Abstract
Molybdenum (Mo)-dependent nitrogenase is a complex metalloprotein that catalyzes the biological reduction of dinitrogen (N2) to ammonia (NH3) at the molybdenum-iron cofactor (FeMoco) site of its molybdenum-iron (MoFe) protein component. Here we report the formation of a homocitrate-free, iron-molybdenum (“FeMo”) cluster on the biosynthetic scaffold of FeMoco, NifEN. Such a NifEN-associated “FeMo” cluster exhibits EPR features similar to those of the NifEN-associated, fully-complemented “FeMoco”, which originate from the presence of Mo in both cluster species; however, “FeMo” cluster and “FeMoco” display different temperature-dependent changes in the line shape and the signal intensity of their respective EPR features, which reflect the impact of homocitrate on the redox properties of these clusters. XAS/EXAFS analysis reveals that the Mo centers in both “FeMo” cluster and “FeMoco” are present in a similar coordination environment, although Mo in “FeMo” cluster is more loosely coordinated as compared to that in “FeMoco” with respect to the MoO distances in the cluster, likely due to the absence of homocitrate that normally serves as an additional ligand for the Mo in the cluster. Subsequent biochemical investigation of the “FeMo” cluster not only facilitates the determination of the sequence of events in the mobilization of Mo and homocitrate during FeMoco maturation, but also permits the examination of the role of homocitrate in the transfer of FeMoco between NifEN and MoFe protein. Combined outcome of these studies provides a platform for future structural analysis of the interactions between NifEN and MoFe protein, which will provide useful insights into the mechanism of cluster transfer between the two proteins.
Introduction
Nitrogenase is a complex metalloprotein that catalyzes the biological reduction of N2 to NH3 (1). The Mo-nitrogenase of Azotobacter vinelandii is composed of two proteins: the α2-dimeric Fe protein, which has one [Fe4S4] cluster bridged between the subunits and one MgATP binding site located within each subunit; and the α2β2-tetrameric MoFe protein, which has one P-cluster ([Fe8S7]) linked between the α and β subunits and one FeMoco ([MoFe7S9X-homocitrate], X = C, N or O) situated within the α subunit (2, 3). Fe protein is the obligate electron donor to MoFe protein, transferring electrons within the Fe protein/MoFe protein complex, from its [Fe4S4] cluster, through the P-cluster, to the FeMoco of MoFe protein, where substrate is reduced (4).
The organic homocitrate entity coordinates the Mo of FeMoco at the active site of MoFe protein. Apart from its structural function, homocitrate has been implicated in nitrogenase catalysis, particularly for its important role in N2 reduction (5). During the recent years, a third function has been proposed for homocitrate, one that is associated with nitrogenase assembly. Structural analysis of a FeMoco-deficient ΔnifB MoFe protein has revealed the presence of a positively charged “funnel” that could provide a path for FeMoco insertion (6). The dominant contribution of homocitrate to the overall negative charge of FeMoco, therefore, suggests that homocitrate plays a key role in the charge-charge interactions between the negative FeMoco and the positive insertion “funnel” (6, 7). Nevertheless, the validity of such a hypothesis clearly requires additional evidence.
Recently, a Mo/homocitrate-free precursor of FeMoco was captured on NifEN (8; also see Fig. S1, Supplementary Information), the scaffold protein for FeMoco assembly that is homologous to MoFe protein both in the primary sequence and the types of metal centers (9–11). This precursor closely resembles the Fe/S core of the mature FeMoco (12) and, further, it can be converted to a mature FeMoco upon the insertion of Mo and homocitrate by Fe protein in an ATP-dependent process (13, 14; also see Fig. S1, Supplementary Information). Following the maturation of precursor, NifEN can serve as a FeMoco source and directly activate the FeMoco-deficient ΔnifB MoFe protein upon protein-protein interactions (13; also see Fig. S1, Supplementary Information). Two questions follow upon such an observation. One, can Mo be inserted in the NifEN-bound precursor without the homocitrate? Two, if such a cluster can be generated on NifEN, can it be delivered to MoFe protein without the assistance of homocitrate? The answer to the latter question is particularly interesting, as it can be used to examine the previously proposed role of homocitrate in the process of FeMoco insertion into the MoFe protein.
Here, we report the formation of a homocitrate-free “FeMo” cluster on NifEN. Such a “FeMo” cluster is similar to, yet distinct from the NifEN-associated “FeMoco” with regard to the spectroscopic and structural properties. The capture of a novel “FeMo” cluster on NifEN is highly interesting from a chemical perspective of cluster synthesis. More importantly, the observation of the inability of NifEN to deliver the “FeMo” cluster to the MoFe protein provides significant insights into the function of homocitrate in the final step of FeMoco biosynthesis.
Experimental procedures
Cell growth and protein purification
All A. vinelandii strains were grown in 180-l batches in a 200-l New Brunswick fermentor (New Brunswick Scientific, Edison, NJ, USA) in Burke’s minimal medium supplemented with 2 mM ammonium acetate. The growth rate was measured by cell density at 436 nm using a Spectronic 20 Genesys (Spectronic Instruments, Westbury, NY, USA). After ammonium consumption, the cells were derepressed for 3 h, followed by harvesting using a flow-through centrifugal harvestor (Cepa, Lahr/Schwarzwald, Germany). The cell paste was washed with 50 mM Tris–HCl (pH 8.0). His-tagged NifEN, His-taggedΔnifB MoFe protein and non-tagged Fe protein were purified from A. vinelandii strains DJ1041, DJ1143 (both DJ strains were generously provided by Professor Dennis Dean of Virginia Tech) and AvOP, respectively, following previously described methods (8, 15, 16). Crude extract containing non-tagged ΔnifB MoFe protein was prepared from A. vinelandii strain UW45 as described earlier (15, 17).
FeMoco maturation assay
The conversion of NifEN-bound precursor to “FeMoco” was performed in a 50 ml maturation assay containing 25 mM Tris–HCl (pH 8.0), 100 mg NifEN containing precursor [designated NifENPrecursor, which was isolated from Azotobacter vinelandii strain DJ1041 (8)], 120 mg Fe protein, 0.4 mM homocitrate, 0.4 mM Na2MoO4, 2.4 mM ATP, 4.8 mM MgCl2, 30 mM creatine phosphate, 24 units/ml creatine phosphokinase, and 20 mM dithionite (Na2S2O4) (13, 18). The conversion of NifEN-bound precursor to a “half-matured” cluster containing only Mo, or, presumably, only homocitrate, was performed using the same procedure as described above, except that homocitrate or Na2MoO4 in the maturation assay was omitted. In all cases, the maturation mixture was stirred for 1 hr at 30°C and then NifEN was re-isolated (designated NifEN“FeMoco”, NifEN“FeMo” and NifENHC, respectively) and subjected to reconstitution, EPR and XAS experiments (see below).
MoFe protein reconstitution analysis
The reconstitution of purified ΔnifB MoFe protein was performed in a 0.8 ml assay containing 25 mM Tris–HCl (pH 8.0), 20 mM Na2S2O4 and 0.5 mg FeMoco-deficient ΔnifB MoFe protein [isolated from A. vinelandii strain DJ1143 (6)]. FeMoco insertion was initiated with the addition of 2 mg NifEN“FeMoco”, 2 mg NifEN“FeMo”, or 2 mg NifENHC to the assay, and the reaction mixture was then incubated for 30 min at 30°C before being terminated and examined for enzymatic activities as described earlier (19). The reconstitution of the crude extract of UW45 (containing non-tagged ΔnifB MoFe protein) was performed by mixing 60 ml UW45 crude extract (12 mg/ml) and 50 mg NifEN“FeMoco” or 50 mg NifEN“FeMo” and stirring the mixture for 1 h at 30 °C. Subsequently, His-tagged NifEN“FeMoco” or NifEN“FeMo” was removed from the incubation mixture by affinity chromatography (8) and the respective flow-through containing the non-tagged ΔnifB MoFe protein was collected and subjected to enzymatic assays (19).
EPR spectroscopy
All electron paramagnetic resonance spectroscopy (EPR) samples were prepared in a Vacuum Atmospheres dry box (Hawthorne, CA, USA) at an oxygen level of less than 4 ppm. The dithionite-reduced samples contained 15 mg/ml protein, 10% glycerol, 2 mM Na2S2O4 and 25 mM Tris–HCl (pH 8.0). The indigodisulfonate (IDS)-oxidized samples were prepared by incubating proteins with excess IDS for 30 min and subsequently removing IDS by an anion-exchange column. Spectra were collected in perpendicular mode using a Bruker ESP 300 Ez spectrophotometer (Bruker, Billerica, MA, USA) interfaced with an Oxford Instruments ESR-9002 liquid helium continuous-flow cryostat (Oxford Instruments, Oxford, UK). All spectra were recorded using a gain of 5 × 104, a modulation frequency of 100 kHz, a modulation amplitude of 5 G, and a microwave frequency of 9.62 GHz. For temperature-dependent experiments, spectra were measured at 4, 6, 10, 15, 20 and 30 K.
XAS data acquisition
X-ray absorption spectroscopy (XAS) data were measured at the 16-pole wiggler BL9-3 biological XAS station at Stanford Synchrotron Radiation Lightsource (SSRL) with storage ring parameters 3 GeV and 80–100 mA. A pre-monochromator flat bent Rh-coated mirror provided rejection of higher harmonics and vertical collimation, and a second post-monochromator Rh-coated toroidal mirror was employed for beam focusing. A Si(220) double-crystal monochromator was used for energy selection. Samples were stored in LN2 prior to data collection and held at a constant temperature of 10 K during data collection via an Oxford Instruments CF1208 liquid-helium continuous-flow cryostat. A Canberra 30-element solid-state Ge detector array was used to record Mo Kα fluorescence data. By using Soller slits and a Zr filter secured between the sample cryostat and the detector window, signal intensity from inelastic scattering and Mo Kβ fluorescence was substantially diminished. Internal energy calibration was performed by simultaneous measurement of the absorption of a Mo foil placed between two ionization chambers filled with Ar located after the sample. The first inflection point of the foil XAS edge was assigned to 20003.9 eV. No signs of photoreduction of the metal sites, as observed by shifts in edge energy with time, were noted. A total of 34 and 38 scans were measured for NifEN“FeMo” and NifEN“FeMoco”, respectively.
XAS data analysis
After inspection of raw data and averaging, the average data files for each sample were normalized using the program PYSPLINE (20) by fitting a second-order polynomial to the pre-edge region and subtracting from the entire data range with control points, followed by fitting a four-region spline function of orders 2, 3, and 3 over the post-edge region. The data were normalized to an edge jump of 1.0 at 20025 eV. Though some data sets extended further, the data range selected for EXAFS fits was limited to the shortest k-range (16 Å−1) to perform internally consistent fits. By means of the least squares fitting program OPT, a component of the EXAFSPAK suite of software (21), EXAFS data of k = 2–16 Å−1 were fit using initial ab initio theoretical phase and amplitude functions calculated from FEFF 7.0 (22) based on the 1M1N (3) crystallographic starting model. Atomic coordinates from the crystal model were adjusted as necessary as fits were further refined. During fit optimization, the inter-atomic distance between the absorbing and backscattering atom (R) and the mean-square thermal and static deviation in R (σ2) were varied for all components. The threshold energy (ΔEo) was allowed to vary for each fit but constrained to the same value for all components. The amplitude reduction factor (S02) was maintained at a value of 1.0 throughout analysis. Coordination numbers (N) were methodically adjusted from crystallographic values to provide the best chemically viable agreement to the EXAFS data and their Fourier transform. Inclusion or exclusion of various scattering paths was systematically tested to fully explore the atomic environment at Mo.
Results and discussion
Generation of a homocitrate-free “FeMo” cluster on NifEN
A “half-matured”, “FeMo” cluster-bound form of NifEN (designated NifEN“FeMo”) was generated by incubating the precursor-bound NifEN (designated NifENPrecursor) (8, 12) with all maturation factors except homocitrate (i.e., Fe protein, MgATP, MoO42− and dithionite) and re-isolating NifEN after such a treatment. In addition, a fully-matured, “FeMoco”-bound form of NifEN (designated NifEN“FeMoco”) was generated by incubating NifENPrecursor with the complete complement of maturation factors (i.e., Fe protein, MgATP, MoO42−, homocitrate and dithionite) and re-isolating NifEN afterwards (13, 14, 18). The “half-matured” NifEN“FeMo” was compared with the fully-matured NifEN“FeMoco” in the following biochemical and spectroscopic analyses.
Like NifEN“FeMoco”, NifEN“FeMo” is capable of activating the FeMoco-deficient ΔnifB MoFe protein. However, the various activities of MoFe protein upon reconstitution by NifEN“FeMo” are considerably and disproportionately lower than those by NifEN“FeMoco” or isolated FeMoco (Table 1). Both NifEN“FeMo” and NifEN“FeMoco” can form a complex with the ΔnifB MoFe protein, as a single band can be observed in the native PAGE upon incubation of ΔnifB MoFe protein with either NifEN“FeMo” (Fig. 1, lane 6) or NifEN“FeMoco” (Fig. 1, lane 7). In contrast, NifENPrecursor cannot complex with the ΔnifB MoFe protein (Fig. 1, lane 5). Consistent with the outcome of native PAGE analysis, portions of the NifEN“FeMo”/ΔnifB MoFe protein complex and the NifEN“FeMoco”/ΔnifB MoFe protein complex can “survive” the lengthy gel filtration procedure; in contrast, no NifENPrecursor/ΔnifB MoFe protein complex can be observed following the same procedure (Fig. S2, Supplementary Information). These observations suggest that the attachment of Mo to the NifEN-associated precursor (in the cases of both NifEN“FeMo” and NifEN“FeMoco”) triggers a conformational change of NifEN that allows the docking of NifEN on ΔnifB MoFe protein. However, the altered substrate-reducing profile of NifEN“FeMo”-activated MoFe protein (Table 1) indicates that the transfer of the cluster between NifEN“FeMo” and ΔnifB MoFe protein is largely impaired compared to that between NifEN“FeMoco” and ΔnifB MoFe protein, most likely due to the absence of homocitrate from the “FeMo” cluster.
Table 1.
Reconstitution of FeMoco-deficient MoFe protein by various FeMoco sources. Nitrogenase reactions under C2H2/Ar, Ar and N2 are C2H2+2H++2e−→C2H4, H++2e−→H2 and N2+8H++8e−→2NH3+H2, respectively. The Mo content of NifEN“FeMo” was indistinguishable from that of NifEN“FeMoco” (13).
| FeMoco Source | Activities (nmol/mg protein/min) |
|||
|---|---|---|---|---|
| C2H4 formation under C2H2/Ar | H2 formation under Ar | NH3 formation under N2 | H2 formation under N2 | |
| NifENPrecursor | 0 | 0 | 0 | 0 |
| NifEN“FeMo” | 44 ± 15 | 58 ± 7 | 38 ± 11 | 56 ± 8 |
| NifEN“FeMoco” | 930 ± 73 | 1054 ± 53 | 333 ± 40 | 254 ± 16 |
| Isolated FeMoco | 1078 ± 87 | 1324 ± 128 | 309 ± 51 | 273 ± 18 |
Fig. 1.
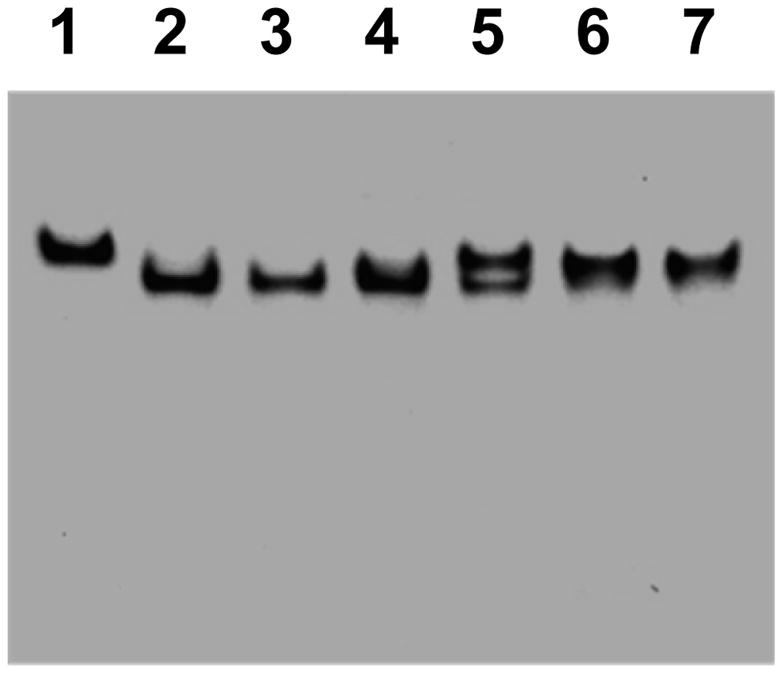
Native PAGE of protein samples. Lane 1, ΔnifB MoFe protein; lane 2, NifENPrecursor; lane 3, NifEN“FeMo”; lane 4, NifEN“FeMoco”; lane 5, NifENPrecursor plus ΔnifB MoFe protein; lane 6, NifEN“FeMo” plus ΔnifB MoFe protein; lane 7, NifEN“FeMoco” plus ΔnifB MoFe protein.
EPR properties of NifEN“FeMo”
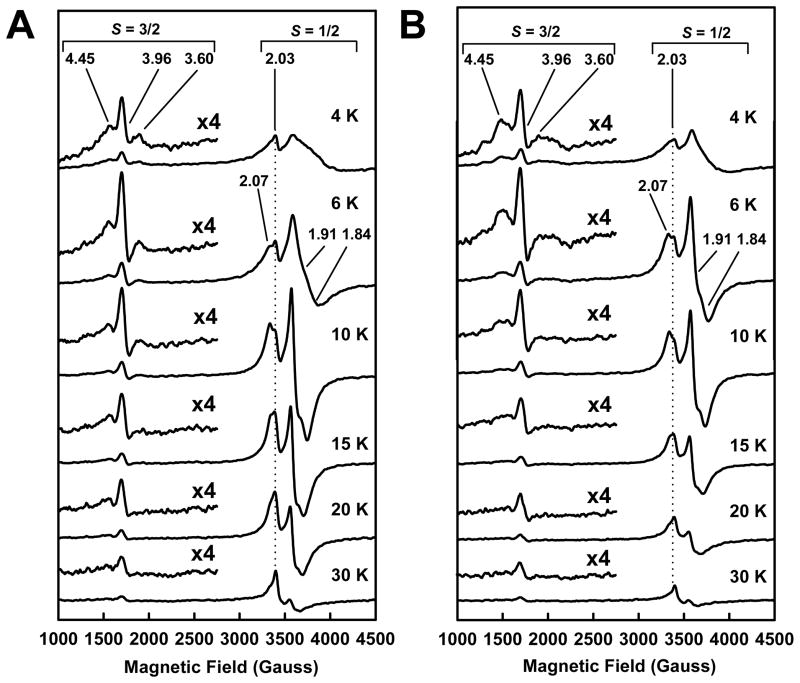
In the dithionite-reduced state, NifEN“FeMo” exhibits an S = 3/2 signal, which has nearly the same line shape and temperature-dependency as the S = 3/2 signal of NifEN“FeMoco” (Fig. 2A and B). These observations suggest that both signals originate from the attachment of Mo to the NifEN-bound precursor, the common denominator in the generation of both forms of NifEN. On the other hand, despite their similarities, the g = 3.60 and g = 4.45 features of the S = 3/2 signal of NifEN“FeMo” is narrower than those of the S = 3/2 signal of NifEN“FeMoco”, which can be best visualized at temperatures below 6 K (Fig. 2A and B). Further, the shape and intensity of the S = 1/2 signals of NifEN“FeMo” and NifEN“FeMoco” are different: the S = 1/2 signal of NifEN“FeMo” is broader in shape at temperatures below 6 K and stronger in intensity at temperatures beyond 6 K than the S = 1/2 signal of NifEN“FeMoco” (Fig. 2A and B). Finally, the ratios between the signal intensities of NifEN“FeMo” and NifEN“FeMoco” in both the S = 3/2 and the S = 1/2 regions have a similar temperature dependency (Fig. S3, A, B, Supplementary Information), which is consistent with a subtle yet reproducible difference between the electronic properties of the two NifEN-bound cluster species.
Fig. 2.
Temperature-dependency of EPR spectra of NifEN“FeMo” (A) and NifEN“FeMoco” (B) in dithionite-reduced states. Spectra were measured at 4, 6, 10, 15, 20 and 30 K, respectively. The S = 3/2 features of NifEN“FeMo” and NifEN“FeMoco” are enlarged, and the g values of the S = 3/2 and S = 1/2 signals are indicated.
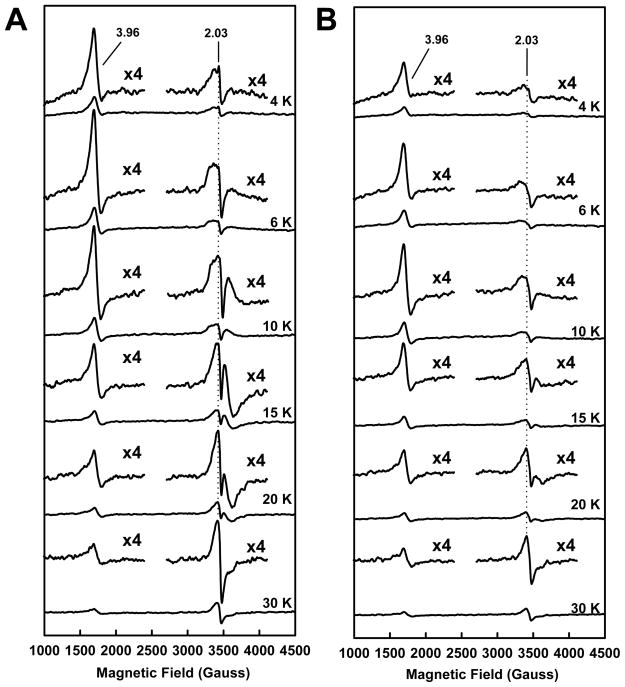
The similarity and dissimilarity between the EPR properties of NifEN“FeMo” and NifEN“FeMoco” are also observed when both proteins are present in the indigodisulfonate (IDS)-oxidized state. The g = 4.45 and 3.60 features of the S = 3/2 signals of both NifEN“FeMo” and NifEN“FeMoco” disappear upon IDS oxidation; however, the g = 3.96 feature remains intact in both cases (Fig. 3A and B). As is observed in the dithionite-reduced state, the g = 3.96 features of NifEN“FeMo” and NifEN“FeMoco” in the IDS-oxidized state are nearly identical in shape but the feature of NifEN“FeMo” is larger than that of NifEN“FeMoco” (Fig. 3A and B). Similarly, the g = 2.03 feature of NifEN“FeMo” is also stronger in intensity than that of NifEN“FeMoco” (Fig. 3A and B). As is observed in the dithionite-reduced spectra, the ratios between the signal intensities of the IDS-oxidized NifEN“FeMo” and NifEN“FeMoco” in the S = 3/2 and the S = 1/2 regions have a similar temperature dependency (Fig. S3, C, D, Supplementary Information). Taken together, the subtle yet distinct differences between NifEN“FeMo” and NifEN“FeMoco” reflect the impact of homocitrate association on the electronic properties of the NifEN-associated cluster.
Fig. 3.
Temperature-dependency of EPR spectra of NifEN“FeMo” (A) and NifEN“FeMoco” (B) in indigodisulfonate (IDS)-oxidized states. Spectra were measured at 4, 6, 10, 15, 20 and 30 K, respectively. The features of NifEN“FeMo” and NifEN“FeMoco” at g = 3.96 and 2.03 are enlarged.
XAS/EXAFS analysis of NifEN“FeMo”
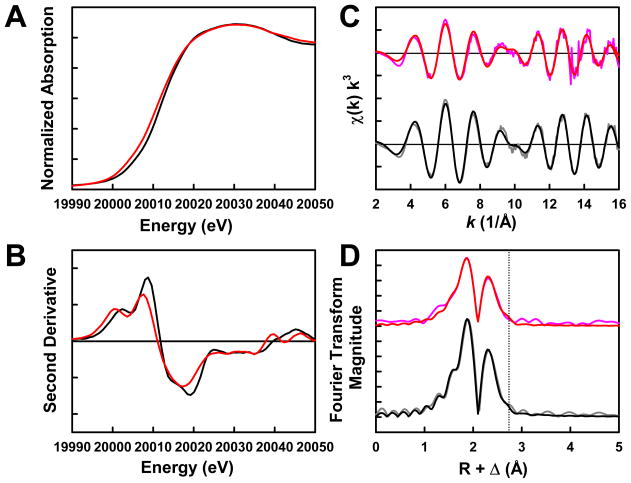
The Mo K-edge XAS spectrum of NifEN“FeMo” assumes a shape that is similar to that of the spectrum of NifEN“FeMoco” both in the rising edge (Fig. 4A) and in the second derivative (Fig. 4B). However, NifEN“FeMo” displays a <1 eV negative edge shift from NifEN“FeMoco” in the absorption spectra (Fig. 4A), suggesting that Mo in the NifEN-bound “FeMo” cluster has a different effective charge and electronic environment than Mo in the NifEN-bound “FeMoco”. The EXAFS data of NifEN“FeMo” and NifEN“FeMoco” are similar in frequency and beat pattern, although the EXAFS intensity of NifEN“FeMo” is lower than that of NifEN“FeMoco” (Fig. 4C). Likewise, Fourier transforms of NifEN“FeMo” and NifEN“FeMoco” are similar in that both display a moderate shoulder in the second shell (Fig. 4D, dashed line), which corresponds to a Mo-Fe scattering path at 2.92 and 2.90 Å, respectively. With the inclusion of this long Mo-Fe interaction, the best fits for the data of NifEN“FeMo” and NifEN“FeMoco” are very similar. In NifEN“FeMo”, Mo is best modeled by placing three S atoms at 2.33 Å, one Fe atom at 2.92 Å, two Fe atoms at 2.73 Å, and three O atoms at 2.24 Å (Table 2). In comparison, Mo in NifEN“FeMoco” is best modeled by positioning three S atoms at 2.37 Å, one Fe atom at 2.90 Å, two Fe atoms at 2.71 Å, one O atom at 2.12 Å, and two O atoms at 2.24 Å (Table 2). While two of the three O atoms in NifEN“FeMoco” represent the association of homocitrate to Mo (18), the three O atoms in NifEN“FeMo” reflect the contribution of either protein ligands or adventitious H2O to the coordination of Mo in the absence of homocitrate. On average, the Mo-O distances are slightly longer in NifEN“FeMo” than those in NifEN“FeMoco”, whereas the Mo-S and Mo-Fe distances in both NifEN“FeMo” and NifEN“FeMoco” are comparable. These results suggest a very similar (with respect to the Mo-S and Mo-Fe distances), yet more loosely coordinated (with respect to the Mo-O distances) Mo site in NifEN“FeMo”, likely reflecting the absence of homocitrate that normally serves as an additional anchor of the cofactor within the protein.
Fig. 4.
Mo K-edge x-ray absorption spectra (A) and smoothed second derivatives (B) of NifEN“FeMo” (red) and NifEN“FeMoco” (black); Mo K-edge EXAFS (C) and Fourier transforms (D) of data (pink) and fits (red) for NifEN“FeMo”, and data (gray) and fits (black) for NifEN“FeMoco”. The dashed line is centered at ~2.90 Å (D).
Table 2.
Final EXAFS fit results over a k range of 2–16 Å−1. The variables are coordination number, N; interatomic distance, R; mean-square thermal and static deviation, σ2; and the shift in the threshold energy from 20025 eV, ΔE0. The estimated uncertainties in R, σ2, and N are ± 0.02 Å, ± 0.0001 Å 2, and ± 20%, respectively. The goodness of fit, F, is defined as F = [Σk6(χexptl. − χcalcd.)2/Σk6(χexptl.)2]0.5. Data of NifEN“FeMoco” were taken from our earlier report (18).
| NifEN“FeMo” | NifEN“FeMoco” | |||||
|---|---|---|---|---|---|---|
| N | R(Å) | σ2(Å2) | N | R(Å) | σ2(Å2) | |
| Scatterer | ||||||
| Mo-O short | - | - | - | 1 | 2.12 | 0.0055 |
| Mo-O long | 3 | 2.24 | 0.0030 | 2 | 2.24 | 0.0012 |
| Mo-S | 3 | 2.33 | 0.0080 | 3 | 2.37 | 0.0059 |
| Mo-Fe short | 2 | 2.73 | 0.0042 | 2 | 2.71 | 0.0029 |
| Mo-Fe long | 1 | 2.92 | 0.0053 | 1 | 2.90 | 0.0058 |
| ΔE0 (eV) | −8.8 | −8.9 | ||||
| Weighted F | 0.301 | 0.150 | ||||
Determination of the sequence of events during the process of FeMoco maturation on NifEN
To investigate the sequence of Mo and homocitrate insertion into the NifEN-associated precursor, another form of NifEN (designated NifENHC, where HC=homocitrate) was prepared by incubating NifENPrecursor with all maturation factors except the Mo source (i.e., Fe protein, MgATP, homocitrate and dithionite) and re-isolating NifEN after such a treatment. Together with NifEN“FeMo”, NifENHC was used in the following complementation experiments designed to determine the sequence of events during the process of FeMoco maturation on NifEN.
In the presence of Fe protein, MgATP and dithionite, NifENPrecursor can be converted to NifEN“FeMoco” when MoO42− and homocitrate are supplied at the same time (Fig. 5A, ➁). In contrast, neither can NifEN“FeMo” be complemented with homocitrate (Fig. 5A, ➃), nor can NifENHC be complemented with MoO42− (Fig. 5A, ➆) for the generation of NifEN“FeMoco”, even if Fe protein, MgATP and dithionite are provided. These observations suggest that Mo and homocitrate are not incorporated stepwise into the NifEN-associated precursor; instead, they are delivered simultaneously to the precursor.
Fig. 5.
(A) Reconstitution of purified ΔnifB MoFe protein by various NifEN species: NifENPrecursor (➀); NifENPrecursor plus MoO42−, homocitrate, Fe protein and MgATP (➁); NifEN“FeMo” (➂); NifEN“FeMo” plus homocitrate, Fe protein and MgATP (➃); NifEN“FeMo” plus MoO42−, homocitrate, Fe protein and MgATP (➄); NifENHC (➅; HC: homocitrate); NifENHC plus MoO42−, Fe protein and MgATP (➆); NifENHC plus MoO42−, homocitrate, Fe protein and MgATP (➇). (B) Percentage activities of the crude extract of UW45 (a nifB-deletion strain) before (➀) and after (➁) incubation with NifEN“FeMo” and upon removal of NifEN“FeMo” (➂). (C) Percentage activities of the crude extract of UW45 before (➀) and after (➁) incubation with NifEN“FeMoco” and upon removal of NifEN“FeMoco” (➂). Consistent with our earlier report (18), the ΔnifB MoFe protein preparation used in this study can be activated to a comparable level by either NifEN“FeMoco” (725±72 nmol C2H4 formation/mg protein/min) or isolated FeMoco (745±51 nmol C2H4 formation/mg protein/min), suggesting that NifEN“FeMoco” is as proficient a FeMoco source as the isolated FeMoco. The maximum (100%) activities of acetylene reduction are set for 725±72, 1.6±0.3 and 22.8±3.8 nmol C2H4 formation/mg protein/min, respectively, in A, B and C.
Interestingly, upon incubation with the full complement of maturation factors (i.e., Fe protein, MgATP, dithionite, MoO42− and homocitrate), there is a minor increase in the capacity of NifEN“FeMo” to activate ΔnifB MoFe protein (Fig. 5A, ➄); whereas the ability of NifENHC to activate ΔnifB MoFe protein is increased to the level of NifEN“FeMoco” upon such a treatment (Fig. 5A, ➇). Apparently, NifEN“FeMo” contains a vast majority of “half-matured” “FeMo” cluster (which cannot be further matured) and a minor portion of precursors (which can be further matured upon the insertion of Mo and homocitrate). In contrast, NifENHC still contains 100% precursors that can be fully matured upon the insertion of Mo and homocitrate (i.e., the precursors on NifENHC has not been converted to a “half-matured”, homocitrate-containing form despite the attempted maturation effort). These results are consistent with our earlier finding that Fe protein, which mobilizes Mo and homocitrate for the maturation of NifEN-associated precursor, can be “loaded” with Mo alone, but not with homocitrate alone (14). Together, these observations imply a sequential pattern of Mo/homocitrate attachment to the Fe protein, i.e., Mo is directly associated to the Fe protein, whereas homocitrate is indirectly attached through Mo in an arrangement of Fe protein—Mo—homocitrate. Such a pattern of attachment of Mo and homocitrate to Fe protein could account for the ability of Fe protein to deliver Mo alone to the precursor (thereby generating NifEN“FeMo”) and its inability to deliver homocitrate alone to the precursor (as is observed in the case of NifENHC).
Examination of the role of homocitrate in the transfer of FeMoco between NifEN and MoFe protein
To investigate the function of homocitrate in the transfer of FeMoco from NifEN to MoFe protein, His-tagged NifEN“FeMo” or NifEN“FeMoco” was incubated with the crude extract of non-tagged ΔnifB MoFe protein and re-isolated afterwards, and the activity of the ΔnifB MoFe protein crude extract is analyzed before, during and after the incubation/re-isolation. The crude extract of ΔnifB MoFe protein can be activated when it is mixed with either NifEN“FeMo” (Fig. 5B, ➁) or NifEN“FeMoco” (Fig. 5C, ➁); however, as is observed with the purified ΔnifB MoFe protein, the ΔnifB MoFe protein crude extract that is mixed with NifEN“FeMo” (1.6±0.3 nmol C2H4 formation/mg protein/min) is significantly less active than that mixed with NifEN“FeMoco” (22.8±3.8 nmol C2H4 formation/mg protein/min). When NifEN“FeMo” or NifEN“FeMoco” is separated from the ΔnifB MoFe protein crude extract, the crude extract treated by NifEN“FeMo” becomes inactive (Fig. 5B, ➂), whereas the crude extract reconstituted by NifEN“FeMoco” retains 80% of the activity (Fig. 5C, ➂). This result suggests that, while the fully-complemented cluster on NifEN“FeMoco” can be transferred to the ΔnifB MoFe protein, the homocitrate-free cluster on NifEN“FeMo” cannot be delivered to the ΔnifB MoFe protein. Interestingly, based on metal and activity analyses, the “FeMo” cluster is absent from NifEN“FeMo” upon re-isolation from the ΔnifB MoFe protein crude extract (data not shown), although the “FeMo” cluster has not been transferred to the ΔnifB MoFe protein (as is suggested by the result in Fig. 5B). The absence of “FeMo” cluster from both NifEN and ΔnifB MoFe protein suggests a relocation of the “FeMo” cluster to the surface of NifEN that is induced by the interactions between NifEN and ΔnifB MoFe protein, and the subsequent loss of the cluster upon the separation of the two proteins.
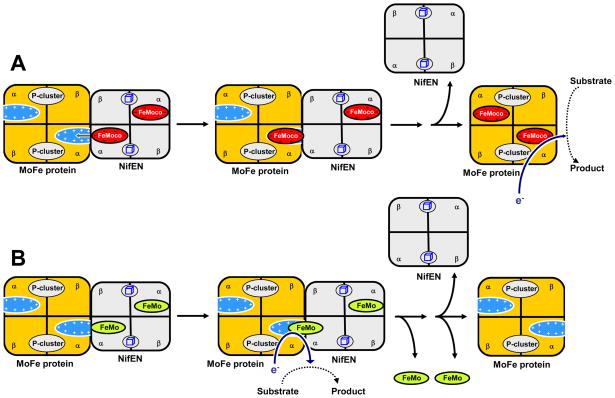
Plausible models depicting the interactions between NifEN“FeMoco” and ΔnifB MoFe protein (Fig. 6A) and those between NifEN“FeMo” and ΔnifB MoFe protein (Fig. 6B) can be proposed on the basis of these results. The presence of homocitrate in NifEN-associated “FeMoco” and its contribution to the overall negative charge of the cluster facilitate the subsequent insertion of this cluster into the positively charged insertion funnel in ΔnifB MoFe protein (6), leading to the formation of a holo-MoFe protein that is fully proficient in catalysis (Fig. 6A). In contrast, the absence of homocitrate from the NifEN-associated “FeMo” cluster results in the attempted (and unsuccessful) delivery of “FeMo” cluster from NifEN to ΔnifB MoFe protein and the generation of a complex between the two proteins [as is suggested by the formation of a single band in the native PAGE upon incubation of ΔnifB MoFe protein with NifEN“FeMo” (see Fig. 1, lane 6)]. A “chimeric” electron transfer chain could be formed in this complex, which utilizes the P-cluster on ΔnifB MoFe protein and the “FeMo” cluster on NifEN for catalysis (Fig. 6B). Such a “chimeric” electron transfer chain may very well account for the minor activation of the ΔnifB MoFe protein crude extract when it is mixed with NifEN“FeMo”. In this scenario, the “FeMo” cluster is “stuck” between NifEN and ΔnifB MoFe protein and “falls out” when the two proteins are pulled apart, which would be consistent with the absence of cluster from both NifEN and ΔnifB MoFe protein upon separation. More importantly, the fact that NifEN“FeMo” is unable to deliver the cluster to theΔnifB MoFe protein points to a critical role of homocitrate in the transfer of FeMoco from NifEN to MoFe protein and the subsequent incorporation of FeMoco into its binding site in MoFe protein. Future work will focus on the structural analysis of the “stuck” complex between NifEN“FeMo” and ΔnifB MoFe protein, which will provide further evidence for the proposed function of homocitrate in the process of FeMoco assembly.
Fig. 6.
Plausible models depicting the interactions between ΔnifB MoFe protein and NifEN“FeMoco” (A) and those between ΔnifB MoFe protein and NifEN“FeMo” (B). For the purpose of simplicity, the interactions between one cofactor assembly site in NifEN and one cofactor binding site in ΔnifB MoFe protein are shown for NifEN“FeMoco” (A) and NifEN“FeMo” (B), respectively. The presence of homocitrate in NifEN-associated “FeMoco” and its contribution to the overall negative charge of the cluster facilitate the subsequent insertion of this cluster into the positively charged insertion funnel in ΔnifB MoFe protein, leading to the formation of a holo-MoFe protein that is fully proficient in catalysis (A). In contrast, the absence of homocitrate from the NifEN-associated “FeMo” cluster results in the attempted (and unsuccessful) delivery of “FeMo” cluster from NifEN to ΔnifB MoFe protein and the generation of a complex between the two proteins (B). A “chimeric” electron transfer chain could be formed in this complex, which utilizes the P-cluster on ΔnifB MoFe protein and the “FeMo” cluster on NifEN for catalysis (B). The “FeMo” cluster that is “stuck” between NifEN and ΔnifB MoFe protein likely “falls out” when the two proteins are pulled apart, which would be consistent with the absence of cluster from both NifEN and ΔnifB MoFe protein upon separation (B).
Supplementary Material
Acknowledgments
This work was supported by NIH Grants GM67626 (M.W.R.) and RR001209 (K.O.H.). SSRL operations are funded by the DOE BES, and the SSRL Structural Molecular Biology Program by NIH NCRR BTP and DOE BER.
Abbreviations
- MoFe protein
molybdenum-iron protein
- Fe protein
iron protein
- Mo
molybdenum
- HC
homocitrate
- FeMoco
iron-molybdenum cofactor
- “FeMo” cluster
iron-molybdenum cluster
- NifEN“FeMoco”
NifEN containing a mature FeMoco
- NifEN“FeMo”
NifEN containing a “FeMo” cluster
- NifENHC
NifEN containing a precursor that is matured with homocitrate alone
Contributor Information
Yilin Hu, Email: yilinh@uci.edu.
Keith Owen Hodgson, Email: hodgson@ssrl.slac.stanford.edu.
Britt Hedman, Email: hedman@ssrl.slac.stanford.edu.
Markus Walter Ribbe, Email: mribbe@uci.edu.
Notes and references
- 1.Burgess BK, Lowe DJ. Chem Rev. 1996;96:2983. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]
- 2.Georgiadis MM, Komiya H, Chakrabarti P, Woo D, Kornuc JJ, Rees DC. Science. 1992;257:1653. doi: 10.1126/science.1529353. [DOI] [PubMed] [Google Scholar]
- 3.Einsle O, Tezcan FA, Andrade SLA, Schmid B, Yoshida M, Howard JB, Rees DC. Science. 2002;297:1696. doi: 10.1126/science.1073877. [DOI] [PubMed] [Google Scholar]
- 4.Schindelin H, Kisker C, Schlessman JL, Howard JB, Rees DC. Nature. 1997;387:370. doi: 10.1038/387370a0. [DOI] [PubMed] [Google Scholar]
- 5.Durrant MC, Francis A, Lowe DJ, Newton WE, Fisher K. Biochem J. 2006;397:261. doi: 10.1042/BJ20060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmid B, Ribbe MW, Einsle O, Yoshida M, Thomas LM, Dean DR, Rees DC, Burgess BK. Science. 2002;296:352. doi: 10.1126/science.1070010. [DOI] [PubMed] [Google Scholar]
- 7.Hu Y, Fay AW, Lee CC, Yoshizawa J, Ribbe MW. Biochemistry. 2008;47:3973. doi: 10.1021/bi7025003. [DOI] [PubMed] [Google Scholar]
- 8.Hu Y, Fay AW, Ribbe MW. Proc Natl Acad Sci USA. 2005;102:3236. doi: 10.1073/pnas.0409201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean DR, Brigle KE. Proc Natl Acad Sci USA. 1985;82:5720. doi: 10.1073/pnas.82.17.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodwin PJ, Agar JN, Roll JT, Roberts GP, Johnson MK, Dean DR. Biochemistry. 1998;37:10420. doi: 10.1021/bi980435n. [DOI] [PubMed] [Google Scholar]
- 11.Dos Santos PC, Dean DR, Hu Y, Ribbe MW. Chem Rev. 2004;104:1159. doi: 10.1021/cr020608l. [DOI] [PubMed] [Google Scholar]
- 12.Corbett MC, Hu Y, Fay AW, Ribbe MW, Hedman B, Hodgson KO. Proc Natl Acad Sci USA. 2006;103:1238. doi: 10.1073/pnas.0507853103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Y, Corbett MC, Fay AW, Webber JA, Hodgson KO, Hedman B, Ribbe MW. Proc Natl Acad Sci USA. 2006;103:17119. doi: 10.1073/pnas.0602647103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y, Corbett MC, Fay AW, Webber JA, Hodgson KO, Hedman B, Ribbe MW. Proc Natl Acad Sci USA. 2006;103:17125. doi: 10.1073/pnas.0602651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess BK, Jacobs DB, Stiefel EI. Biochim Biophys Acta. 1980;614:196. doi: 10.1016/0005-2744(80)90180-1. [DOI] [PubMed] [Google Scholar]
- 16.Ribbe MW, Hu Y, Guo M, Schmid B, Burgess BK. J Biol Chem. 2002;277:23469. doi: 10.1074/jbc.M202061200. [DOI] [PubMed] [Google Scholar]
- 17.Shah VK, Davis LC, Gordon JK, Orme-Johnson WH, Burris WJ. Biochim Biophys Acta. 1973;292:246. doi: 10.1016/0005-2728(73)90269-7. [DOI] [PubMed] [Google Scholar]
- 18.Yoshizawa JM, Blank MA, Fay AW, Lee CC, Wiig JA, Hu Y, Hodgson KO, Hedman B, Ribbe MW. J Am Chem Soc. 2009;131:9321. doi: 10.1021/ja9035225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribbe MW, Burgess BK. Proc Natl Acad Sci USA. 2001;98:5521. doi: 10.1073/pnas.101119498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenderholt A. PYSPLINE. Stanford Synchrotron Radiation Laboratory; Stanford, CA: 2006. [Google Scholar]
- 21.George GN. EXAFSPAK. Stanford Synchrotron Radiation Laboratory; Stanford, CA: 1990. [Google Scholar]
- 22.Rehr JJ, Albers RC. Rev Mod Phys. 2000;72:621. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.