Abstract
Norwalk virus (NV) replicon-harboring cells have provided an excellent tool to the development of antivirals. Previously we demonstrated that the expression levels of replicon RNA and proteins were significantly reduced in the presence of various interferons (IFNs) including IFN-α and IFN-γ in a dose-dependent manner in the NV replicon-harboring cells, and suggested that IFNs could be therapeutic options for norovirus infection. It was also demonstrated that innate immunity including IFNs is crucial in the replication and pathogenicity of murine norovirus (MNV) in vitro (RAW267.4 cells) and in vivo. IFNs have a short half-life in vitro and in vivo due to low stability. Thus it is important to have a good delivery system to improve the stability of IFNs. Nanogels are nanosized networks of chemically cross-linked polymers that swell in physiologic solutions and provide improved stability and bioavailability to drugs. We have synthesized nanogels based on cross-linked polyethyleneimine (PEI)-polyethylenglycol (PEG). The PEI/PEG nanogels were further acetylated (AcNg) to reduce cellular penetration and cytotoxicity. The IFN-AcNg complex was prepared by incubating two components together at 4 °C and lyophilization. The IFN activity of IFN-AcNg was evaluated in the NV- and HCV-replicon-harboring cells and against MNV-1 in RAW267.4 cells in comparison to IFN without AcNg. The AcNg improved the stability of IFN stored at 4 °C, and was well tolerated in the cells. Furthermore, the activity of IFN was significantly higher when combined with AcNg in the replicon-harboring cells and against MNV-1 in RAW267.4 cells. We concluded that AcNg may be pursued further as a vehicle for oral delivery of IFNs in norovirus infection.
Keywords: antiviral, interferon, nanogel, Norwalk virus, replicon-harboring cells, murine norovirus
1. Introduction
Noroviruses are a leading cause of food- or water-borne gastroenteritis outbreaks. Studies have demonstrated that noroviruses are responsible for more than 60% of all food-water-borne gastroenteritis outbreaks (Fankhauser et al., 1998) with an estimated 23 million cases annually in the US causing 50 000 hospitalizations and 300 deaths (Mead et al., 1999). Studies of the replication of human noroviruses have been severely hampered by the absence of a cell culture system (Duizer et al., 2004). To overcome this limitation, we have previously generated Norwalk virus (NV), the prototype norovirus strain, replicon-harboring cells in BHK21 and Huh-7 cells (Chang et al., 2006). This replicon system has provided an excellent tool to study the replication of noroviruses, and served as a platform to screen potential antiviral drugs (Chang, 2009; Chang and George, 2007b; Chang et al., 2006). Using the NV replicon-harboring cells, we have previously demonstrated that the expression levels of replicon RNA and proteins were significantly reduced in the presence of various interferons (IFNs) including IFN-α and IFN-γ in a dose-dependent manner, and suggested IFNs could be therapeutic options for norovirus infection (Chang, 2009; Chang and George, 2007b; Chang et al., 2006). The important roles of IFN in replication and pathogenicity of noroviruses were also demonstrated using murine norovirus (MNV) in vitro (RAW267.4 cells) and in vivo (Changotra et al., 2009; Karst et al., 2003; Mumphrey et al., 2007). Among the noroviruses, only MNVs including MNV-1 strain have been successfully propagated in cell culture (Wobus et al., 2004), and provided a cell culture model for norovirus research.
IFN therapy has been a part of standard treatment regime in some viral diseases such as hepatitis B and C virus infection (Foster, 2010; Liu and Kao, 2006). The importance of IFN in respiratory viral infection including influenza virus has also been studied extensively (Herzog et al., 1983; Isomura et al., 1982; Merigan et al., 1973; Phillpotts et al., 1983). However, the short half-life of IFN (up to 8.5 hr) in the serum requires repeated administration to maintain effective concentration in targeted organs. To increase the half-life, IFN can be chemically conjugated with poly(ethylene glycol) (PEG) which is inert, water-soluble, and nontoxic, and does not adversely affect the safety profile of IFN (Foster, 2010; Kozlowski et al., 2001; Luxon et al., 2002; Sharieff et al., 2002; Shiffman, 2001). The pegylated IFNs significantly increase the stability of IFN and are used for treatment of hepatitis B and C virus infection commonly in combination with ribavirin (Aghemo et al., 2010; Foster, 2010; Husa and Husova, 2001; Liu and Kao, 2006). The parenteral administration of high dose IFNs (several million international units [IU]) is associated with side effects, one of the major causes of treatment failure (Negro, 2010). However, systemic administration of IFN may not be required for viral mucosa infection, such as respiratory or intestinal infection. Therefore, oral or intranasal administration of IFNs has been explored as a way to reduce significant side effects and to introduce IFNs directly to the affected mucosal surfaces [review (Beilharz, 2010)].
Nanogels are nanosized networks of chemically cross-linked polymers that swell in a solvent and provide improved stability and bioavailability to drugs (Vinogradov et al., 1999; Vinogradov et al., 2006). We have synthesized nanogels based on cross-linked polyethyleneimine (PEI)-PEG, which were further acetylated to reduce cytotoxicity (AcNg). The IFN-AcNg complexes were prepared by incubating two components together at 4 °C and lyophilization. The prepared IFN-AcNg complexes of approximately 200 nm in diameter were evaluated for stability and antiviral effects of IFN in the NV- or HCV- replicon-harboring cells and MNV-1 in RAW267.4 cells. The AcNg improved the stability of IFN stored at 4 °C, and the antiviral activity of IFN was significantly higher in IFN-AcNg complex compared to IFN alone in the replicon-harboring cells or against MNV-1 in RAW267.4 cells. The preliminary study using rats suggested that IFN-AcNg did not cause side effects by oral or systemic administration, and AcNg did not cause IFN carryover to systemic circulation after oral administration of IFN-AcNg. We concluded that AcNg has a potential to be pursued as a vehicle for oral delivery of IFNs in norovirus infection.
2. Materials and methods
2.1. Cells, viruses, and reagents
The Vero cells, Huh-7, HG23 (Huh-7 based NV replicon-harboring cells), 1A7 (Huh-7 based hepatitis C virus replicon-harboring cells) and murine macrophage-like RAW267.4 cells were maintained in Dulbecco's minimal essential medium (DMEM) containing 10% fetal bovine serum and antibiotics (chlortetracycline [25 μg/ml], penicillin [250 U/ml], and streptomycin [250 μg/ml]) (DMEM-C). Murine norovirus-1 was provided by Dr. H. Virgin (Washington University in St Louis, MO), and maintained in RAW267.4 cells. Recombinant IFN type I (human IFN-αA+D fusion protein), mouse IFN-β, and human IFN-αA were purchased from Sigma-Aldrich (St Louis, MO). The polyclonal antibody specific for NV or MNV-1 proteinase-polymerase (ProPol) was described in previous reports (Chang et al., 2006; Sosnovtsev et al., 2006). Antibodies specific for neomycin phosphotransferase II (NPT II) or β-actin were obtained from Santa Cruz biotechnology (Santa Cruz, CA) or Cell Signaling Technology (Danvers, MA), respectively. PEI (~25 kDa), PEG (8 kDa), dichloromethane, actonitrile, acetic anhydride, 1,1′-carbonyldiimidazole, N,N′-dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide, and 1-hydroxybenzotriazole (HOBT) were purchased from Sigma-Aldrich.
2.2. Preparation of PEG/PEI nanogel
Nanogel PEG-PEI was prepared by following a method described previously (Ganta et al., 2008). Briefly, 7.0 g of PEI (MW ~25 kDa) in 20 ml of deionized water was loaded into Sephacryl S200 chromatographic column. The middle fractions (based on weight distribution) were collected and lyophilized to give 3.64 g (0.146 mmol) of PEI (molecular weight [MW] ~25 kDa). To activate PEG, 2.0 g of PEG (MW 8 kDa) in 7 ml of dry acetonitrile under argon was added to 0.41 g (2.5 mmol) of 1, 1′-carbonyldimidazole, and the solution was stirred at 40 °C for 2 hrs. The crude product was purified by dialysis using a MW cut-off (MWCO) 2 kDa membrane with 10 % ethanol in deionized water at 4 °C for 4 hrs. The solution was lyophilized to give 1.84 g of activated PEG. Nanogel PEG-PEI was prepared by following a similar micellar method (Vinogradov et al., 2006). Synthesis started from activated PEG (~63 μmol) and PEI (~40 μmol). The PEI in 300 ml of deionized water was added dropwise to a solution of activated PEG (MW ~8 kDa) in 2 ml of dichloromethane. The reaction solution was sonicated in a water bath for 10 min, and the organic solvent was removed on a rotary evaporator resulting in a transparent solution. The solution was dialyzed with a MWCO 12K – 14 K membrane in 800 ml of 10 % ethanol in deionized water for 1 day at 25 °C and lyophilized to give nanogel PEG-PEI. For the acetylation of nanogel, 0.2 ml of acetic anhydride was added to a solution of 100 mg of nanogel in 1 ml of acetonitrile under argon. The solution was stirred at 50 °C for 12 hrs and dialyzed with a MWCO 12 K – 14 K membrane in 100 ml of 10 % ethanol in deionized water at 25 °C for 12 hrs. The solution was lyophilized to give 95 mg of AcNg as white solids.
2.3. Preparation of IFN-AcNg
IFN-αA+D or mouse IFN-β (for in vitro studies), or human IFN-αA (for in vivo study) was encapsulated with AcNg. For encapsulation, 3 mg of AcNg in 0.5 ml of deionized water was mixed with the same volume of IFN (3×103, 3×104, or 107 IU in PBS) at 4 °C for 5 min, and then the mixture was lyophilized. The lyophilized white solids were stored at -80 °C. The encapsulated IFNs were resuspended with 1 ml of PBS to a final concentration of 104 IU/μl for in vivo study, or 3 or 30 IU/μl for in vitro studies. As a control, IFNs were prepared by the same method without AcNg (final concentration is 104, 3 or 30 IU/μl). For AcNg control, AcNg was prepared by the same method without IFN. The nonspecific cytotoxic effects of AcNg or IFN-AcNg in Vero, HG23 and RAW267.4 cells were monitored by observation under a microscopy and CytoTox 96 Non-radioactive cytotoxicity assay (Promega, Madison, WI).
2.4. Atomic force microscope (AFM) experiments
The particles of AcNg and IFN-AcNg were observed under an AFM (Veeco, Santa Barbara CA). The images were collected using a tapping mode with a high aspect ratio tip (Veeco Nanoprobe TM tips, Model TESP-HAR). A small aliquot (20 μl) of AcNg or IFN-AcNg was placed onto freshly cleaved mica, washed twice with deionized water, and dried with N2. AFM images on different locations of the mica were then obtained from a Nanoscope IIIa SPM instrument (Veeco).
2.5. Stability of IFN with or without AcNg
The lyophilized AcNg, IFN30 (30 IU/ μl), IFN3 (3 IU/ μl), IFN30-AcNg and IFN3-AcNg were solubilized with PBS and stored at 4 °C for up to 14 days. Each preparation was added daily to one-day old, 80-90% confluent NV harboring HG23 cells at the final concentration of IFN 3 or 30 IU/ml, and then viral RNA levels were analyzed at 24 or 48 hrs after treatment using qRT-PCR. The reduction of RNA level by each preparation was calculated by the comparison to that of day 0.
2.6. IFN or AcNg-IFN treatment on ISRE-luciferase response in Vero and Huh-7 cells
The IFN activities of IFN and IFN-AcNg were assessed by the reporter assay with IFN-sensitive response element (ISRE) in Vero and HG23 cells using plasmids, pISRE-TA-Luc, pNF-kB-TA-Luc (both from Clontech), and pRL-CMV (form Promega). One-day old (~90% confluent) Vero or HG23 cells in 12 well plates were transfected with pISRE-TA-Luc or pNF-kB-TA-Luc, and pRL-CMV. The pRL-CMV (for renillar luciferase under CMV promoter) served as a control for transfection efficiency and for standardization of luciferase expression levels. Cells were incubated for 4 hrs before mock-medium, and AcNg, IFN (final concentration of 3 or 30 IU/ml) or IFN-AcNg (3 or 30 IU/ml) was added. After an additional 18 hrs, cells were harvested for analysis of luciferase expression. The luciferase assay was carried out with Dual Glo luciferase assay system (Promega) in a luminometer (Promega). The luciferase expression (firefly luciferase) from each reporter plasmid was normalized against the expression level of the renilla luciferase encoded in pRL-CMV.
2.7. Treatment of NV- or HCV-harboring cells with IFNα or IFN-AcNg
The effects of IFNα with or without AcNg on the replication of NV or HCV in the replicon-harboring cells were examined. Mock-medium, AcNg, IFN (final concentration of 3 or 30 IU/ml) or IFN-AcNg (3 or 30 IU/ml) were added to one-day old, 80-90% confluent HG23 or 1A7 cells, and viral protein and genome expression levels were examined at 24 or 48 hr after treatment. The inhibitory effects of AcNg, IFN and IFN-AcNg on the genome expression levels of NV or HCV replicon by qRT-PCR were compared to those of Mock-medium. The NV protein expression levels were examined by Western blot analysis, as described below.
2.8. The effects of IFN or IFN-AcNg on the replication of MNV-1 in RAW267.4 cells
The effects of mouse IFN-β of IFN-AcNg on the replication of MNV-1 in RAW267.4 cells were examined in comparison to IFN alone. Confluent RAW267.4 cells were inoculated with MNV-1 at a MOI of 5 or 0.05 for 1 hr, and medium was replaced with medium containing mock-medium, AcNg, IFN (final concentration of 3 or 30 IU/ml) and IFN-AcNg (3 or 30 IU/ml). The virus infected cells were further incubated for up to 48 hrs, and the replication of MNV-1 was measured by TCID50 assay with the 10-fold dilution of each sample used for virus titration (Reed and Muench, 1938). MNV-1 ProPol was detected by Western blot analysis.
2.9. Detection of viral RNA and proteins
2.9.1. Real-Time qRT-PCR
The quantity of NV or HCV genome in the replicon-harboring cells was measured by real-time qRT-PCR with One-step Platinum qRT-PCR kit (Invitrogen, Carlsbad, CA), following an established protocol with specific primers and probes . For qRT-PCR, the total RNA in cells (in 6-well plate) was extracted with RNeasy kit (Qiagen, Valencia, CA). The primer sequences for NV were: Forward 5’-CGYTGGATGCGITTYCATGA-3’ and reverse 5’-CTTAGACGCCATCATCATTYAC-3’. The probe sequence used was: FAM-5’-AGATYGCGITCICCTGTCCA - 3’-Iowa Black. The primer sequences for HCV were: Forward 5’-CGGGAGAGCCATAGTGGTCTGCG-3’ and reverse 5’-CTCGAAGCACCCTATCAGGCAGTA -3’. The probe sequence used was: FAM-5’-GCGAGCCACCGGAATTGCCT- 3’-Iowa Black. The qRT-PCR amplification was performed in a SmartCycler (Cepheid, Sunnyvale, CA) with the following parameters: 45 °C for 30 min, and 95 °C 10 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 50 °C for 1 min and elongation at 72 °C for 30 s. For quantity control, qRT-PCR for β-actin was performed as described previously (Spann et al., 2004). The relative genome levels in cells with various treatments were calculated after the RNA levels were normalized with those of β-actin.
2.9.2. Western blot analysis
Protein samples of HG23 cells or MNV-1 infected RAW 267.4 cells with various treatments were prepared in SDS-PAGE sample buffer containing 1% β-mercaptoethanol, and sonicated for 20 sec. The proteins were resolved in a 10% Novex Tris-Bis gel (Invitrogen) and transferred to a nitrocellulose membrane. The membranes were probed with guinea pig antibodies specific for NV ProPol protein and the binding of the antibodies was detected with peroxidase-conjugated, goat anti-guinea pig IgG (Sigma-Aldrich). In addition, membranes were probed with rabbit antiserum specific for β-actin and peroxidase-conjugated, goat anti-rabbit IgG as a loading control. Following incubation with a chemiluminescent substrate (SuperSignal West Pico Chemiluminescent Substrate, Pierce Biotechnology, Rockford, IL), signals were detected with X-ray film.
2.10. Oral and intravenous administration of IFN or AcNg-IFN in Sprague-Dawley rats
Four-week old female Sprague-Dawley rats were housed and studied under an Institutional Animal Care and Use Committee-approved protocol. The study was designed to determine if the oral administration (oral gavage) of 100 μl of human IFN-αA alone (106 IU total) or AcNg-IFN (100 ug AcNg combined with 106 IU IFN-αA) leads to carry over of IFN to blood. The intravenous injection of IFN or AcNg-IFN served as a control. The animals were weighed and randomly assigned to 4 groups of 3 animals each. Each group was assigned for intravenous or oral gavage administration of AcNg or AcNg-IFN. Rats are cannulated via carotid artery to collect blood. Blood samples were collected at 30 min, 1, 4, and 24 hr following the administration and sera were separated and stored at -80 °C. The concentration of IFN in each serum was measured using IFNα detection kit (Human IFNα ELISA kit, Interferonsournce, Piscataway, NJ). Animals were monitored daily by behavioral observation and euthanized at 1 week following the administration for gross necropsy.
2.11. Statistical analysis
The effects of IFN or IFN-AcNg on NV, HCV or MNV-1 replication were analyzed by Student's t test. Results were considered statistically significant when the P value was <0.05.
3. Results
3.1. Nanogel preparation
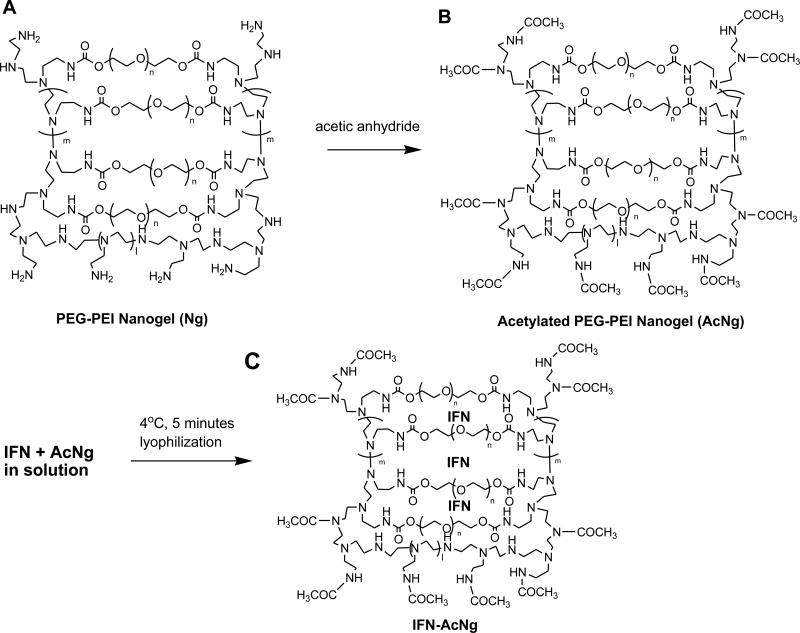
The acetylated PEG-PEI cross-linked nanogel has a molecular weight of ~23 kDa (for each mole of PEI, there is ~0.4 mole of PEG attached) determined by a size exclusion chromatography. The encapsulation of IFN by AcNg was performed at 4 °C for 5 min, followed by lyophilization. The lyophilized AcNg and AcNg-IFN were white powder, and easily solubilized in PBS or water. The putative scheme of acetylated nanogel with entrapped IFNs is shown in figure 1. The AFM images of solubilized AcNg and AcNg-IFN showed that both contained circular aggregates up to 200 nm in diameter (Figure 2, left panels). Various concentrations of IFN were used for the encapsulation with AcNg, and their AFM images were similar in appearance. The non-acetylated nanogel was cytotoxic at 0.6 mg/ml. However, AcNg was not cytotoxic at up to 10 mg/ml.
Figure 1.
Schematic drawing of acetylated nanogel (AcNg) and entrapped interferon. The cross-link of PEI and PEG molecules in aqueous solutions forms nanogels (A). The nanogels are acetylated at the active sites (NH and NH2) to prevent cellular penetration (B). Incubation of AcNg with IFN at 4 °C for 5 min produces IFN entrapment in AcNg (C).
Figure 2.
Atomic force microscope (AFM) images of AcNg only (A) and IFNα- AcNg complexes (B). The AFM image (left) of each panel shows AcNg particles with or without IFN, and its height and width are shown in the right images (up to 200 nm in diameters).
3.2. The stability of IFN stored at 4 °C in IFN-AcNg complex
The anti-norovirus activity of IFN stored at 4 °C without AcNg showed a steep drop (by 80%) in a 3-day of incubation, while the IFN activity of IFN-AcNg complex decreased only by up to 17% during the same period (Figure 3). This stability result shows that AcNg delayed the decrease in IFN activity. The AcNg alone did not show any antiviral effects (not shown).
Figure 3.
Stability of IFN or IFN-AcNg. The lyophilized AcNg, IFN30 (30 IU/μl), IFN3 (3 IU/μl), IFN30-AcNg or IFN3-AcNg was solubilized with PBS and stored at 4 °C for up to 14 days. Each preparation was added daily to one-day old, 80-90% confluent NV harboring HG23 cells which were analyzed for viral RNA level at 24 or 48 hr after treatment using qRT-PCR. The reduction of genome by each preparation was calculated by the comparison to that of day 0.
3.3. The effects of IFN or AcNg-IFN on ISRE-luciferase response in Vero and Huh-7 cells
IFN at 30 IU/ml without AcNg significantly increased the luciferase expression in pISRE-luc system in Vero and Huh-7 cells compared to mock-treatment, while IFN at 3 IU/ml without AcNg did not (Figure 4). However, IFN at both concentrations (3 and 30 IU/ml) combined with AcNg induced significant increase in luciferase expression levels in both cell lines compared to mock-treatment. In addition, AcNg significantly increased IFN-induced luciferase expression at both IFN concentrations, compared to IFN alone (Figure 4). The treatment of IFN or IFN-AcNg in the cells transfected with pNFkB-TA-luc did not induce luciferase expression (not shown).
Figure 4.
Induction of luciferase activity under the control of IFN-sensitive response element using Vero and Huh-7 cells. One-day old (~90% confluent) Vero or HG23 cells in 12 well plates were transfected with reporter plasmid (pISRE-TA-Luc) and pRL-CMV. At 4 hr-post transfection, the cells were incubated with mock, AcNg, or IFN (3 or 30 IU/ml) with or without AcNg. After 18 hrs, luciferase expression was measured. Data were presented as fold increase of luciferase expression. The luciferase expression (firefly luciferase) from reporter plasmid was normalized against the expression level of the renilla luciferase encoded in pRL-CMV. The Luciferase expression was significantly (P<0.05) increased by the treatment of IFN at 30 IU/ml or IFN-AcNg (3 and 30 IU/ml) compared to mock-treatment (single and double asterisks). IFN-AcNg also significantly decreased the luciferase expression level compared to IFN alone at both concentrations (double asterisks). Bars represent standard deviations of at least 3 independent experiments.
3.4. Antiviral activity of IFN or AcNg-IFN on NV- or HCV-replicon-harboring cells
The treatment of IFN alone at 30 IU/ml or IFN-AcNg containing 3 or 30 IU/ml of IFN for 24 or 48 hrs significantly reduced the RNA levels of NV in HG23 or HCV in 1A7 cells (Figure 5). The longer incubation of cells with IFN or IFN-AcNg resulted in greater reduction of viral RNA in both cells. Similar to the results of the luciferase expression with pISRE-luc, IFN-AcNg was significantly more effective than IFN alone at both concentrations of 3 and 30 IU/ml (Figure 5). The NV RNA levels in HG23 cells by 3 or 30 IU/ml of IFN-AcNg were reduced on average to 71% or 35% of mock-treatment at 24 hr and 59% or 21% of mock-treatment at 48 hr, respectively. However, with IFN alone at 3 or 30 IU/ml, they were reduced to 81% or 46% of mock-treatment at 24 hr and 69% or 35% of mock-treatment at 48 hr, respectively (Figure 5.A). In 1A7 cells, HCV RNA levels were reduced by 3 or 30 IU/ml of IFN-AcNg to 78% or 40% of mock-treatment at 24 hr and 60% or 15% of mock-treatment at 48 hr, respectively. With IFN only, they were 83% or 65% of mock-treatment at 24 hr and 65% or 40% of mock-treatment at 48 hr, respectively (Figure 5.B). However, AcNg alone did not significantly change the viral RNA levels in HG23 and 1A7 cells compared to mock-treatment (Figure 5.A and B). The western blot shows the decrease in NV protein expression by IFN in a dose dependent manner (Figure 7.A).
Figure 5.
Effects of IFN with or without AcNg on NV or HCV replication in NV- and HCV-replicon harboring cells. One-day old, semiconfluent NV replicon-harboring HG23 or HCV replicon-harboring 1A7 cells were incubated with mock, AcNg, or IFN (3 or 30 IU/ml) with or without AcNg for 24 or 48 hrs, and then total RNA was prepared for real-time qRT-PCR to detect the NV genome (A) or HCV genome (B). The reduction of genomes by various treatments was calculated by the comparison to that with mock (medium) treatment. The RNA levels were significantly decreased (P<0.05) in the cells by the treatment of IFN at 30 IU/ml or IFN-AcNg (3 and 30 IU/ml) compared to mock-treatment (single and double asterisks). IFN-AcNg also significantly decreased NV or HCV RNA levels compared to IFN alone at both concentrations (double asterisks). Error bars represent standard deviations from at least three independent experiments.
Figure 7.
Effects of various IFN treatments with or without AcNg on the expression of NV and MNV-1 proteins in HG23 (A) and RAW267.4 (B) cells, respectively. Cell lysates were prepared after incubation with mock medium, AcNg alone, or IFN 3 or 30 IU/ml with or without AcNg for 48 hrs in NV-harboring HG23 cells or following MNV-1 infection in RAW267.4 cells. Western blot analysis of cell lysates was performed with antibodies against NV or MNV-1 ProPol or β-actin. NV-Pol or MNV-1 Pol indicates the polymerases of NV or MNV-1 detected by the antibodies against NV or MNV-1 ProPol, respectively.
3.5. The effects of IFN or IFN-AcNg on the replication of MNV-1
We used mouse IFN-β to examine the effects of IFN or IFN-AcNg on the replication of MNV-1. Both concentrations of IFN (3 or 30 IU/ml) with or without AcNg significantly reduced the replication of MNV-1 at 12 or 24 hr-post infection compared to mock treatment (Figure 6). Similar to the results with NV- and HCV-replicon-harboring cells, the IFN activities of IFN-AcNg against MNV-1 were significantly higher than IFN alone at both IFN concentrations (Figure 6). The western blot shows that higher dose of IFN or IFN-AcNg reduced NV protein expression more than the lower dose of IFN or IFN alone, respectively (Figure 7.B).
Figure 6.
The effects of mouse IFN-β in IFN-AcNg preparations on the replication of MNV-1 in RAW267.4 cells were examined in comparison to IFN alone. Confluent RAW267.4 cells were inoculated with MNV-1 at a MOI of 5 or 0.05 for 1 hr, and medium was replaced with medium containing mock-medium, AcNg, IFN (3 or 30 IU/ml) or IFN-AcNg (3 or 30 IU/ml). The virus infected cells were further incubated for up to 48 hr, and the replication of MNV-1 was measured by TCID50 assay. Error bars represent standard deviations from at least three independent experiments. The MLV-1 titers in cells were significantly (P<0.05) decreased by the treatment of IFN (3 and 30 IU/ml) or IFN-AcNg (3 and 30 IU/ml) compared to mock-treatment (single and double asterisks). IFN-AcNg also significantly decreased MLV-1 titers compared to IFN alone at both IFN concentrations (double asterisks).
3.6. Oral administration of AcNg-IFN in vivo
Up to 250 pg/ml (approximately 25 IU/ml) of IFN was detected in the blood 30 min post-administration in rats administered with IFN or IFN-AcNg intravenously. However, IFN was not detected in the blood following oral administration of IFN or IFN-AcNg up to 24 hrs, indicating that AcNg did not cause carryover of IFN to systemic circulation. There was neither significant behavioral change by daily observation or gross pathology on necropsy at 7 days post-administration in all animals.
4. Discussion
Norovirus infection continues to be an important cause of gastroenteritis in humans, the leading cause of food-borne disease followed by Salmonella contamination among laboratory-confirmed single etiologic agent cases (CDC, 2010). In most cases, norovirus infection leads to acute illness, even though recent findings demonstrated that the infection could last longer than several days or even several months, especially in immunocompromised patients (Nilsson et al., 2003). Since noroviruses are very contagious and require only a very low dose to cause an infection, they are classified as category B bioterrorism agents. However, there is no vaccine or treatment specific for norovirus infection except for supportive therapy including fluid administration to correct dehydration. Previously, we have used the recently developed NV-replicon harboring cells, and demonstrated that IFN-α and γ were effective inhibitors of NV replication (Chang et al., 2006). We also suggested that NV may not have strong means to counteract IFN systems, which further suggests the potential use of IFN for the control of noroviruses (Chang et al., 2006). Other researchers have found that the replication of MNV-1 was sensitive to IFN system in vivo as well as in vitro (Changotra et al., 2009; Karst et al., 2003). These results suggest that IFNs could be potential therapeutic agents for norovirus infection.
IFN treatment in various virus infections including respiratory viruses or human hepatitis viruses has been studied. Currently, the standard therapy for chronic HCV infection is the combination therapy of IFN-α and ribavirin (Aghemo et al., 2010). For treatment of HCV infection, IFNs chemically conjugated with branched or linear PEG (pegylated IFN) were recently licensed for longer half-life of IFN in parenteral administration (Jen et al., 2001). PEG is inert, water-soluble, and nontoxic, and does not adversely affect the safety profile of IFN. PEG exists in a multitude of molecular weights, and can be attached to IFN with size up to 40kD (Sharieff et al., 2002). It was reported that higher molecular weight of PEG yields longer half-life of the conjugated IFN. However, due to physical conjugation to IFN, PEG reduces the bioactivity of IFN (Bailon et al., 2001; Monkarsh et al., 1997). The parenteral administration of high dose of IFN (in HCV infection, the standard dose for IFN is above 1 million IU) could induce significant side effects such as influenza-like syndrome with fever, chills, myalgia, and malaise (Negro, 2010).
Due to these side effects associated with high dose of parenteral administration of IFN, oral administration of IFN has been explored for virus infection at mucosal surfaces. In natural settings, humans and animals encounter various viruses and bacteria on daily basis and secret IFN in oral and nasal cavity (Beilharz, 2010). Interestingly, the oral administration of IFN-α was reported to be associated with no significant side effects (Dec and Puchalski, 2008) [review (Beilharz, 2010)]. The oral IFN studies showed conflicting results on systemic effects of IFN. In humans, oral administration of high dose of IFN-β (2.5 and 7.5 mg or 4.5 × 108 and 1.35 × 109 IU) to healthy volunteers did not significantly induce IFN-responding proteins in serum (Witt et al., 1992). However, interestingly, low oral dose of IFN-α (125 IU) were more effective than intramuscular dose of millions (IU) in inducing 2’, 5’-OAS activity in the blood (Uno et al., 2006), and oromucosal delivery of IFN-β at 100 IU was immunostimulating and high dose (107 IU) was immunosuppressive (Gonzalez-Cabanas et al., 1996). These findings suggest that feedback mechanism of interferon might be important in oral administration of IFN (Beilharz, 2010).
In influenza virus infection, low dose of intranasal administration of IFN (up to 5000 IU/dose) were found effective in preventing or alleviating symptoms without serious side effects (Arnaoudova, 1976; Imanishi et al., 1980; Isomura et al., 1982). Interestingly, other studies of higher doses of IFN (1000~10,000 times more per day) showed no efficacy against influenza viruses (Hayden et al., 1983; Merigan et al., 1973; Phillpotts et al., 1984; Saito et al., 1985; Treanor et al., 1987), suggesting that the dose of IFN is an important consideration in assessing antiviral effectiveness. In animals, recent studies showed that IFN orally or intranasally delivered to mice (Beilharz et al., 2007; Grimm et al., 2007; Tumpey et al., 2007), guinea pig (Van Hoeven et al., 2009) or ferrets (Kugel et al., 2009) reduced influenza virus replication. Interestingly, oral low-dose IFN (100 IU) effectively protected mice from a lethal challenge of influenza virus (Beilharz et al., 2007).
Nanogels are hydrogel particles of less than 1 μm in diameter formed by physically or chemically cross-linked polymer networks. These nanogels can load large amounts of small molecules and/or proteins through spontaneous electrostatic, van der Waals and/or hydrophobic interactions, and show high stability in vitro and in vivo, thus they have great potential as drug-delivery carriers. It has been shown that nanoencapsulation of peptides and protein colloidal particles protects them against the harsh environment of the gastrointestinal tract due to their covalent nature (Lowe and Temple, 1994). One of the potential nanogel applications is to deliver functional proteins such as IFN which would be easily degraded in biological solution. Nanogels based on chemically cross-linked networks with PEG and PEI were previously synthesized, and it was demonstrated that they are a good tool to deliver small molecules such as antisense oligonucleotides (Vinogradov et al., 1999). Despite the beneficial properties of PEG/PEI nanogel, it has been associated with in vitro cytotoxicity depending on the ratio of PEG/PEI (Ganta et al., 2008). To overcome this, we acetylated PEG/PEI nanogel (AcNg) to block the reactive groups (NH and NH2) on the surface of the nanogel, reducing cytotoxicity. Enteric viruses such as norovirus replicate in intestinal epithelial cells where the receptors for IFN are located on the luminal cell surface, and acetylated nanogel-IFN was postulated to activate innate immunity against noroviruses in the intestinal wall without the risk of systemic toxicity. Furthermore, IFN combined with AcNg is released as an intact form, thus bioavailability of IFN is not reduced, compared with pegylated IFN. The encapsulation of IFN with AcNg requires a simple procedure: incubation of AcNg and IFN for 5 min at 4 °C. This simple drug-loading property of AcNg offers an advantage by getting rid of use of organic solvents, surfactants, or evaporation that might affect the properties of the drugs.
The advantage of AcNg as a carrier of IFN is it requires simple mixing of IFN and AcNg and requires no solvents to achieve the IFN-AcNg complexes. In this study, the IFN-AcNg complexes were prepared by simple incubating two components together at 4 °C and lyophilization. Once lyophilized, the complexes could be stored at 4 °C or -20 °C for long period of time without losing IFN activity. Importantly, AcNg was well-tolerable in cultured cells: the incubation of AcNg in various cells did not lead to cytotoxicity at up to 10 mg/ml (compared to non-acetylated nanogel which is cytotoxic at 0.6 mg/ml). The round AcNg particles in the presence or absence of IFN (up to ~ 200 nm diameter) were stable (observed by AFM) for 14 days in PBS solution stored at room temperature (data not shown). The IFN activity was assayed using various methods including the reporter assay with IFN-sensitive response element (ISRE), and against norovirus replication using a replicon-harboring cells and MNV-1. Using various assay, we demonstrated that AcNg significantly increased the stability of IFN, and also significantly enhanced IFN activities against noroviruses (MNV-1 and NV-replicon harboring cells) and HCV in cell culture system, compared to IFN alone. Furthermore, the preliminary study using rats suggested that IFN-AcNg did not cause side effects by oral or systemic administration. In summary, these results suggest that IFN could be used as a therapeutic agent against norovirus infection, and AcNg could be a potential drug carrier of IFN by oral route.
Acknowledgement
This work was partly supported by NIH U01 AI081891. We thank David George for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Aghemo A, Rumi MG, Colombo M. Pegylated interferons alpha2a and alpha2b in the treatment of chronic hepatitis C. Nat Rev Gastroenterol Hepatol. 2010 doi: 10.1038/nrgastro.2010.101. [DOI] [PubMed] [Google Scholar]
- Arnaoudova V. Treatment and prevention of acute respiratory virus infections in children with leukocytic interferon. Virologie. 1976;27(2):83–8. [PubMed] [Google Scholar]
- Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung WJ, Porter JE, Ehrlich GK, Pan W, Xu ZX, Modi MW, Farid A, Berthold W, Graves M. Rational design of a potent, long-lasting form of interferon: a 40 kDa branched polyethylene glycol-conjugated interferon alpha-2a for the treatment of hepatitis C. Bioconjug Chem. 2001;12(2):195–202. doi: 10.1021/bc000082g. [DOI] [PubMed] [Google Scholar]
- Beilharz MW, Cummins JM, Bennett AL. Protection from lethal influenza virus challenge by oral type 1 interferon. Biochem Biophys Res Commun. 2007;355(3):740–4. doi: 10.1016/j.bbrc.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Beilharz MWC,MJ, Bennett AL, Cummins JM. Oromucosal Administration of Interferon to Humans. Pharmaceuticals. 2010;3:323–344. doi: 10.3390/ph3020323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Surveillance for Foodborne Disease Outbreaks --- United States, 2007 Morbidity and mortality weekly report. 2010. pp. 973–979. [PubMed]
- Chang KO. Role of cholesterol pathways in norovirus replication. J Virol. 2009;83(17):8587–95. doi: 10.1128/JVI.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KO, George DW. Bile acids promote the expression of hepatitis C virus in replicon-harboring cells. J Virol. 2007a;81(18):9633–40. doi: 10.1128/JVI.00795-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KO, George DW. Interferons and ribavirin effectively inhibit Norwalk virus replication in replicon-bearing cells. J Virol. 2007b;81(22):12111–8. doi: 10.1128/JVI.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KO, Sosnovtsev SV, Belliot G, King AD, Green KY. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. Virology. 2006;353(2):463–73. doi: 10.1016/j.virol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Changotra H, Jia Y, Moore TN, Liu G, Kahan SM, Sosnovtsev SV, Karst SM. Type I and type II interferons inhibit the translation of murine norovirus proteins. J Virol. 2009;83(11):5683–92. doi: 10.1128/JVI.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dec M, Puchalski A. Use of oromucosally administered interferon-alpha in the prevention and treatment of animal diseases. Pol J Vet Sci. 2008;11(2):175–86. [PubMed] [Google Scholar]
- Duizer E, Schwab KJ, Neill FH, Atmar RL, Koopmans MP, Estes MK. Laboratory efforts to cultivate noroviruses. J Gen Virol. 2004;85(Pt 1):79–87. doi: 10.1099/vir.0.19478-0. [DOI] [PubMed] [Google Scholar]
- Fankhauser RL, Noel JS, Monroe SS, Ando T, Glass RI. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J Infect Dis. 1998;178(6):1571–8. doi: 10.1086/314525. [DOI] [PubMed] [Google Scholar]
- Foster GR. Pegylated interferons for the treatment of chronic hepatitis C: pharmacological and clinical differences between peginterferon-alpha-2a and peginterferon-alpha-2b. Drugs. 2010;70(2):147–65. doi: 10.2165/11531990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Ganta C, Shi A, Battina SK, Pyle M, Rana S, Hua DH, Tamura M, Troyer D. Combination of nanogel polyethylene glycol-polyethylenimine and 6(hydroxymethyl)-1,4-anthracenedione as an anticancer nanomedicine. J Nanosci Nanotechnol. 2008;8(5):2334–40. doi: 10.1166/jnn.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cabanas R, Miro A, Ferrero J, Gonzalez R, Marin N, Maccias C, Lopez-Saura P. Biological effects of oral leukocyte interferon-β in healthy volunteers. Eur.Cytokine netw. 1996;7:650. [Google Scholar]
- Grimm D, Staeheli P, Hufbauer M, Koerner I, Martinez-Sobrido L, Solorzano A, Garcia-Sastre A, Haller O, Kochs G. Replication fitness determines high virulence of influenza A virus in mice carrying functional Mx1 resistance gene. Proc Natl Acad Sci U S A. 2007;104(16):6806–11. doi: 10.1073/pnas.0701849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden FG, Mills SE, Johns ME. Human tolerance and histopathologic effects of long-term administration of intranasal interferon-alpha 2. J Infect Dis. 1983;148(5):914–21. doi: 10.1093/infdis/148.5.914. [DOI] [PubMed] [Google Scholar]
- Herzog C, Just M, Berger R, Havas L, Fernex M. Intranasal interferon for contact prophylaxis against common cold in families. Lancet. 1983;2(8356):962. doi: 10.1016/s0140-6736(83)90470-1. [DOI] [PubMed] [Google Scholar]
- Husa P, Husova L. Treatment of chronic hepatitis C patients with combination of alpha-interferon and ribavirin, consensus and pegylated interferons. Bratisl Lek Listy. 2001;102(5):248–52. [PubMed] [Google Scholar]
- Imanishi J, Karaki T, Sasaki O, Matsuo A, Oishi K, Pak CB, Kishida T, Toda S, Nagata H. The preventive effect of human interferon-alpha preparation on upper respiratory disease. J Interferon Res. 1980;1(1):169–78. doi: 10.1089/jir.1980.1.169. [DOI] [PubMed] [Google Scholar]
- Isomura S, Ichikawa T, Miyazu M, Naruse H, Shibata M, Imanishi J, Matsuo A, Kishida T, Karaki T. The preventive effect of human interferon-alpha on influenza infection; modification of clinical manifestations of influenza in children in a closed community. Biken J. 1982;25(3):131–7. [PubMed] [Google Scholar]
- Jen JF, Glue P, Ezzet F, Chung C, Gupta SK, Jacobs S, Hajian G. Population pharmacokinetic analysis of pegylated interferon alfa-2b and interferon alfa-2b in patients with chronic hepatitis C. Clin Pharmacol Ther. 2001;69(6):407–21. doi: 10.1067/mcp.2001.115872. [DOI] [PubMed] [Google Scholar]
- Karst SM, Wobus CE, Lay M, Davidson J, Virgin H.W.t. STAT1-dependent innate immunity to a Norwalk-like virus. Science. 2003;299(5612):1575–8. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- Kozlowski A, Charles SA, Harris JM. Development of pegylated interferons for the treatment of chronic hepatitis C. BioDrugs. 2001;15(7):419–29. doi: 10.2165/00063030-200115070-00001. [DOI] [PubMed] [Google Scholar]
- Kugel D, Kochs G, Obojes K, Roth J, Kobinger GP, Kobasa D, Haller O, Staeheli P, von Messling V. Intranasal administration of alpha interferon reduces seasonal influenza A virus morbidity in ferrets. J Virol. 2009;83(8):3843–51. doi: 10.1128/JVI.02453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CJ, Kao JH. Pegylated interferons for the treatment of chronic hepatitis B. Recent Pat Antiinfect Drug Discov. 2006;1(1):85–94. doi: 10.2174/157489106775244154. [DOI] [PubMed] [Google Scholar]
- Lowe PJ, Temple CS. Calcitonin and insulin in isobutylcyanoacrylate nanocapsules: protection against proteases and effect on intestinal absorption in rats. J Pharm Pharmacol. 1994;46(7):547–52. doi: 10.1111/j.2042-7158.1994.tb03854.x. [DOI] [PubMed] [Google Scholar]
- Luxon BA, Grace M, Brassard D, Bordens R. Pegylated interferons for the treatment of chronic hepatitis C infection. Clin Ther. 2002;24(9):1363–83. doi: 10.1016/s0149-2918(02)80042-x. [DOI] [PubMed] [Google Scholar]
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5(5):607–25. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan TC, Reed SE, Hall TS, Tyrrell DA. Inhibition of respiratory virus infection by locally applied interferon. Lancet. 1973;1(7803):563–7. doi: 10.1016/s0140-6736(73)90714-9. [DOI] [PubMed] [Google Scholar]
- Monkarsh SP, Ma Y, Aglione A, Bailon P, Ciolek D, DeBarbieri B, Graves MC, Hollfelder K, Michel H, Palleroni A, Porter JE, Russoman E, Roy S, Pan YC. Positional isomers of monopegylated interferon alpha-2a: isolation, characterization, and biological activity. Anal Biochem. 1997;247(2):434–40. doi: 10.1006/abio.1997.2128. [DOI] [PubMed] [Google Scholar]
- Mumphrey SM, Changotra H, Moore TN, Heimann-Nichols ER, Wobus CE, Reilly MJ, Moghadamfalahi M, Shukla D, Karst SM. Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J Virol. 2007;81(7):3251–63. doi: 10.1128/JVI.02096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negro F. Adverse effects of drugs in the treatment of viral hepatitis. Best Pract Res Clin Gastroenterol. 2010;24(2):183–92. doi: 10.1016/j.bpg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Hedlund KO, Thorhagen M, Larson G, Johansen K, Ekspong A, Svensson L. Evolution of human calicivirus RNA in vivo: accumulation of mutations in the protruding P2 domain of the capsid leads to structural changes and possibly a new phenotype. J Virol. 2003;77(24):13117–24. doi: 10.1128/JVI.77.24.13117-13124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillpotts RJ, Higgins PG, Willman JS, Tyrrell DA, Freestone DS, Shepherd WM. Intranasal lymphoblastoid interferon (“Wellferon”) prophylaxis against rhinovirus and influenza virus in volunteers. J Interferon Res. 1984;4(4):535–41. doi: 10.1089/jir.1984.4.535. [DOI] [PubMed] [Google Scholar]
- Phillpotts RJ, Scott GM, Higgins PG, Wallace J, Tyrrell DA, Gauci CL. An effective dosage regimen for prophylaxis against rhinovirus infection by intranasal administration of HuIFN-alpha 2. Antiviral Res. 1983;3(2):121–36. doi: 10.1016/0166-3542(83)90034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. American Journal of Hygiene. 1938;27:493–497. [Google Scholar]
- Saito H, Takenaka H, Yoshida S, Tsubokawa T, Ogata A, Imanishi F, Imanishi J. Prevention from naturally acquired viral respiratory infection by interferon nasal spray. Rhinology. 1985;23(4):291–5. [PubMed] [Google Scholar]
- Sharieff KA, Duncan D, Younossi Z. Advances in treatment of chronic hepatitis C: ‘pegylated’ interferons. Cleve Clin J Med. 2002;69(2):155–9. doi: 10.3949/ccjm.69.2.155. [DOI] [PubMed] [Google Scholar]
- Shiffman ML. Pegylated interferons: what role will they play in the treatment of chronic hepatitis C? Curr Gastroenterol Rep. 2001;3(1):30–7. doi: 10.1007/s11894-001-0038-z. [DOI] [PubMed] [Google Scholar]
- Sosnovtsev SV, Belliot G, Chang KO, Prikhodko VG, Thackray LB, Wobus CE, Karst SM, Virgin HW, Green KY. Cleavage map and proteolytic processing of the murine norovirus nonstructural polyprotein in infected cells. J Virol. 2006;80(16):7816–31. doi: 10.1128/JVI.00532-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann KM, Tran KC, Chi B, Rabin RL, Collins PL. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected]. J Virol. 2004;78(8):4363–9. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor JJ, Betts RF, Erb SM, Roth FK, Dolin R. Intranasally administered interferon as prophylaxis against experimentally induced influenza A virus infection in humans. J Infect Dis. 1987;156(2):379–83. doi: 10.1093/infdis/156.2.379. [DOI] [PubMed] [Google Scholar]
- Tumpey TM, Szretter KJ, Van Hoeven N, Katz JM, Kochs G, Haller O, Garcia-Sastre A, Staeheli P. The Mx1 gene protects mice against the pandemic 1918 and highly lethal human H5N1 influenza viruses. J Virol. 2007;81(19):10818–21. doi: 10.1128/JVI.01116-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno K, Suginoshita Y, Kakimi K, Moriyasu Y, Nakano K, Nakamura N, Fujita T, Horino Y, Sato T, Kishida T. Clinical utility of 2',5'-oligoadenylate synthetase activity measurement: using whole blood as a highly sensitive method to detect the effects of IFN. J Virol Methods. 2006;136(1-2):185–92. doi: 10.1016/j.jviromet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Van Hoeven N, Belser JA, Szretter KJ, Zeng H, Staeheli P, Swayne DE, Katz JM, Tumpey TM. Pathogenesis of 1918 pandemic and H5N1 influenza virus infections in a guinea pig model: antiviral potential of exogenous alpha interferon to reduce virus shedding. J Virol. 2009;83(7):2851–61. doi: 10.1128/JVI.02174-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Batrakova E, Kabanov A. Poly(ethylene glycol)–polyethyleneimine NanoGel™ particles: novel drug delivery systems for antisense oligonucleotides. Colloids and Surfaces B: Biointerfaces. 1999;16(1-4):291–304. [Google Scholar]
- Vinogradov SV, Kohli E, Zeman AD. Comparison of nanogel drug carriers and their formulations with nucleoside 5'-triphosphates. Pharm Res. 2006;23(5):920–30. doi: 10.1007/s11095-006-9788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt PL, Goldstein D, Storer BE, Grossberg SE, Flashner M, Colby CB, Borden EC. Absence of biological effects of orally administered interferon-beta ser. J Interferon Res. 1992;12(6):411–3. doi: 10.1089/jir.1992.12.411. [DOI] [PubMed] [Google Scholar]
- Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. Replication of Norovirus in Cell Culture Reveals a Tropism for Dendritic Cells and Macrophages. PLoS Biol. 2004;2(12):e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]