Abstract
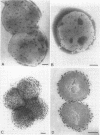
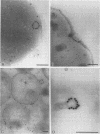
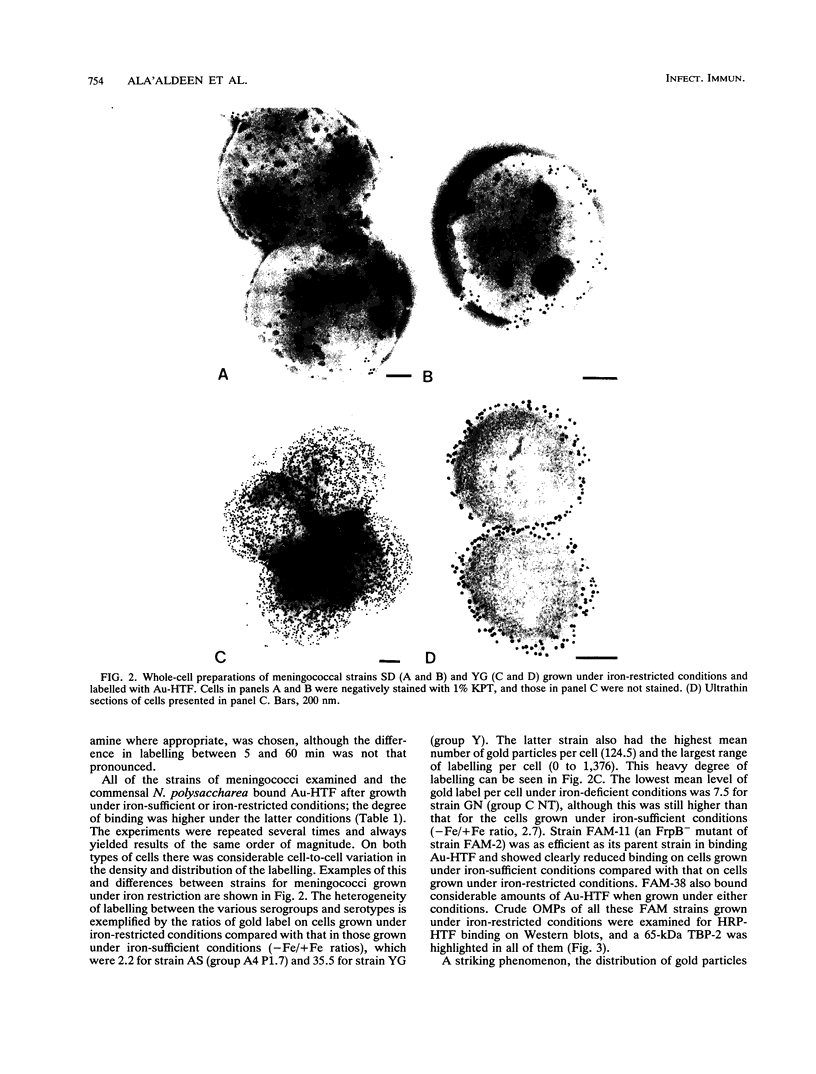
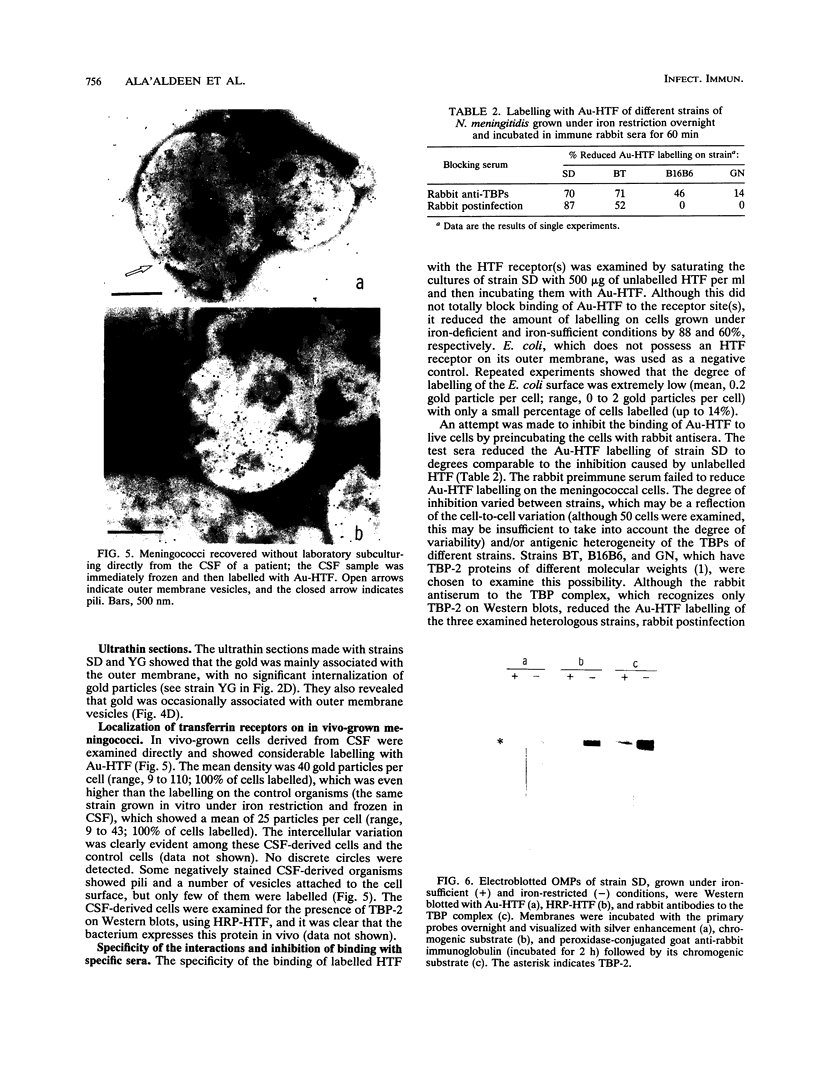
The interaction between gold-labelled human transferrin (Au-HTF) with live meningococci after growth in vivo or in different in vitro conditions was examined by electron microscopy to localize and quantify the numbers of HTF-binding sites on the cell surface. It was clearly demonstrated that HTF binds to the surface of live meningococci (of different serogroups and serotypes) after growth in either iron-sufficient or iron-restricted cultures, although the degree of labelling was always higher (2- to 35-fold) in the latter case. The commensal Neisseria polysaccharea behaved similarly. Ultrathin sections showed that Au-HTF was localized predominantly on the outer membrane of the cells and vesicles, with hardly any internalization. Au-HTF labelling on meningococci was significantly reduced after incubation with unlabelled HTF or with rabbit antiserum containing antibodies against transferrin-binding proteins (TBPs), demonstrating the specificity of the interaction. These sera also blocked binding between HTF and outer membrane proteins on Western immunoblots. Direct evidence of the expression of the TBPs (Western blots) and localization of the HTF receptor (electron microscopy) on in vivo-grown meningococci was obtained from organisms derived without laboratory culturing from the cerebrospinal fluid of a patient. There was considerable cell-to-cell variation in the amount of labelling present on cells of the same sample (in vitro- or in vivo-grown organisms) and between different strains. The degree of binding varied with time of incubation of the cells with Au-HTF. The gold particles frequently formed discrete circles on the cell surfaces of the in vitro-grown organisms; these circles appear to be associated with outer membrane vesicle formation. The results show that the TBPs, which form part of the active components of the HTF receptor(s), are expressed in vivo and are surface exposed and immunogenic and that antibodies against them can interfere with the HTF binding of the meningococcal cells, which may affect iron utilization. This study further supports the concept of regarding the TBPs as future vaccine candidates.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ala'Aldeen D. A., Davies H. A., Wall R. A., Borriello S. P. The 70 kilodalton iron regulated protein of Neisseria meningitidis is not the human transferrin receptor. FEMS Microbiol Lett. 1990 May;57(1-2):37–42. doi: 10.1016/0378-1097(90)90409-j. [DOI] [PubMed] [Google Scholar]
- Ala'Aldeen D. A., Wall R. A., Borriello S. P. Immunogenicity and cross-reactivity of the 70-Kda iron-regulated protein of Neisseria meningitidis in man and animals. J Med Microbiol. 1990 Aug;32(4):275–281. doi: 10.1099/00222615-32-4-275. [DOI] [PubMed] [Google Scholar]
- Banerjee-Bhatnagar N., Frasch C. E. Expression of Neisseria meningitidis iron-regulated outer membrane proteins, including a 70-kilodalton transferrin receptor, and their potential for use as vaccines. Infect Immun. 1990 Sep;58(9):2875–2881. doi: 10.1128/iai.58.9.2875-2881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates G. W., Billups C., Saltman P. The kinetics and mechanism of iron (3) exchange between chelates and transferrin. I. The complexes of citrate and nitrilotriacetic acid. J Biol Chem. 1967 Jun 25;242(12):2810–2815. [PubMed] [Google Scholar]
- Bjune G., Høiby E. A., Grønnesby J. K., Arnesen O., Fredriksen J. H., Halstensen A., Holten E., Lindbak A. K., Nøkleby H., Rosenqvist E. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991 Nov 2;338(8775):1093–1096. doi: 10.1016/0140-6736(91)91961-s. [DOI] [PubMed] [Google Scholar]
- DeVoe I. W. The meningococcus and mechanisms of pathogenicity. Microbiol Rev. 1982 Jun;46(2):162–190. doi: 10.1128/mr.46.2.162-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer D. W., West E. P., McKenna W., Thompson S. A., Sparling P. F. A pleiotropic iron-uptake mutant of Neisseria meningitidis lacks a 70-kilodalton iron-regulated protein. Infect Immun. 1988 Apr;56(4):977–983. doi: 10.1128/iai.56.4.977-983.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreirós C. M., Criado M. T., Del Río M. C., Pintor M. Analysis of the expression of outer-membrane proteins in Neisseria meningitidis in iron-replete and iron-deficient media. FEMS Microbiol Lett. 1990 Sep 1;59(1-2):49–54. doi: 10.1016/0378-1097(90)90031-k. [DOI] [PubMed] [Google Scholar]
- Griffiths E., Stevenson P., Ray A. Antigenic and molecular heterogeneity of the transferrin-binding protein of Neisseria meningitidis. FEMS Microbiol Lett. 1990 May;57(1-2):31–36. doi: 10.1016/0378-1097(90)90408-i. [DOI] [PubMed] [Google Scholar]
- Mickelsen P. A., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981 Aug;33(2):555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E. N., Jr, McGee Z. A., Buchanan T. M., Blake M. S., Hitchcock P. J. Probing the surface of Neisseria gonorrhoeae: simultaneous localization of protein I and H.8 antigens. Infect Immun. 1987 May;55(5):1190–1197. doi: 10.1128/iai.55.5.1190-1197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers A. B., Lee B. C. Comparative analysis of the transferrin and lactoferrin binding proteins in the family Neisseriaceae. Can J Microbiol. 1989 Mar;35(3):409–415. doi: 10.1139/m89-063. [DOI] [PubMed] [Google Scholar]
- Schryvers A. B., Morris L. J. Identification and characterization of the transferrin receptor from Neisseria meningitidis. Mol Microbiol. 1988 Mar;2(2):281–288. doi: 10.1111/j.1365-2958.1988.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Simonson C., Brener D., DeVoe I. W. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitidis. Infect Immun. 1982 Apr;36(1):107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stevenson P., Williams P., Griffiths E. Common antigenic domains in transferrin-binding protein 2 of Neisseria meningitidis, Neisseria gonorrhoeae, and Haemophilus influenzae type b. Infect Immun. 1992 Jun;60(6):2391–2396. doi: 10.1128/iai.60.6.2391-2396.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J., Dyer D. W., Sparling P. F. Loss of transferrin receptor activity in Neisseria meningitidis correlates with inability to use transferrin as an iron source. Infect Immun. 1988 Dec;56(12):3132–3138. doi: 10.1128/iai.56.12.3132-3138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R. A., Davies H. A., Borriello S. P. Epitopes of serogroup B Neisseria meningitidis analysed in vitro and directly from cerebrospinal fluid. FEMS Microbiol Lett. 1989 Nov;53(1-2):129–135. doi: 10.1016/0378-1097(89)90379-0. [DOI] [PubMed] [Google Scholar]
- West S. E., Sparling P. F. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985 Feb;47(2):388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Boslego J., Moran E., Garcia J., Cruz C., Ruiz S., Brandt B., Martinez M., Arthur J., Underwood P. Meningococcal serogroup B vaccine protection trial and follow-up studies in Chile. The Chilean National Committee for Meningococcal Disease. NIPH Ann. 1991 Dec;14(2):211–213. [PubMed] [Google Scholar]