Abstract
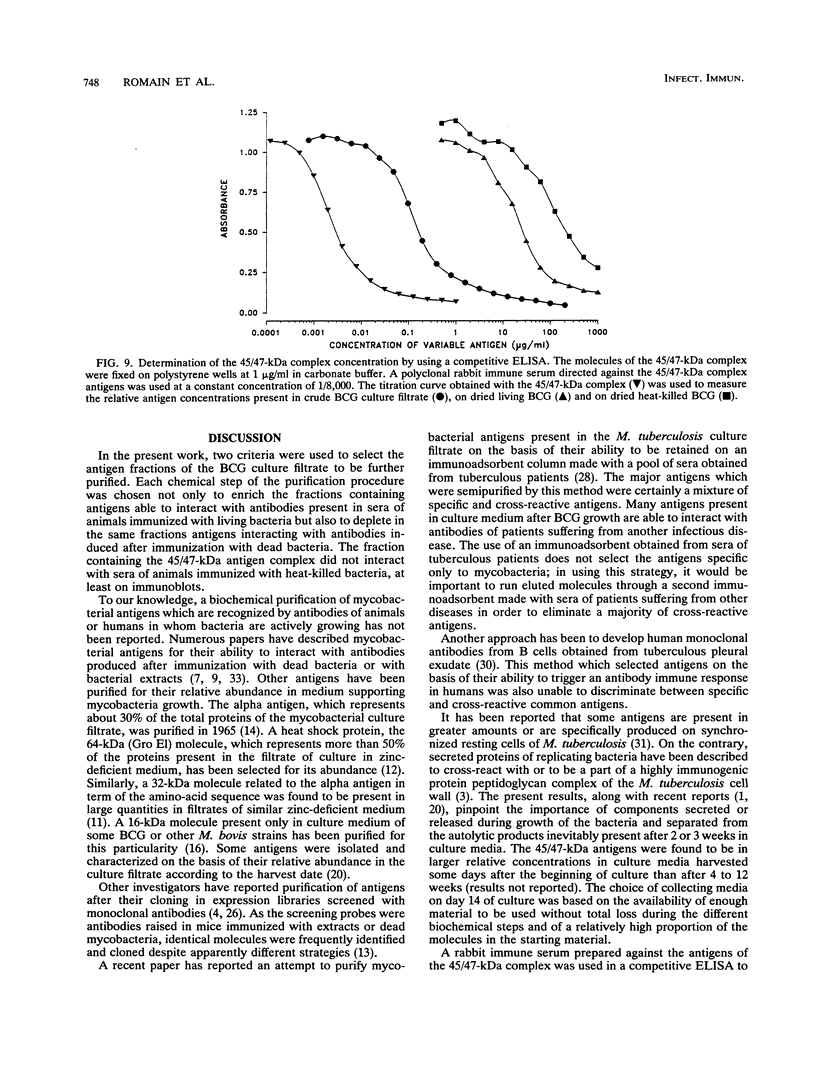
Increased protection against a virulent challenge with Mycobacterium tuberculosis is induced mainly by a previous immunization with living avirulent mycobacteria, usually Mycobacterium bovis BCG. Only a transient and marginal protection is obtained after immunization with bacterial extracts or dead bacteria. Both living and heat-killed bacteria share a number of common antigens. In order to identify mycobacterial molecules which are dominant antigens during immunization with living bacteria, a two-step selection method was used. Two groups of guinea pigs were immunized either with living or with heat-killed BCG. Sera were then collected and used to select and counterselect antigens present in BCG culture filtrates. Each major fraction eluted from a series of high-pressure liquid chromatography columns (gel filtration, DEAE, and reverse-phase chromatography) was run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred on polyvinylidene difluoride sheets. The molecules present on twin immunoblots were stained with antibodies raised in guinea pigs immunized either with living or with heat-killed BCG. Cross-reactive antigens stained in twin immunoblots were eliminated. Major antigens interacting with antibodies raised after immunization only with living bacteria were further purified. A complex of 45- and 47-kDa major molecules (45/47-kDa complex) was thus identified and further purified. The complex was found to interact only with antibodies present in sera of guinea pigs immunized with living bacteria and not at all with antibodies raised after immunization with dead bacteria. The 45/47-kDa antigen complex molecules were resolved on two-dimensional electrophoresis in three major and seven minor proteins detected with silver staining. All the molecules interacted with the antibodies present in sera of guinea pigs immunized with living BCG. The three major proteins (two at 47 kDa and one at 45 kDa) were amino-terminal sequenced. The sequence A-P-E-P-A-P-P-V-P-P-A-A-A-A-P-P-A, which was not previously reported, was the same for these three molecules. By using a competitive enzyme-linked immunosorbent assay, the concentrations of the 45/47-kDa antigen complex were measured in BCG culture filtrates, freeze-dried BCG, and dried heat-killed BCG; they were, respectively, 2, 0.01, and 0.001% of the total mass. The low or very low values compared with the high antibody concentration emphasized the ability of the 45/47-kDa complex delivered through live BCG to trigger an antibody response.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Askgaard D., Ljungqvist L., Bentzon M. W., Heron I. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect Immun. 1991 Apr;59(4):1558–1563. doi: 10.1128/iai.59.4.1558-1563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Bloch A. B., Davidson P. T., Snider D. E., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991 Jun 6;324(23):1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Mehra V., Hirschfield G. R., Fong S. J., Abou-Zeid C., Rook G. A., Hunter S. W., Brennan P. J., Modlin R. L. Characterization of T cell antigens associated with the cell wall protein-peptidoglycan complex of Mycobacterium tuberculosis. J Immunol. 1989 Oct 15;143(8):2656–2662. [PubMed] [Google Scholar]
- Britton W. J., Hellqvist L., Basten A., Inglis A. S. Immunoreactivity of a 70 kD protein purified from Mycobacterium bovis Bacillus Calmette-Guerin by monoclonal antibody affinity chromatography. J Exp Med. 1986 Sep 1;164(3):695–708. doi: 10.1084/jem.164.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol Today. 1991 Jul;12(7):228–233. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- De Bruyn J., Huygen K., Bosmans R., Fauville M., Lippens R., Van Vooren J. P., Falmagne P., Weckx M., Wiker H. G., Harboe M. Purification, characterization and identification of a 32 kDa protein antigen of Mycobacterium bovis BCG. Microb Pathog. 1987 May;2(5):351–366. doi: 10.1016/0882-4010(87)90077-5. [DOI] [PubMed] [Google Scholar]
- De Bruyn J., Weckx M., Beumer-Jochmans M. P. Effect of zinc deficiency on Mycobacterium tuberculosis var. bovis (BCG). J Gen Microbiol. 1981 Jun;124(2):353–357. doi: 10.1099/00221287-124-2-353. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Hirai T., Uchida T., Yoneda M. Extracellular proteins of tubercle bacilli. IV. Alpha and beta antigens as major extracellular protein products and as cellular components of a strain (H37Rv) of Mycobacterium tuberculosis. Biken J. 1965 Dec;8(4):189–199. [PubMed] [Google Scholar]
- Harboe M., Nagai S. MPB70, a unique antigen of Mycobacterium bovis BCG. Am Rev Respir Dis. 1984 Mar;129(3):444–452. doi: 10.1164/arrd.1984.129.3.444. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Nagai S., Wiker H. G., Harboe M., Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991 Jan;59(1):372–382. doi: 10.1128/iai.59.1.372-382.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988 Dec;56(12):3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romain F., Versmisse E., Pescher P., Augier J. Preparation of tuberculin antigen L. Ann Inst Pasteur Microbiol. 1985 Sep-Oct;136B(2):235–248. doi: 10.1016/s0769-2609(85)80048-x. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Stabel L. F., Suykerbuyk M. E., De Wit M. Y., Klatser P. R., Kolk A. H., Hartskeerl R. A. A major immunogenic 36,000-molecular-weight antigen from Mycobacterium leprae contains an immunoreactive region of proline-rich repeats. Infect Immun. 1990 Jan;58(1):80–87. doi: 10.1128/iai.58.1.80-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongkrajai P., Lulitanon V., Chamnanvanakit C. Improved ELISA with immunoabsorbent-purified mycobacterial antigen for serodiagnosis of tuberculosis. J Med Microbiol. 1989 Oct;30(2):101–104. doi: 10.1099/00222615-30-2-101. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS D. W., DUBOS R. J. Antituberculous immunity induced in mice by vaccination with killed tubercle bacilli or with a soluble bacillary extract. J Exp Med. 1955 Mar 1;101(3):313–330. doi: 10.1084/jem.101.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S., Alde S. L., Havlir D. V., Amir-Tahmasseb M. H., Daniel T. M., Ellner J. J. Identification of antigens of Mycobacterium tuberculosis using human monoclonal antibodies. J Clin Invest. 1989 Jul;84(1):214–219. doi: 10.1172/JCI114143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Sramek H. A. Antigenic differences between extracts of actively replicating and synchronized resting cells of Mycobacterium tuberculosis. Infect Immun. 1979 May;24(2):363–370. doi: 10.1128/iai.24.2.363-370.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiker H. G., Harboe M., Bennedsen J., Closs O. The antigens of Mycobacterium tuberculosis, H37Rv, studied by crossed immunoelectrophoresis. Comparison with a reference system for Mycobacterium bovis, BCG. Scand J Immunol. 1988 Feb;27(2):223–239. doi: 10.1111/j.1365-3083.1988.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Wiker H. G., Sletten K., Nagai S., Harboe M. Evidence for three separate genes encoding the proteins of the mycobacterial antigen 85 complex. Infect Immun. 1990 Jan;58(1):272–274. doi: 10.1128/iai.58.1.272-274.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Kaufmann S. H., Hermans P. W., Thole J. E. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992 Jan;6(2):133–145. doi: 10.1111/j.1365-2958.1992.tb01994.x. [DOI] [PubMed] [Google Scholar]