Abstract
Background
Abstinent drug users remain at risk for relapse long after withdrawal subsides. Animal studies indicate that responses to drug-related cues not only persist, but increase, with abstinence, a phenomenon termed “incubation of drug craving”. It is unknown if cue-induced craving increases, decreases, or remains constant with abstinence in humans. We investigated effects of abstinence on cue-induced craving in cigarette smokers.
Methods
Eighty-six non-treatment-seeking, adult smokers (≥ 10 cigarettes daily) were paid to abstain for 7 (Group 1), 14 (Group 2), or 35 (Groups 3, 4) days. Abstinence was verified daily. Groups 1, 2, and 3 underwent a single cue session on the final abstinence day (7, 14, or 35). Group 4 viewed cues on days 7, 14, and 35.
Results
Between and within groups, smoking-cue-induced craving increased with abstinence on some measures. Cue-induced craving was greater in Group 3 (35-day) compared to Group 1 (7-day). Within Group 4, cue-induced craving was greater at 35 than 14 days. Cue-induced craving did not decrease with abstinence on any measure.
Conclusions
We present initial evidence of incubation of cue-induced craving in humans. The observation that cue-induced craving increases with abstinence, even as “background” craving and withdrawal symptoms subside, may have treatment implications.
Keywords: cue-induced craving, incubation of craving, addiction, relapse, cigarette smoking
INTRODUCTION
Relapse is a persistent problem for abstaining drug users. Commonly, relapse occurs soon after quitting when withdrawal symptoms are pronounced [1]. However, many users relapse long after withdrawal has abated, implicating processes other than withdrawal relief in relapse [1–2]. Factors underlying time-delayed relapse are poorly understood [2]. One factor thought to play a role is conditioned craving induced by cues associated with drugs [3]. Drug-related cues robustly increase subjective cravings [3–4], and, to a lesser extent, physiological indices [4]. Stimuli paired with drugs reliably reinstate drug seeking in laboratory animals trained to self-administer drugs [5]. In humans, the relationship between cue reactivity and relapse appears less direct [6], and may be modulated by variables such as the ability to inhibit habitual behavior. Nevertheless, there is evidence that drug-related cues play a role in relapse [7–8].
Although cue-induced craving appears to precipitate relapse, little is known about the time course of cue-induced craving in humans. In rodents [9] and non-human primates [10], cue-induced drug seeking increases with longer abstinence, a phenomenon termed “incubation of drug craving” [9]. Incubation of craving has been reported in rats trained to self-administer cocaine [9], heroin [11], methamphetamine [12], and nicotine [13]. However, incubation of drug craving has not been demonstrated in humans. Indeed, conventional wisdom and the decreasing time course of relapse rates [1] suggest that responses to drug-paired cues abate, rather than incubate, with abstinence. Some treatment strategies reflect this idea, advising newly abstinent users to avoid exposure to drug-related cues [14]. However, studies in which abstinence lengths are controlled have not been undertaken; it thus remains unclear whether cue-induced craving increases, decreases, or remains stable with abstinence duration in humans.
In this translational study, we employed an experimental approach to investigate the time course of cue-induced craving in humans. We studied smokers because tobacco is a commonly used addictive substance. Based on laboratory animal findings, we hypothesized that smoking cue-induced craving would increase with abstinence after both single and repeated cue exposure.
METHODS
Non-treatment-seeking, healthy male and female adult smokers (≥10 cigarettes daily) were recruited. Candidates were excluded for medical or psychiatric contraindications (see Supplement). Participants provided written informed consent as approved by the University of Chicago and NIDA IRP Institutional Review Boards.
The main design was between-subjects. Participants were randomly allocated to three groups: Group 1 abstained from smoking for 7 days, Group 2 for 14 days, and Group 3 for 35 days. Participants were exposed to a single cue session on the final abstinence day. Group 4, enrolled as a separate cohort for a within-subjects component, abstained for 35 days, with cues on days 7, 14, and 35. During abstinence, participants attended the laboratory daily for biochemical abstinence verification and self-report measurement of withdrawal (see Supplement). Participants were paid $30 per day; those not abstaining were removed from the study.
During cue sessions, neutral and smoking cues were presented in random order in separate rooms. For smoking cues, participants viewed 30 smoking-related photographs while holding a lit cigarette. For neutral cues, participants viewed neutral pictures while holding a pencil cut to cigarette length. Cigarette smoke provided an olfactory smoking cue, and a scented candle provided the neutral olfactory cue. Measures collected before and after cues included heart rate and blood pressure, salivary cortisol, and subjective measures. Subjective measures were the Tobacco Craving Questionnaire – Short Form (TCQ-SF) [15] Total, the Brief Questionnaire of Smoking Urges (QSU-B) [16] Factor 1 and 2 subscales, and the Positive and Negative Affect Schedule (PANAS) [17] Positive and Negative Affect subscales.
To assess whether smoking cues were effective, we combined data from Groups 1–3, and used t-tests comparing within-session change scores (post-cue minus pre-cue) for smoking versus neutral cues. Only outcome measures sensitive to smoking cues were included in main analyses. Group differences were assessed with ANOVAs, using polynomial contrasts testing for linear trends (Group 1>2>3). Planned contrasts compared Groups 1 and 2, and 1 and 3. Dependent variables were within-session change scores, with smoking and neutral cues analyzed separately. A similar approach tested group differences in baseline cue session craving. In Group 4, we assessed cue-reactivity and cue baseline scores in repeated-measures regressions (Proc Mixed) using day (7, 14, and 35) as the predictor; Tukey-Kramer pairwise comparisons were made between days. Analyses were done with SPSS 17.0 and SAS 9.0. Alpha was 0.05, two-tailed.
RESULTS
Eighty-six participants completed the study (Supplement: Figure S1, Tables S1 and S2). About half the participants began but did not complete abstinence; this proportion did not differ across groups (see Supplement). Compared to neutral cues, smoking cues increased TCQ-SF Total, QSU-B Factors 1 and 2, and PANAS Negative Affect; these were included in further analyses. Smoking cues did not preferentially affect physiological measures. As expected, daily withdrawal symptoms progressively decreased with abstinence (Supplement: Figure S2).
Between-Group Analyses
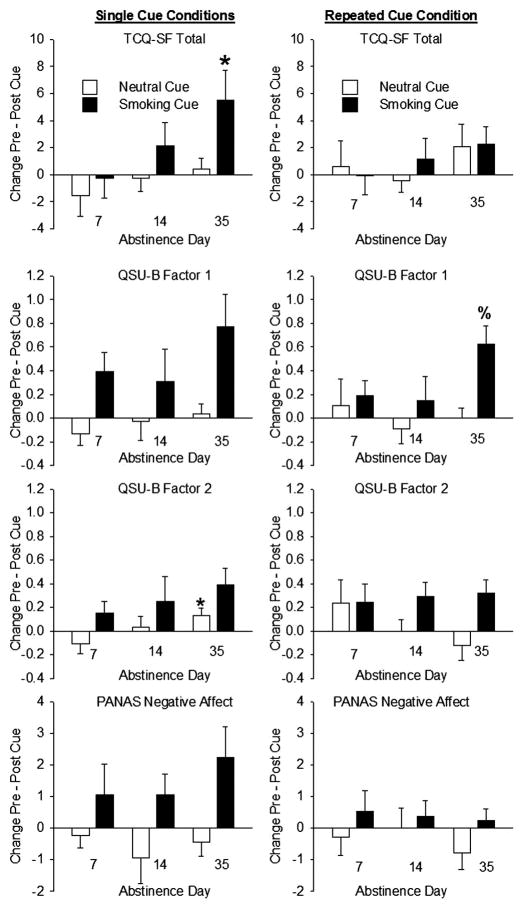
For TCQ-SF Total smoking-cue reactivity (within-session change scores), there was a significant linear trend across Groups 1, 2, and 3 (F(1,59) = 4.9, p = 0.03; effect-size r = .28); as assigned abstinence length increased, smoking-cue response increased proportionally. TCQ-SF Total smoking-cue reactivity was higher in the 35-day than 7-day group (t(59) = 2.2, p = 0.03). Although groups did not differ on QSU-B Factor 1, there was a linear trend for an increase in neutral cue response measured with QSU-B Factor 2 (F(1,59) = 4.3, p = 0.04; effect-size r = .26); change scores after neutral cues were higher in the 35-day than 7-day group (t(59) = 2.1, p = 0.04). However, these differences were small (the 7-day group decreased −0.1 (± 0.1) on a scale of 1 to 7; the 35-day group increased 0.1 (± 0.1); Figure 1).
Figure 1.
Effects of exposure to smoking and neutral cues on self-reported craving as a function of length of abstinence. Left Column: Single cue conditions (Groups 1, 2 and 3). Right column: Repeated cue condition (Group 4). Top Row: Tobacco Craving Questionnaire – Short Form (TCQ-SF) Total score response to cues. Second Row: Brief Questionnaire of Smoking Urges (QSU-B) Factor 1 score cue response. Third Row: QSU-B Factor 2 score cue response. Bottom Row: Positive and Negative Affect (PANAS) Negative Affect score cue response. Asterisks denote a significant difference from Group 1 (7-day abstinence), p < 0.05. % denotes a significant difference from Group 2 (14-day abstinence), p < 0.05. Data are group means (±S.E.M.).
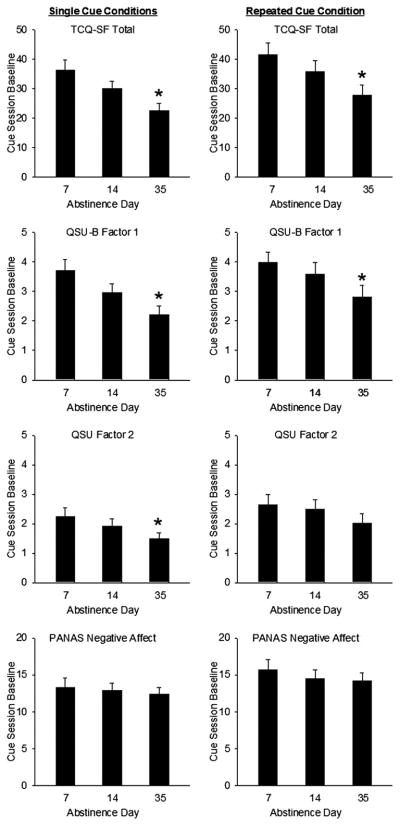
Participants’ ratings of craving before cue presentations decreased with abstinence. Scores on TCQ-SF Total, and QSU-B Factors 1 and 2 declined as assigned abstinence increased (TCQ-SF Total: F(1,59) = 11.6, p = 0.001, effect-size r = .41; QSU-B Factor 1: F(1,59) = 11.1, p = 0.002, effect-size r = .40; QSU-B Factor 2: F(1,59) = 4.4, p = 0.04, effect-size r = .26; Figure 2).
Figure 2.
Effects of length of abstinence on baseline (non-provoked) cigarette craving. Left Column: Single cue conditions (Groups 1, 2 and 3). Right column: Repeated cue condition (Group 4). Top Row: Tobacco Craving Questionnaire – Short Form (TCQ-SF) Total score baseline. Second Row: Brief Questionnaire of Smoking Urges (QSU-B) Factor 1 score cue baseline. Third Row: QSU-B Factor 2 score baseline. Bottom Row: Positive and Negative Affect (PANAS) Negative Affect score baseline. Asterisks denote significant decreases from 7 days to 35 days, p < 0.05. Data are group means (±S.E.M.).
Within-Group Analyses
Although abstinence length did not affect cue-induced TCQ-SF Total, QSU-B Factor 2 or PANAS Negative Affect, there was a marginal effect on QSU-B Factor 1 (F(2,43) = 3.0, p = 0.06), with more pronounced cue responses on day 35 than 14 (Tukey-Kramer p = 0.001; Figure 1).
As in between-group analysis, pre-cue craving ratings on TCQ-SF Total (F(2,43) = 5.8, p = 0.006) and QSU-B Factor 1 (F(2,44) = 4.4, p = 0.02) scores declined with days of abstinence (Figure 2).
DISCUSSION
Here, we present initial evidence that cue-induced craving in cigarette smokers does not decline with increasing abstinence up to 35 days. Even as daily craving declined, craving in response to smoking cues remained robust, and on some measures, actually increased with abstinence. This finding is broadly consistent with incubation studies in nonhumans [9, 11], which use lever-pressing to measure drug-seeking or “craving”. Time-dependent increases in conditioned craving occurred despite progressively decreasing baseline (non-provoked) craving and withdrawal symptoms.
Incubation was evident on some, but not all, measures. This variability may relate to the relatively modest effect of the cues. Smoking cues did not preferentially affect all self-report measures, and did not increase any physiological variables. Relatively modest effects of smoking cues, by subjective or physiological measures, have been reported previously [4]. Additionally, incubation of cue-induced craving was weaker in the within-subjects than in between-subjects assessment. This is likely due to some extinction of the conditioned response in the laboratory in the within-subject group. However, it is notable that the time-dependent increases in cue-induced craving observed occurred while participants, living in their normal environments, were presumably exposed to smoking-related stimuli. The fact that an incubation-like phenomenon was detectable in the laboratory, despite day-to-day cue exposure, suggests that the incubation of craving phenomenon is real and robust. This effect may be even more evident in situations where incidental drug cue exposure is minimized, such as in inpatient settings.
These initial findings suggest several avenues for future enquiry. Incubation of cue-induced craving may also occur in users of other drugs, and may be especially evident when drug users are protected from cues in a controlled environment. It will be important to determine whether the phenomenon also occurs in treatment seekers, and how it relates to relapse. Future research may also elucidate the neurobiological substrates of time-dependent changes in provoked craving in humans. In rats, incubation involves neuronal activity and synaptic plasticity in the nucleus accumbens, central amygdala, and ventral medial prefrontal cortex [18–19], areas also implicated in cue-induced craving in humans [20].
In sum, we have demonstrated that cue-induced craving does not decrease over an extended period of abstinence, and may, as in animal models, actually increase with longer abstinence duration. It is notable that the increase in cue-induced craving occurred even as baseline craving ratings declined. These findings have significant clinical implications, suggesting that clinicians and users should be aware that risk of relapse precipitated by cue-induced craving may persist or increase with abstinence, even as background craving and withdrawal subsides.
Supplementary Material
Acknowledgments
Thanks to Patricia Kreigel, Jamie Golden, Erin Prater, Alex Gomes and Rebecca Evans for technical assistance and Drs. Royce Lee and Karran Phillips for medical support. Many thanks to the participants. Supported by NIDA (R21DA020773) and NIDA Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. J Clin Psychol. 1971;27:455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 2.Herd N, Borland R, Hyland A. Predictors of smoking relapse by duration of abstinence: Findings from the International Tobacco Control (ITC) Four Country Survey. Addiction. 2009;104:2088–2099. doi: 10.1111/j.1360-0443.2009.02732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi J, Jun W, Zhao LY, Xue YX, Zhang XY, Kosten TR, Lu L. Effect of rapamycin on cue-induced drug craving in abstinent heroin addicts. Eur J Pharmacol. 2009;615:108–112. doi: 10.1016/j.ejphar.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- 5.Cromba H, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: A review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drummond DC. What does cue-reactivity have to offer clinical research? Addiction. 2000;95:S129–S144. doi: 10.1080/09652140050111708. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X, Nonnemaker J, Sherill B, Gilsenan AW, Coste F, West R. Attempts to quit smoking and relapse: Factors associated with success or failure from the ATTEMPT cohort study. Addict Behav. 2009;34:365–373. doi: 10.1016/j.addbeh.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Heinz A, Beck A, Grusser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009;14:108–118. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weerts EM, Goodwin AK, Kaminski BJ, Hienz RD. Environmental cues, alcohol seeking, and consumption in baboons: Effects of response requirement and duration of alcohol abstinence. Alcohol Clin Exp Res. 2006;30:2026–2035. doi: 10.1111/j.1530-0277.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 11.Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug-seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- 12.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 13.Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine seeking is associated with enhanced PKA-regulated signaling of DARPP-32 in the insular cortex. Eur J Neurosci. doi: 10.1111/j.1460-9568.2010.07114.x. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirby K, Lamb RJ, Iquichi MY, Husband SD, Platt JJ. Situations occasioning cocaine use and cocaine abstinence strategies. Addiction. 1995;90:1241–1252. doi: 10.1046/j.1360-0443.1995.90912418.x. [DOI] [PubMed] [Google Scholar]
- 15.Heishman SE, Singleton EG, Pickworth W. Reliability and validity of a Short Form of the Tobacco Craving Questionnaire. Nicotine Tob Res. 2008;10:643–651. doi: 10.1080/14622200801908174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanderson Cox L, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 17.Watson D, Clark LA, Tellegen A. Development and validation if brief measures of positive and negative affect : The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 18.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu L, Hope BT, Dempsey J, Liu Sy, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 20.Koya E, Uejima J, Wihbey K, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56:117–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.