Chem. 2000;275:35893–35900. doi: 10.1074/jbc.M006741200. [DOI] [PubMed] [Google Scholar]
- 92.Suzuma I, Mandai M, Takagi H, Suzuma K, Otani A, Oh H, Kobayashi K, Honda Y. 17-Estradiol increases VEGF receptor-2 and promotes DNA synthesis in retinal microvascular endothelial cells. Invest Opthalmol Vis Sci. 1999;40:2122–2129. [PubMed] [Google Scholar]
- 93.Geraldes P, Sirois MG, Bernatchez PN, Tanguay JF. Estrogen regulation of endothelial and smooth muscle cell migration and proliferationw. Role of p38 and p42/44 mitogen-activate protein kinase. Arterioscler Thromb Vasc Biol. 2002;22:1585–1590. doi: 10.1161/01.atv.0000035393.11854.6a. [DOI] [PubMed] [Google Scholar]
- 94.Spyridopoulos I, Sullivan AB, Kearney M, Isner JM, Losordo DW. Estrogen-receptor-mediated inhibition of human endothelial cell apoptosis. Estradiol as a survival factor. Circulation. 1997;95:1505–1514. doi: 10.1161/01.cir.95.6.1505. [DOI] [PubMed] [Google Scholar]
- 95.Shang W, Konidari I, Schomberg DW. 2-Methoxyestradiol, an endogenous estradiol metabolite, differentially inhibits granulosa and endothelial cell mitosis: a potential follicular antiangiogenic regulator. Biol Reprod. 2001;65:622–627. doi: 10.1095/biolreprod65.2.622. [DOI] [PubMed] [Google Scholar]
- 96.Dubey RK, Tofovic SP, Jackson EK. Cardiovascular pharmacology of estradiol metabolites. J Pharm Exp Ther. 2004;308:403–409. doi: 10.1124/jpet.103.058057. [DOI] [PubMed] [Google Scholar]
- 97.Tomaszewski J, Adamiak A, Skorupski P, Rzeski W, Rechberger T. Effects of 17 beta-estradiol and phytoestrogen daidzein on the proliferation of pubocervial fascia and skin fibroblast derived from women suffering from stress urinary incontinence. Ginekologia Polska. 2003;74:1410–1414. [PubMed] [Google Scholar]
- 98.Kondo H, Kasuga H, Noumura T. Effects of various steroids on in vitro lifespan and cell growth of human fetal lung fibroblasts (WI-38) Mech Ageing Dev. 1983;21:335–344. doi: 10.1016/0047-6374(83)90050-7. [DOI] [PubMed] [Google Scholar]
- 99.Kamath K, Okouneva T, Larson G, Panda D, Wilson L, Jordan MA. 2-Methoxyestradiol suppresses microtubule dynamics and arrests mitosis without depolymerizing microtubules. Mol Cancer Ther. 2006;5:2225–2233. doi: 10.1158/1535-7163.MCT-06-0113. [DOI] [PubMed] [Google Scholar]
- 100.Seeger H, Mueck AO, Lippert TH. Effects of estradiol metabolites on prostacyclin synthesis in human endothelial cell cultures. Life Sci. 1999;65:167–170. doi: 10.1016/s0024-3205(99)00383-5. [DOI] [PubMed] [Google Scholar]
- 101.Tsukamoto A, Kaneko Y, Yoshida T, Han K, Ichinose M, Kimura S. 2-Methoxyestradiol, an endogenous metabolite of estrogen, enhances apoptosis and beta-galactosidase expression in vascular endothelial cells. Biochem Biophys Res Commun. 1998;248:9–12. doi: 10.1006/bbrc.1998.8902. [DOI] [PubMed] [Google Scholar]
- 102.Dubey RK, Jackson EK, Keller PJ, Imthum B, Roselli M. Estradiol metabolites inhibit endothelin synthesis by an estrogen receptor-independent mechanism. Hypertension. 2001;37:640–644. doi: 10.1161/01.hyp.37.2.640. [DOI] [PubMed] [Google Scholar]
- 103.Fenoy FJ, Hernandez ME, Hernandez M, Quesada T, Salom MG, Hernandez I. Acute effects of 2-methoxyestradiol on endothelial aortic NO release in male and ovariectomized female rats. Nitric Oxide. 2010;23:12–19. doi: 10.1016/j.niox.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 104.Fotsis T, Zhang Y, Pepper MS, Adlercreutz H, Montesano R, Nawroth PP, Schweigere L. The endogenous oestrogen metabolite 2-methoxyestradiol inhibits angiogenesis and suppresses tumor growth. Nature. 1994;17:237–239. doi: 10.1038/368237a0. [DOI] [PubMed] [Google Scholar]
- 105.Yue TL, Wang X, Louden CS, Gupta S, Pillarisetti K, Gu JK, Hart TK, Lysko PG, Freurstein GZ. 2-Methoxyestradiol, an endogenous estrogen metabolite, induces apoptosis in endothelial cells and inhibits angiogenesis: possible role for stress-activated protein kinase signaling pathway and Fas expression. Mol Pharmacol. 1997;51:951–962. doi: 10.1124/mol.51.6.951. [DOI] [PubMed] [Google Scholar]
- 106.Barchiesi F, Jackson EK, Fingerle J, Gillespie DG, Odermatt B, Dubey RK. 2-Methoxyestradiol, an estradiol metabolite, inhibits neointima formation and smooth muscle cell growth via double blockade of the cell cycle. Circ Res. 2006;99:266–274. doi: 10.1161/01.RES.0000233318.85181.2e. [DOI] [PubMed] [Google Scholar]
- 107.Ricker JL, Chen Z, Yang XP, Pribluda VS, Swartz GM, Van Waes C. 2-methoxyestradiol inhibits hypoxia-inducible factor 1alpha, tumor growth and angiogenesis and augments paclitaxel efficacy in heart and neck squamous cell carcinoma. Clin Cancer Res. 2004;10:8665–8673. doi: 10.1158/1078-0432.CCR-04-1393. [DOI] [PubMed] [Google Scholar]
- 108.Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–75. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 109.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 110.Lai Y-L, Law TC. Chronic hypoxia and monocrotaline-induced elevation of hypoxia-inducible factor-1a levels and pulmonary hypertension. J Biomed Sci. 2004;11:315–321. doi: 10.1007/BF02254435. [DOI] [PubMed] [Google Scholar]
- 111.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, Semenza GL. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, Carmelier P. Heterozygous deficiency of hypoxia-inducible factor-2α protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest. 2003;111:1519–1527. doi: 10.1172/JCI15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Salama SA, Kamel MW, Botting S, Salih SM, Borahay MA, Hamed AA, Kilic GS, Saeed M, Williams MY, Diaz-Arrastia CR. Catechol-O-Methyltransferase expression and 2-methoxyestradiol affect microtubule dynamics and modify steroid receptor signaling in leiomyoma cells. PLoS ONE. 2009;4:e7356. doi: 10.1371/journal.pone.0007356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vijayanathan V, Venkiteswaran S, Nair SK, Verma A, Thomas TJ, Zhu BT, Thomas T. Physiologic levels of 2-methoxyestradiol interfere with nongenomic signaling of 17β-estradiol in human breast cancer cells. Clin Cancer Res. 2006;12(7):2038–2048. doi: 10.1158/1078-0432.CCR-05-2172. [DOI] [PubMed] [Google Scholar]
- 115.Tofovic SP, Eman S, Mady H, Jackson EK, Melhem M. Estradiol metabolites attenuates monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol. 2005;46:430–437. doi: 10.1097/01.fjc.0000175878.32920.17. [DOI] [PubMed] [Google Scholar]
- 116.Tofovic SP, Zhang X, Jones T, Jackson EK, Petrusevska G. 2-Methoxyestradiol attenuates the development and retards the progression of chronic hypoxia-induced pulmonary hypertension in rats. Circulation. 2005;112:98–99. [Google Scholar]
- 117.Tofovic SP, Jackson EK, Zhang X. 2-Methoxyestradiol attenuates pulmonary hypertension induced by α-naphthylthiourea. FASEB J. 2005;19(5):A1333. [Google Scholar]
- 118.Tofovic SP, Jones TJ, Petrusevska G. Dose-Dependent Therapeutic Effects of 2-Methoxyestradiol on Monocrotaline-Induced Pulmonary Hypertension and Vascular remodeling. Prilozi (Contributions) 2010;31 (in press) [PubMed] [Google Scholar]
- 119.Tofovic SP, Jones TJ, Bilan V, Jackson EK, Petrusevska G. Synergistic effects of 2-methoxyestradiol with either sildenafil or bosentan on amelioration of monocrotaline-induced pulmonary hypertension and vascular remodeling. J Cardiovasc Pharmacol. 2010 doi: 10.1097/FJC.0b013e3181f215e7. (in press) [DOI] [PubMed] [Google Scholar]
- 120.Tofovic SP, Zhang X, Jackson EK, Dacic S, Petrusevska G. 2-Methoxyestradiol mediates the protective effects of estradiol in monocrotaline-induced pulmonary hypertension. Vasc Pharmacol. 2006;45:358–367. doi: 10.1016/j.vph.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 121.Welter BH, Hansen EL, Saner KJ, Wei Y, Price TM. Membrane-bound Progesterone Receptor Expression in Human Aortic Endothelial Cells. J Histochem Cytochem. 2003;51:1049–1055. doi: 10.1177/002215540305100808. [DOI] [PubMed] [Google Scholar]
- 122.Barberis MC, Veronese S, Bauer D, De Juli E, Harari S. Immunocytochemical detection of progesterone receptors. A study in a patient with primary pulmonary hypertension. Chest. 1995;107:869–872. doi: 10.1378/chest.107.3.869. [DOI] [PubMed] [Google Scholar]
- 123.Vazquez F, Rodriguez-Menzaneque JC, Lydon JP, Edwards DP, O’Malley BW, Iruela-Arispe ML. Progesterone regulates proliferation of endothelial cells. Biol Chem. 1999;274:2185–2192. doi: 10.1074/jbc.274.4.2185. [DOI] [PubMed] [Google Scholar]
- 124.Morey AK, Pedram A, Razandi M, Prins BA, Hu RM, Biesiada E, Levin ER. Estrogen and progesterone inhibit vascular smooth muscle proliferation. Endocrinology. 1997;138:3300–3339. doi: 10.1210/endo.138.8.5354. [DOI] [PubMed] [Google Scholar]
- 125.Lee WS, Harder JA, Yoshizumi M, Lee ME, Haber E. Progesterone inhibits arterial smooth muscle cell proliferation. Nat Med. 1997;3:1005–1008. doi: 10.1038/nm0997-1005. [DOI] [PubMed] [Google Scholar]
- 126.Lee W-S, Liu C-W, Juan S-H, Liang Y-C, Ho P-Y, Lee Y-H. Molecular mechanism of progesterone-induced antiproliferation in rat aortic smooth muscle cells. Endocrinology. 2003;144:2785–2790. doi: 10.1210/en.2003-0045. [DOI] [PubMed] [Google Scholar]
- 127.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Hormone Metab Res. 2001;33:645–52. doi: 10.1055/s-2001-18689. [DOI] [PubMed] [Google Scholar]
- 128.Li H-F, Zhen T-Z, Li W, Qu S-Y, Zhang C-L. Effects of progesterone on the contractile response of isolated pulmonary artery in rabbits. Can J Physiol Pharmacol. 2001;79:545–550. [PubMed] [Google Scholar]
- 129.Tofovic SP, Zhang X, Petrusevska G. Progesterone inhibits vascular remodeling and attenuates monocrotaline-induced pulmonary hypertension in estrogen deficient rats. Prilozi (Contributions) 2009;30:5–24. [PubMed] [Google Scholar]
- 130.Jones TJ, Zhang X, Jackson EK, Tofovic SP. Medroxyprogesterone acetate attenuates and tibolone prevents the development of monocrotaline-induced pulmonary hypertension. FASEB J. 2006;20:A402. [Google Scholar]
- 131.Jones RD, English KM, Pugh PJ, Morice AH, Jones TH, Channer KS. Pulmonary vasodilatory action of testosterone: Evidence of a calcium antagonistic action. J Cardiovasc Pharmacol. 2002;39:814–23. doi: 10.1097/00005344-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 132.Smith AM, Bennett RT, Jones HT, Cowen ME, Channer KS, Jones DS. Characterization of the vasodilatory action of testosterone in the human pulmonary circulation. Vasc Health Risk Manag. 2008;4:1459–1466. doi: 10.2147/vhrm.s3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Horm Metab Res. 2001;33:645–52. doi: 10.1055/s-2001-18689. [DOI] [PubMed] [Google Scholar]
- 134.Hampl V, Bíbová J, Povysilová, Herget J. Dehydroepiandrosterone sulfate reduces chronic hypoxic pulmonary hypertension in rats. Eur Respir J. 2003;21:862–865. doi: 10.1183/09031936.03.00084503. [DOI] [PubMed] [Google Scholar]
- 135.Bonnet S, Dumas-de-La-Roque E, Bégueret H, Marthan R, Fayon M, Santos PD, Savineau J-P, Baulieu E-E. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci. 2003;100:9488–9493. doi: 10.1073/pnas.1633724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oka M, Karoor V, Homma N, Nagaoka T, Sakao E, Golembeski SM, Limbird J, Imamura M, Gebb SA, Fagan KA, McMurtry IF. Dehydroepiandrosterone upregulates soluble guanylate cyclase and inhibits hypoxic pulmonary hypertension. Cardiovasc Res. 2007;74:377–387. doi: 10.1016/j.cardiores.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Homma N, Nagaoka T, Karoor V, Imamura M, Taraseviciene-Stewart L, Walker LA, Fagan K, McMurtry IF, Oka M. Involvement of RhoA/Rho kinase signaling in protection against monocrotaline-induced pulmonary hypertension in pneumonectomized rats by dehydroepiandrosterone. Am J Physiol Lung Cell Mol Physiol. 2008;295:L71–L78. doi: 10.1152/ajplung.90251.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alzoubi A, Toba M, Abe K, Fagan KA, McMurtry IF, Oka M. Dehydroepiandrosterone attenuates established severe pulmonary arterial hypertension in rats. Am J Respir Crit Care Med. 2010;181:A6302. [Google Scholar]
- 139.Sweeney LB, Bogaard HJ, Natarajan R, Kraskauskas D, Voelkel NF. Female Gender Protects Against Angioproliferative Pulmonary Hypertension Induced by VEGF Receptor Inhibition and Hypoxic Exposure. Am J Respir Crit Care Med. 2009;179:A1826. [Google Scholar]
- 140.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–2754. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 141.Meyrick B, Gamble W, Reid L. Development of Crotalaria pulmonary hypertension: hemodynamic and structural study. Am J Physiol Heart Circ Physiol. 1980;239:H692–H702. doi: 10.1152/ajpheart.1980.239.5.H692. [DOI] [PubMed] [Google Scholar]
- 142.Rosenberg H, Rabinovitch M. Endothelial injury and vascular reactivity in monocrotaline pulmonary hypertension. Am J Physiol. 1988;255:H1484–H1491. doi: 10.1152/ajpheart.1988.255.6.H1484. [DOI] [PubMed] [Google Scholar]
- 143.Stenmark KR, Meyrick B, Galie N, Mooi WY, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1013–L1032. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 144.Meyrick BO, Perkett EA. The sequence of cellular and hemodynamic changes of chronic pulmonary hypertension induced by hypoxia and other stimuli. Am Rev Respir Dis. 1989;140:1486–1489. doi: 10.1164/ajrccm/140.5.1486. [DOI] [PubMed] [Google Scholar]
- 145.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 146.Bull TM, Coldren CD, Geraci MW, Voelkel NF. Gene expression profiling in pulmonary hypertension. Proc Am Thorac Soc. 2007;4:117–120. doi: 10.1513/pats.200605-128JG. [DOI] [PubMed] [Google Scholar]
- 147.Okada K, Tanaka Y, Bernstein M, Zhang W, Patterson GA, Botney MD. Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension. Am J Pathol. 1997;151:1019–1025. [PMC free article] [PubMed] [Google Scholar]
- 148.Nishimura T, Faul JL, Berry GJ, Veve I, Pearl RG, Kao PN. 40-O-(2-hydroxyethyl)-rapamycin attenuates pulmonary arterial hypertension and neointimal formation in rats. Am J Respir Crit Care Med. 2001;163:498–502. doi: 10.1164/ajrccm.163.2.2006093. [DOI] [PubMed] [Google Scholar]
- 149.White RJ, Meoli DF, Swarthout RF, Kallop DY, Galaria II, Harvey JL, Miller CM, Blaxall BC, Hall CM, Pierce RA, Cool CD, Taubman MB. Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L583–L590. doi: 10.1152/ajplung.00321.2006. [DOI] [PubMed] [Google Scholar]
- 150.Said SI, Hamidi SA, Dickman KG, Szema AM, Lyubsky S, Lin RZ, Jiang YP, Chen JJ, Waschek JA, Kort S. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation. 2007;15:1260–1268. doi: 10.1161/CIRCULATIONAHA.106.681718. [DOI] [PubMed] [Google Scholar]
- 151.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–244. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Greenway S, van Suylen RJ, Du Marchie Sarvaas G, Kwan E, Ambartsumian N, Lukanidin E, Rabinovitch M. S100A4/Mts1 produces murine pulmonary artery changes resembling plexogenic arteriopathy and is increased in human plexogenic arteriopathy. Am J Pathol. 2004;164:253–262. doi: 10.1016/S0002-9440(10)63115-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Merklinger SL, Wagner RA, Spiekerkoetter E, Hinek A, Knutsen RH, Kabir MG, Desai K, Hacker S, Wang L, Cann GM, Ambartsumian NS, Lukanidin E, Bernstein D, Husain M, Mecham RP, Starcher B, Yanagisawa H, Rabinovitch M. Increased fibulin-5 and elastin in S100A4/Mts1 mice with pulmonary hypertension. Circ Res. 2005;97:596–604. doi: 10.1161/01.RES.0000182425.49768.8a. [DOI] [PubMed] [Google Scholar]
- 154.Song Y, Jones JE, Beppu H, Keaney JF, Jr, Loscalzo J, Zhang Y-Y. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation. 2005;112:553–562. doi: 10.1161/CIRCULATIONAHA.104.492488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ivy DD, McMurtry IF, Colvin K, Imamura M, Oka M, Lee D-S, Gebb S, Jones PL. Development of occlusive neointimal lesions in distal pulmonary arteries of endothelin B receptor-deficient rats: a new model of severe pulmonary arterial hypertension. Circulation. 2005;111:2988–2996. doi: 10.1161/CIRCULATIONAHA.104.491456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mueck AO, Seeger H. 2-Methoxyestradiol—biology and mechanism of action. Steroids. 2010;75:625–631. doi: 10.1016/j.steroids.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 157.Tofovic SP, Jackson EK, Piche C. Pharmacokinetics and safety of a subcutaneously injected long-acting formulation of 2-methoxyestradiol (2ME) in healthy volunteers. Basic Clin Pharmacol Toxicol. 2009;105(Suppl. 1):107. [Google Scholar]