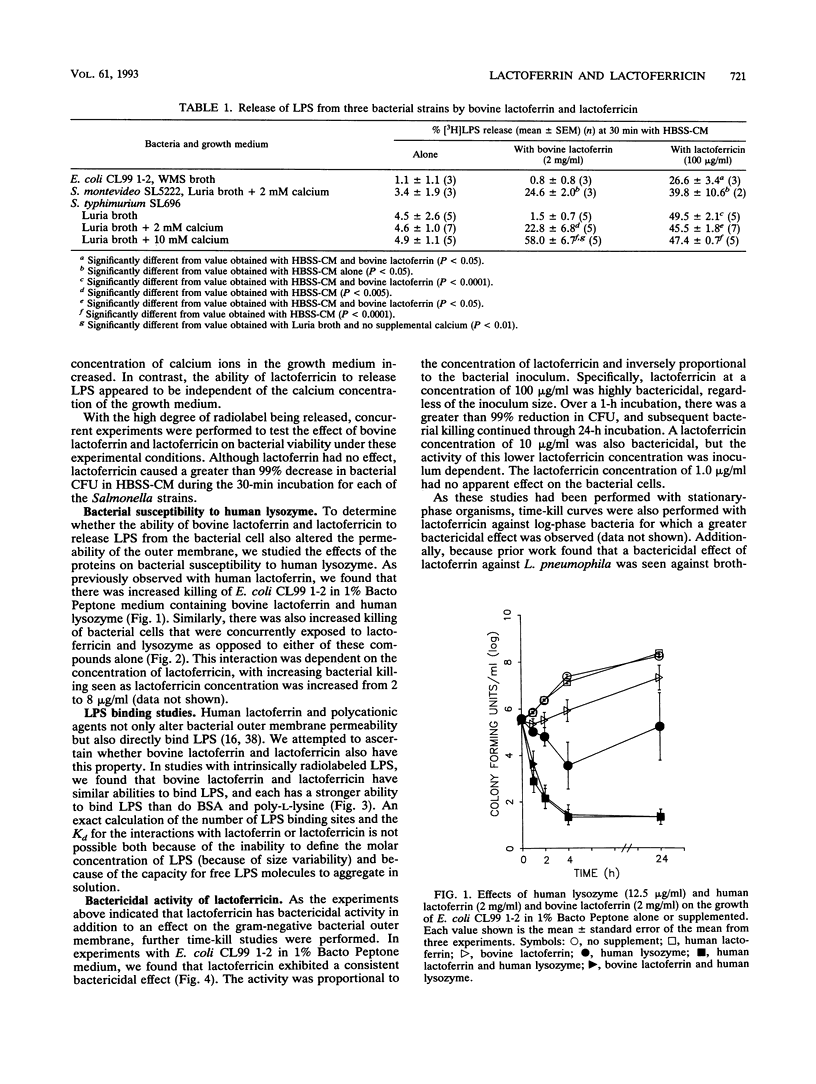
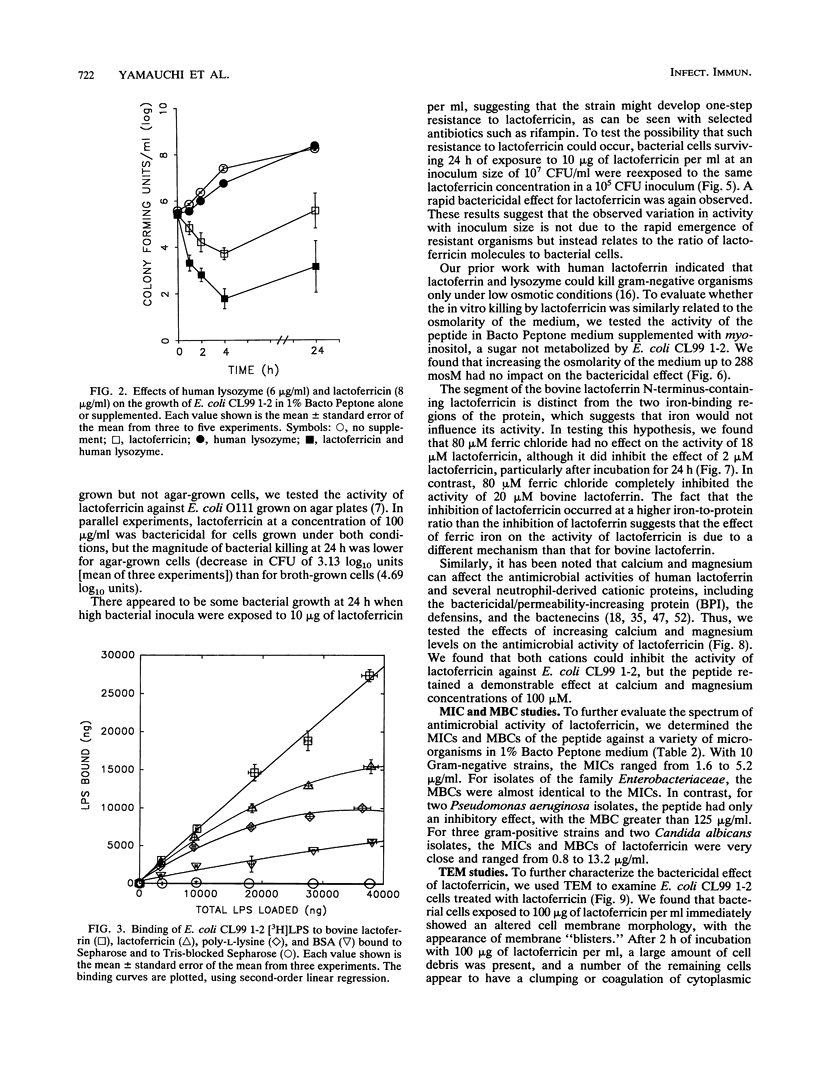
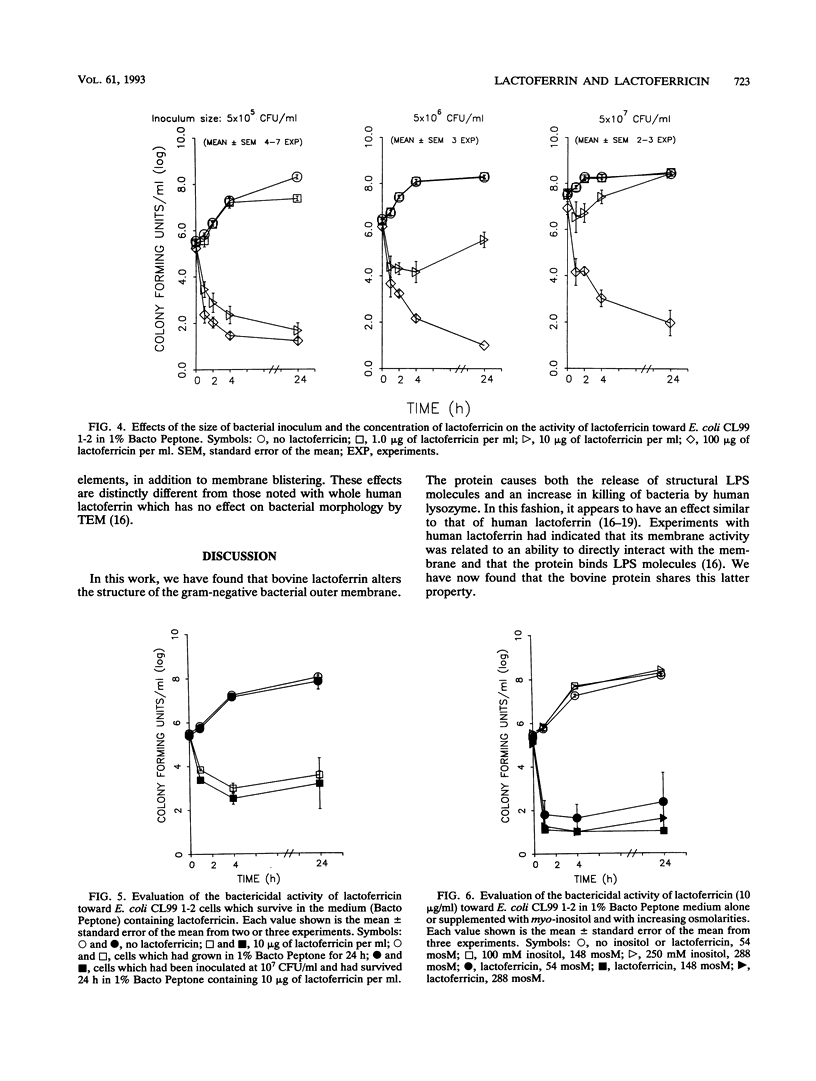
Abstract
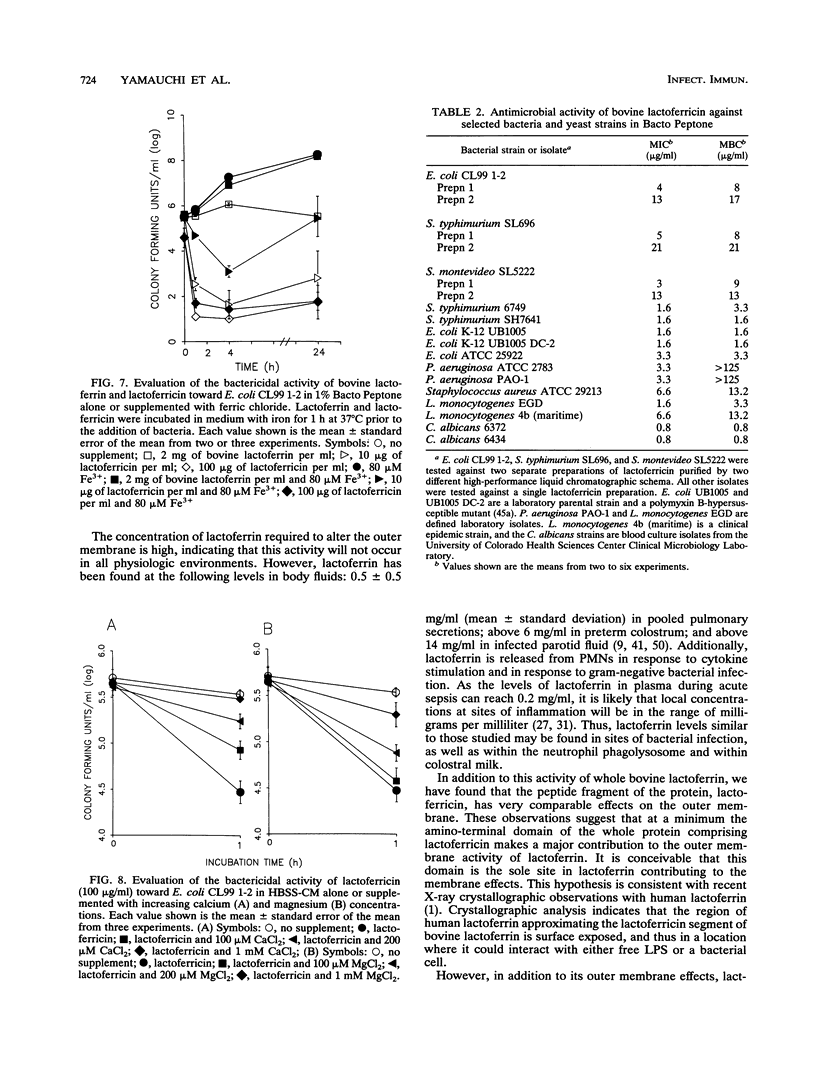
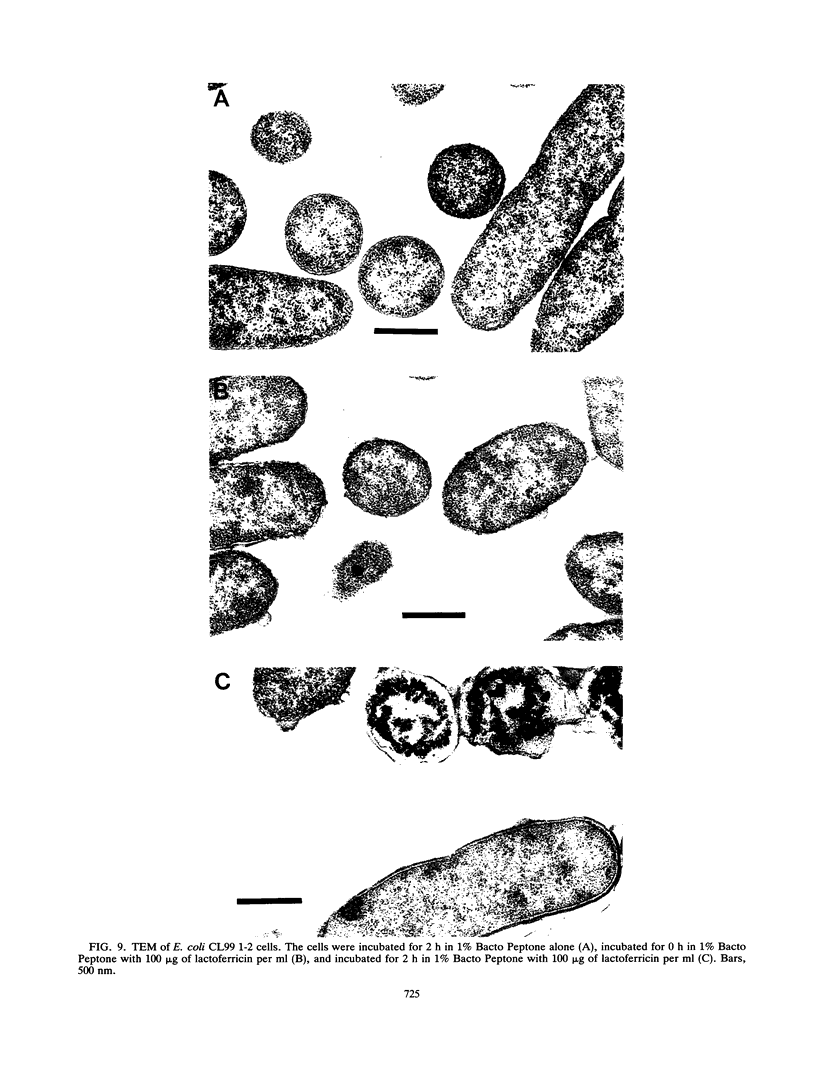
Although the antimicrobial activity of lactoferrin has been well described, its mechanism of action has been poorly characterized. Recent work has indicated that in addition to binding iron, human lactoferrin damages the outer membrane of gram-negative bacteria. In this study, we determined whether bovine lactoferrin and a pepsin-derived bovine lactoferrin peptide (lactoferricin) fragment have similar activities. We found that both 20 microM bovine lactoferrin and 20 microM lactoferricin release intrinsically labeled [3H]lipopolysaccharide ([3H]LPS) from three bacterial strains, Escherichia coli CL99 1-2, Salmonella typhimurium SL696, and Salmonella montevideo SL5222. Under most conditions, more LPS is released by the peptide fragment than by whole bovine lactoferrin. In the presence of either lactoferrin or lactoferricin there is increased killing of E. coli CL99 1-2 by lysozyme. Like human lactoferrin, bovine lactoferrin and lactoferricin have the ability to bind to free intrinsically labeled [3H]LPS molecules. In addition to these effects, whereas bovine lactoferrin was at most bacteriostatic, lactoferricin demonstrated consistent bactericidal activity against gram-negative bacteria. This bactericidal effect is modulated by the cations Ca2+, Mg2+, and Fe3+ but is independent of the osmolarity of the medium. Transmission electron microscopy of bacterial cells exposed to lactoferricin show the immediate development of electron-dense "membrane blisters." These experiments offer evidence that bovine lactoferrin and lactoferricin damage the outer membrane of gram-negative bacteria. Moreover, the peptide fragment lactoferricin has direct bactericidal activity. As lactoferrin is exposed to proteolytic factors in vivo which could cleave the lactoferricin fragment, the effects of this peptide are of both mechanistic and physiologic relevance.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. F., Baker H. M., Norris G. E., Rice D. W., Baker E. N. Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 A resolution. J Mol Biol. 1989 Oct 20;209(4):711–734. doi: 10.1016/0022-2836(89)90602-5. [DOI] [PubMed] [Google Scholar]
- Arnold R. R., Brewer M., Gauthier J. J. Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect Immun. 1980 Jun;28(3):893–898. doi: 10.1128/iai.28.3.893-898.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. R., Cole M. F., McGhee J. R. A bactericidal effect for human lactoferrin. Science. 1977 Jul 15;197(4300):263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- Baker E. N., Anderson B. F., Baker H. M., Haridas M., Jameson G. B., Norris G. E., Rumball S. V., Smith C. A. Structure, function and flexibility of human lactoferrin. Int J Biol Macromol. 1991 Jun;13(3):122–129. doi: 10.1016/0141-8130(91)90036-t. [DOI] [PubMed] [Google Scholar]
- Bellamy W., Takase M., Yamauchi K., Wakabayashi H., Kawase K., Tomita M. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992 May 22;1121(1-2):130–136. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- Bortner C. A., Arnold R. R., Miller R. D. Bactericidal effect of lactoferrin on Legionella pneumophila: effect of the physiological state of the organism. Can J Microbiol. 1989 Nov;35(11):1048–1051. doi: 10.1139/m89-174. [DOI] [PubMed] [Google Scholar]
- Bortner C. A., Miller R. D., Arnold R. R. Bactericidal effect of lactoferrin on Legionella pneumophila. Infect Immun. 1986 Feb;51(2):373–377. doi: 10.1128/iai.51.2.373-377.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogan T. D., Ryley H. C., Neale L., Yassa J. Soluble proteins of bronchopulmonary secretions from patients with cystic fibrosis, asthma, and bronchitis. Thorax. 1975 Feb;30(1):72–79. doi: 10.1136/thx.30.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Lactoferrin inhibits or promotes Legionella pneumophila intracellular multiplication in nonactivated and interferon gamma-activated human monocytes depending upon its degree of iron saturation. Iron-lactoferrin and nonphysiologic iron chelates reverse monocyte activation against Legionella pneumophila. J Clin Invest. 1991 Oct;88(4):1103–1112. doi: 10.1172/JCI115409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanelli D., Detmers P. A., Nathan C. F., Gabay J. E. Azurocidin and a homologous serine protease from neutrophils. Differential antimicrobial and proteolytic properties. J Clin Invest. 1990 Mar;85(3):904–915. doi: 10.1172/JCI114518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Britigan B. E., French M., Bean K. Preliminary observations on lactoferrin secretion in human vaginal mucus: variation during the menstrual cycle, evidence of hormonal regulation, and implications for infection with Neisseria gonorrhoeae. Am J Obstet Gynecol. 1987 Nov;157(5):1122–1125. doi: 10.1016/s0002-9378(87)80274-0. [DOI] [PubMed] [Google Scholar]
- Diamond G., Zasloff M., Eck H., Brasseur M., Maloy W. L., Bevins C. L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer P. B., Harwig S. S., Lehrer R. I. Cryptdins: antimicrobial defensins of the murine small intestine. Infect Immun. 1992 Sep;60(9):3556–3565. doi: 10.1128/iai.60.9.3556-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison R. T., 3rd, Giehl T. J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest. 1991 Oct;88(4):1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison R. T., 3rd, Giehl T. J., LaForce F. M. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect Immun. 1988 Nov;56(11):2774–2781. doi: 10.1128/iai.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison R. T., 3rd, LaForce F. M., Giehl T. J., Boose D. S., Dunn B. E. Lactoferrin and transferrin damage of the gram-negative outer membrane is modulated by Ca2+ and Mg2+. J Gen Microbiol. 1990 Jul;136(7):1437–1446. doi: 10.1099/00221287-136-7-1437. [DOI] [PubMed] [Google Scholar]
- Ellison R. T., 3rd, Luo Q., Reller L. B. Enhancement of the activity of cefotaxime by iron-binding proteins. J Antimicrob Chemother. 1990 Mar;25(3):479–481. doi: 10.1093/jac/25.3.479. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Sciortino C. V., McIntosh M. A. Role of iron in microbe-host interactions. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S759–S777. doi: 10.1093/clinids/5.supplement_4.s759. [DOI] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro R., Skerlavaj B., Romeo D. Purification, composition, and activity of two bactenecins, antibacterial peptides of bovine neutrophils. Infect Immun. 1989 Oct;57(10):3142–3146. doi: 10.1128/iai.57.10.3142-3146.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Goodman R. E., Schanbacher F. L. Bovine lactoferrin mRNA: sequence, analysis, and expression in the mammary gland. Biochem Biophys Res Commun. 1991 Oct 15;180(1):75–84. doi: 10.1016/s0006-291x(05)81257-4. [DOI] [PubMed] [Google Scholar]
- Gutteberg T. J., Haneberg B., Jørgensen T. The latency of serum acute phase proteins in meningococcal septicemia, with special emphasis on lactoferrin. Clin Chim Acta. 1984 Jan 31;136(2-3):173–178. doi: 10.1016/0009-8981(84)90289-4. [DOI] [PubMed] [Google Scholar]
- Hukari R., Helander I. M., Vaara M. Chain length heterogeneity of lipopolysaccharide released from Salmonella typhimurium by ethylenediaminetetraacetic acid or polycations. Eur J Biochem. 1986 Feb 3;154(3):673–676. doi: 10.1111/j.1432-1033.1986.tb09450.x. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Goldman R., Schmetz M., Berger M., Hammer C. H., Frank M. M., Leive L. A quantitative analysis of C3 binding to O-antigen capsule, lipopolysaccharide, and outer membrane protein of E. coli 0111B4. J Immunol. 1984 Jan;132(1):369–375. [PubMed] [Google Scholar]
- Kalmar J. R., Arnold R. R. Killing of Actinobacillus actinomycetemcomitans by human lactoferrin. Infect Immun. 1988 Oct;56(10):2552–2557. doi: 10.1128/iai.56.10.2552-2557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaForce F. M., Boose D. S. Release of lactoferrin by polymorphonuclear leukocytes after aerosol challenge with Escherichia coli. Infect Immun. 1987 Sep;55(9):2293–2295. doi: 10.1128/iai.55.9.2293-2295.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law B. A., Reiter B. The isolation and bacteriostatic properties of lactoferrin from bovine milk whey. J Dairy Res. 1977 Oct;44(3):595–599. doi: 10.1017/s0022029900020550. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Barton A., Daher K. A., Harwig S. S., Ganz T., Selsted M. E. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest. 1989 Aug;84(2):553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Selsted M. E., Babior B. M., Curnutte J. T. Neutrophils and host defense. Ann Intern Med. 1988 Jul 15;109(2):127–142. doi: 10.7326/0003-4819-109-2-127. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Szklarek D., Selsted M. E. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J Clin Invest. 1988 Jun;81(6):1829–1835. doi: 10.1172/JCI113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima M. F., Kierszenbaum F. Lactoferrin effects on phagocytic cell function. I. Increased uptake and killing of an intracellular parasite by murine macrophages and human monocytes. J Immunol. 1985 Jun;134(6):4176–4183. [PubMed] [Google Scholar]
- Mannion B. A., Weiss J., Elsbach P. Separation of sublethal and lethal effects of the bactericidal/permeability increasing protein on Escherichia coli. J Clin Invest. 1990 Mar;85(3):853–860. doi: 10.1172/JCI114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra M. N., Wilde C. G., Griffith J. E., Snable J. L., Scott R. W. Bactericidal/permeability-increasing protein has endotoxin-neutralizing activity. J Immunol. 1990 Jan 15;144(2):662–666. [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Prignot J. J., Wauters G. Immunohistochemical localization and bacteriostatic properties of an iron-binding protein from bronchial mucus. Thorax. 1966 Nov;21(6):538–544. doi: 10.1136/thx.21.6.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur N. B., Dwarkadas A. M., Sharma V. K., Saha K., Jain N. Anti-infective factors in preterm human colostrum. Acta Paediatr Scand. 1990 Nov;79(11):1039–1044. doi: 10.1111/j.1651-2227.1990.tb11380.x. [DOI] [PubMed] [Google Scholar]
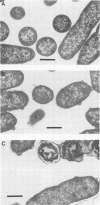
- Odeberg H., Olsson I. Mechanisms for the microbicidal activity of cationic proteins of human granulocytes. Infect Immun. 1976 Dec;14(6):1269–1275. doi: 10.1128/iai.14.6.1269-1275.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A., Colavizza D., Benaissa M., Maes P., Tartar A., Montreuil J., Spik G. Molecular cloning and sequence analysis of bovine lactotransferrin. Eur J Biochem. 1991 Feb 26;196(1):177–184. doi: 10.1111/j.1432-1033.1991.tb15801.x. [DOI] [PubMed] [Google Scholar]
- Rainard P. Bacteriostasis of Escherichia coli by bovine lactoferrin, transferrin and immunoglobulins (IgG1, IgG2, IgM) acting alone or in combination. Vet Microbiol. 1986 Feb;11(1-2):103–115. doi: 10.1016/0378-1135(86)90011-8. [DOI] [PubMed] [Google Scholar]
- Reiter B. The biological significance of lactoferrin. Int J Tissue React. 1983;5(1):87–96. [PubMed] [Google Scholar]
- Silva M. T., Silva M. N., Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathog. 1989 May;6(5):369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- Skerlavaj B., Romeo D., Gennaro R. Rapid membrane permeabilization and inhibition of vital functions of gram-negative bacteria by bactenecins. Infect Immun. 1990 Nov;58(11):3724–3730. doi: 10.1128/iai.58.11.3724-3730.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spik G., Cheron A., Montreuil J., Dolby J. M. Bacteriostasis of a milk-sensitive strain of Escherichia coli by immunoglobulins and iron-binding proteins in association. Immunology. 1978 Oct;35(4):663–671. [PMC free article] [PubMed] [Google Scholar]
- Stephens S., Dolby J. M., Montreuil J., Spik G. Differences in inhibition of the growth of commensal and enteropathogenic strains of Escherichia coli by lactotransferrin and secretory immunoglobulin A isolated from human milk. Immunology. 1980 Nov;41(3):597–603. [PMC free article] [PubMed] [Google Scholar]
- Tabak L., Mandel I. D., Herrera M., Baurmash H. Changes in lactoferrin and other proteins in a case of chronic recurrent parotitis. J Oral Pathol. 1978 Apr;7(2):91–99. doi: 10.1111/j.1600-0714.1978.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Tomita M., Bellamy W., Takase M., Yamauchi K., Wakabayashi H., Kawase K. Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. J Dairy Sci. 1991 Dec;74(12):4137–4142. doi: 10.3168/jds.S0022-0302(91)78608-6. [DOI] [PubMed] [Google Scholar]
- Weiss J., Franson C., Schmeidler K., Elsbach P. Reversible envelope effects during and after killing of Escherichia coli w by a highly-purified rabbit polymorpho-nuclear leukocyte fraction. Biochim Biophys Acta. 1976 Jun 4;436(1):154–169. doi: 10.1016/0005-2736(76)90227-3. [DOI] [PubMed] [Google Scholar]
- Weiss J., Muello K., Victor M., Elsbach P. The role of lipopolysaccharides in the action of the bactericidal/permeability-increasing neutrophil protein on the bacterial envelope. J Immunol. 1984 Jun;132(6):3109–3115. [PubMed] [Google Scholar]
- Weiss J., Victor M., Elsbach P. Role of charge and hydrophobic interactions in the action of the bactericidal/permeability-increasing protein of neutrophils on gram-negative bacteria. J Clin Invest. 1983 Mar;71(3):540–549. doi: 10.1172/JCI110798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]