Abstract
We report the unusually rapid and spontaneous normalisation of low diffusivity that accompanied resolution of acute neurological deficits in a stroke patient who underwent two magnetic resonance imaging examinations within 24 h of symptom onset. Diffusion weighted imaging obtained within hours of onset of left sided weakness demonstrated a focal right capsular area of low diffusivity that resolved within 24 h, coinciding with resolution of the patient’s symptoms.
Background
This case adds to the growing literature confirming that focal transient ischaemia may produce spontaneously reversible low diffusivity in the brain, a phenomenon originally reported only in experimental animal models of stroke but now increasingly recognised as a part of the clinical spectrum of human neurovascular disease.1
Case presentation
A 76-year-old right handed man with a history of diabetes and hypercholesterolaemia presented to the emergency room with a sudden onset of mild left sided weakness. In the approximate time span of 2–3 h that passed between the notification of the emergency medical services, transportation to the hospital, and examination by the emergency medicine and neurology residents, his weakness had fluctuated twice between near resolution of symptoms and a dense left hemiparesis involving face, arm and leg. Mental status examination was normal, and there were no cranial nerve findings other than mild blunting of the right nasolabial fold. There were no sensory or cerebellar deficits. His examination stabilised with an ataxic hemiparesis.
Investigations
A computed tomography (CT) angiogram of the head and neck performed in the emergency room was normal.
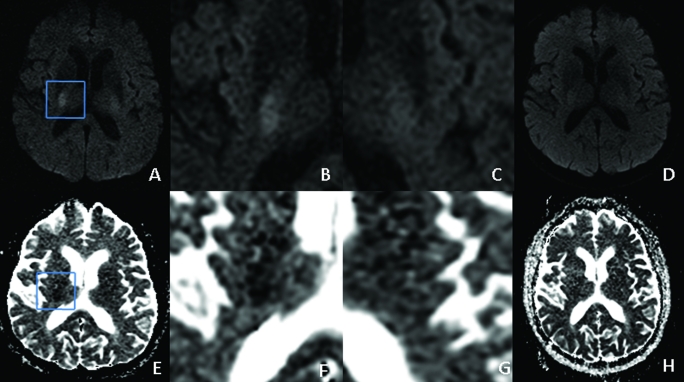
Subsequently, an acute stroke magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) examination showed a small area of very low diffusivity in the right internal capsule and adjacent thalamus (fig 1A–D). Apparent diffusion coefficient within the area of ischaemia and contralateral internal capsule was 490×10−6 mm2/s and 734×10−6 mm2/s, respectively, with an rADC of 0.67. A follow-up MRI was performed 19 h later, to evaluate for potential brain stem ischaemic lesions as a cause for the patient’s ataxia (fig 1E–H). This study, performed when the patient was asymptomatic, demonstrated normal diffusivity throughout the brain, with the apparent diffusion coefficient in the previously measured regions of interest measuring 778×10−6 mm2/s and 787×10−6 mm2/s, respectively, with an rADC of 0.99.
Figure 1.
Axial magnetic resonance imaging (MRI) in a 76-year-old man with transient left ataxic hemiparesis. (A–D) Diffusion weighted imaging (DWI) shows restricted diffusion in the posterior limb of the right internal capsule (A), with magnified views of this region (B) and the normal contralateral side (C). Follow-up MRI performed 19 h later shows complete resolution (D), reflecting the patient’s resolved symptoms. (E–H) Corresponding apparent diffusion coefficient (ADC) maps.
Discussion
Fisher described the lacunar infarct syndrome of ‘ataxic hemiparesis’ first in 1965, and later included other strokes that present with dysmetria that is out of proportion and ipsilateral to the side of weakness.2 A number of these patients presented with stuttering symptoms similar to the case reported here. Lesions that simultaneously interrupt pyramidal systems and adjoining frontopontocerebellar systems produce ataxic hemiparesis. Common locations are both anterior and posterior limb of the internal capsule, upper pons, and more rarely corona radiata, lentiform nucleus and thalamus.2,3
Gorman et al have argued that lacunar infarctions originating in the capsule cannot be distinguished clinically from pontine stroke.4 It was for this reason that the follow-up MRI was performed with thin section coronal imaging through the brainstem. However, by the time the follow-up study was performed the patient’s symptoms had improved dramatically and the diffusion weighted image (DWI) lesion resolved concurrently. The brainstem was still unremarkable. Upon discharge 24 h later, the examination revealed no weakness and only subtle residual deficits with rapid alternating movements and orbiting (making circular motions with the arms as if boxing). The patient’s gait was stable and he was deemed safe to ambulate unassisted.
Prior case reports have demonstrated reversal of abnormality on DWI in patients with transient ischaemic attack (TIA) when imaged soon after symptom onset.5–7 A larger series published subsequently found that four of nine patients with TIAs had no residual evidence of infarct on follow-up imaging.8
However, a still more recent study demonstrated focal atrophy and T2 prolongation in nearly 80% of TIA related DWI abnormalities suggesting that the vast majority of such small areas of low diffusivity result in permanent tissue injury.9
A substantial small animal MRI literature indicates that reversible ischaemic abnormality on DWI is due transient decrease in mitochondrial ATP production and resulting cell membrane sodium–potassium pump dysfunction. In rat models, variation in the duration and intensity of ischaemia leads to wide variation in the percentage of affected neurons that die, but some cell death is seen even in brief ischaemia accompanied by complete normalisation of diffusivity.1,10
This supports the hypothesis that TIAs in human patients result from a degree or duration of ischaemia that causes relatively little permanent cell death.11 Likewise studies of average apparent diffusion coefficient (ADC) in regions of abnormality on DWI have shown higher ADC in patients with TIA when compared with stroke patients.12 On the other hand, recent human imaging studies have shown that improving the spatial resolution of clinical DWI sequences substantially increases the detection of ischaemic low diffusivity lesions on MRI of patients with stroke, raising the possibility that clinical TIA without detectable abnormality on DWI may actually represent infarcts too small to be detected by current clinical DWI techniques.13
While ongoing advances in imaging fuel controversy about both the pathophysiology and appropriate nosology of transient ischaemic clinical syndromes with detectable abnormality on DWI, there is emerging agreement that this entity requires prompt aggressive investigation and treatment because it signals an increased early risk for additional TIA and stroke.14,15 Our report contributes to this growing literature by providing a clear, well documented and temporally correlated case of normalisation of clinical and DWI abnormality within a very short time in an untreated patient with a stuttering presentation of ataxic hemiparesis.
Learning points
‘Ataxic hemiparesis’ implies dysmetria that is out of proportion to weakness and can result from a lacunar stroke or transient ischaemic attack (TIA).
Focal transient ischaemia may produce reversible abnormalities on diffusion weighted imaging (DWI) of the brain, emphasising the continuum between TIA and stroke.
Transient ischaemic clinical syndromes with abnormalities on DWI warrant aggressive investigation and treatment to avoid additional ischaemic injury.
Footnotes
Competing interests: None.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Li F, Liu KF, Silva MD, et al. Transient and permanent resolution of ischemic lesions on diffusion-weighted imaging after brief periods of focal ischemia in rats: correlation with histopathology. Stroke 2000; 31: 946–54 [DOI] [PubMed] [Google Scholar]
- 2.Fisher CM. Ataxic hemiparesis. A pathologic study. Arch Neurol 1978; 35: 126–8 [DOI] [PubMed] [Google Scholar]
- 3.Sanguineti I, Tredici G, Beghi E, et al. Ataxic hemiparesis syndrome: clinical and CT study of 20 new cases and review of the literature. Ital J Neurol Sci 1986; 7: 51–9 [DOI] [PubMed] [Google Scholar]
- 4.Gorman MJ, Dafer R, Levine SR. Ataxic hemiparesis: critical appraisal of a lacunar syndrome. Stroke 1998; 29: 2549–55 [DOI] [PubMed] [Google Scholar]
- 5.Lecouvet FE, Duprez TPJ, Raymackers JM, et al. Resolution of early diffusion-weighted and FLAIR MRI abnormalities in a patient with TIA. Neurology 1999; 52: 1085–7 [DOI] [PubMed] [Google Scholar]
- 6.Neumann-Haefelin T, Wittsack HJ, Wenserskiet F, et al. Diffusion- and perfusion-weighted MRI in a patient with a prolonged reversible ischaemic neurological deficit. Neuroradiology 2000; 42: 444–7 [DOI] [PubMed] [Google Scholar]
- 7.Terasawa Y, Iguchi Y, Kimura K, et al. Reversible diffusion-weighted lesion in a TIA patient without arterial recanalization: a case report. J Neurol Sci 2008; 272: 183–5 [DOI] [PubMed] [Google Scholar]
- 8.Kidwell CS, Alger JR, Di Salle F, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke 1999; 30: 1174–80 [DOI] [PubMed] [Google Scholar]
- 9.Oppenheim C, Lamy C, Touze E, et al. Do transient ischemic attacks with diffusion weighted imaging abnormalities correspond to brain infarctions? AJNR Am J Neuroradiol 2006; 27: 1782–7 [PMC free article] [PubMed] [Google Scholar]
- 10.Ringer TM, Neumann–Haefelin T, Sobel RA, et al. Reversal of early diffusion-weighted magnetic resonance imaging abnormalities does not necessarily reflect tissue salvage in experimental cerebral ischemia. Stroke 2001; 32: 2362–9 [DOI] [PubMed] [Google Scholar]
- 11.Saver JL, Kidwell C. Neuroimaging of TIAs. Neurology 2004; 62: S22–5 [DOI] [PubMed] [Google Scholar]
- 12.Winbeck K, Bruckmaier K, Etgen T, et al. Transient ischemic attack and stroke can be differentiated by analyzing early diffusion-weighted imaging signal intensity changes. Stroke 2004; 35: 1095–9 [DOI] [PubMed] [Google Scholar]
- 13.Benameur K, Bykowski JL, Luby M, et al. Higher prevalence of cortical lesions observed in patients with acute stroke using high–resolution diffusion-weighted imaging. AJNR Am J Neuroradiol 2006; 27: 1987–9 [PMC free article] [PubMed] [Google Scholar]
- 14.Caplan LR. Transient ischemic attack with abnormal diffusion-weighted imaging results: what’s in a name? Arch Neurol 2007; 64: 1080–2 [DOI] [PubMed] [Google Scholar]
- 15.Prabhakaran S, Chong JY, Sacco RL. Impact of abnormal diffusion-weighted imaging results on short–term outcome following transient ischemic attack. Arch Neurol 2007; 64: 1105–9 [DOI] [PubMed] [Google Scholar]