Abstract
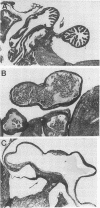
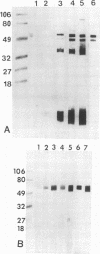
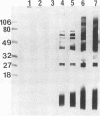
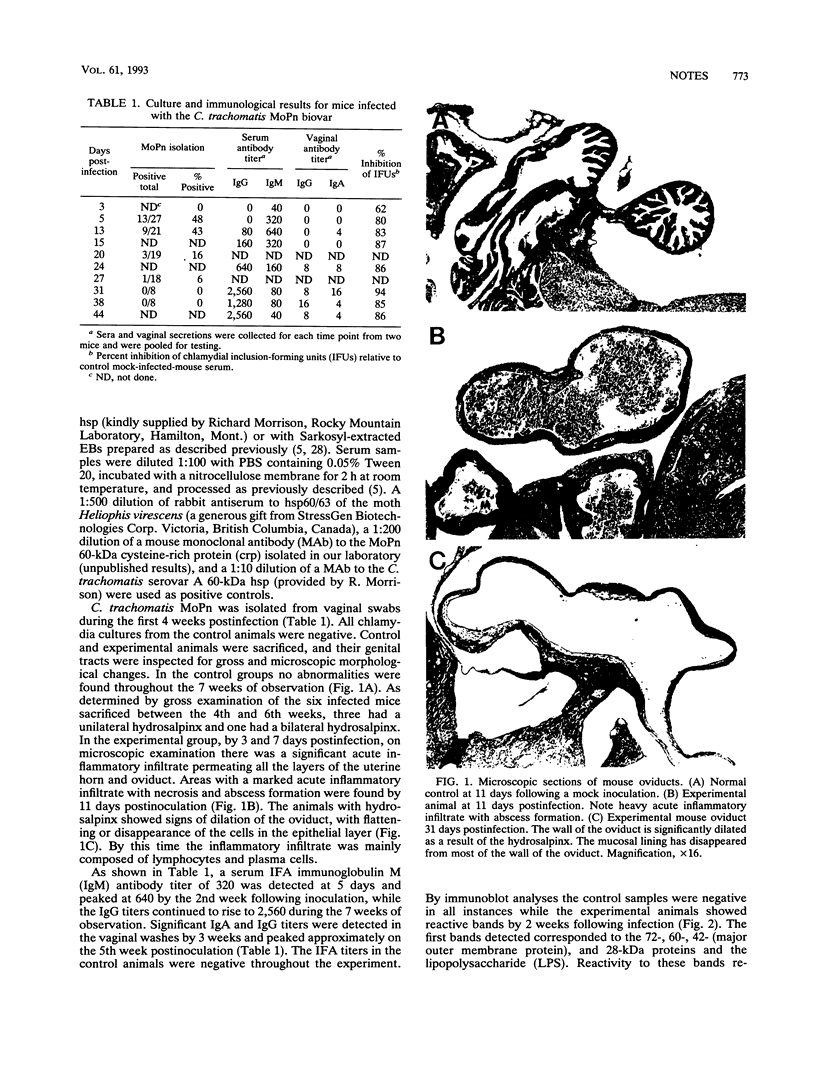
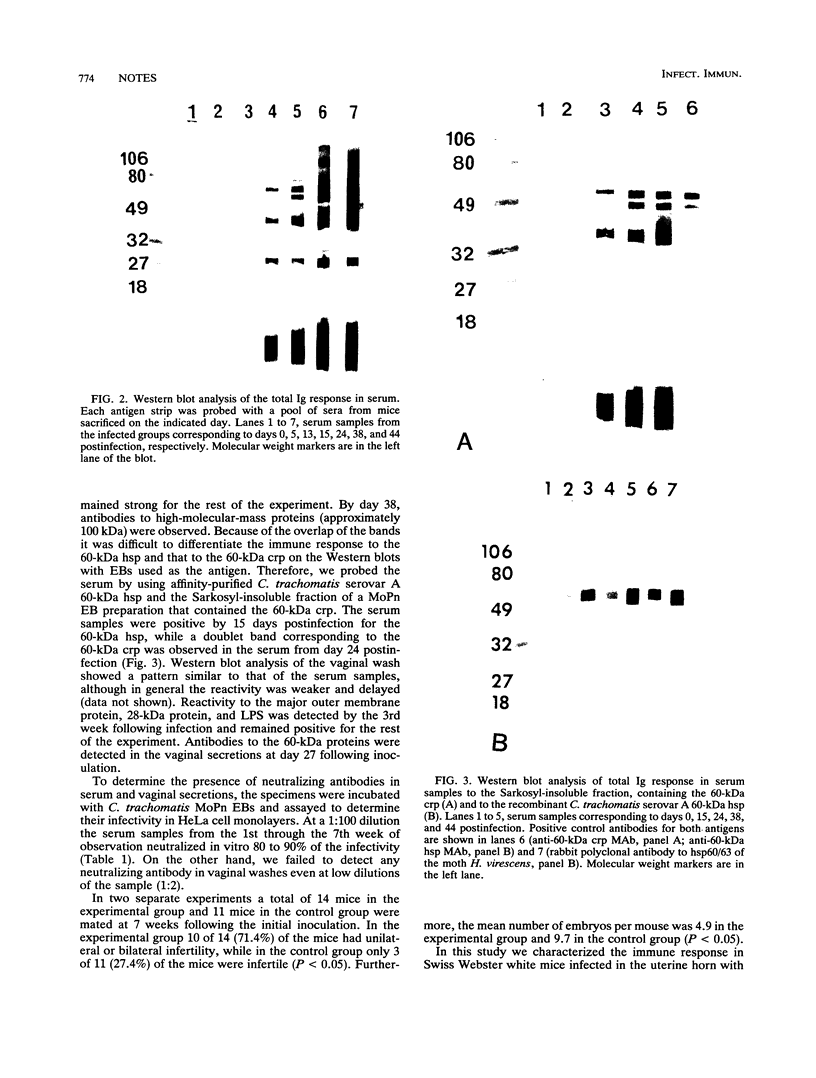
A Swiss Webster white mouse model of salpingitis was used to characterize the immune response following an intrauterine infection with the Chlamydia trachomatis mouse pneumonitis biovar. Western blot (immunoblot) analyses of the serum samples showed that the immunodominant bands corresponded to molecular masses of 72, 60, 42, and 28 kDa and to the lipopolysaccharide. Antibodies to the 60-kDa heat shock protein and to the 60-kDa cysteine-rich protein were detected at 2 and 3 weeks postinfection, respectively. Neutralization was observed in an in vitro assay with serum samples as early as the 3rd day postinfection and remained high for the 7 weeks of observation. The mice were mated in the 7th week following infection. Of the infected experimental mice, 71.4% were found to be either unilaterally or bilaterally infertile, whereas only 27.4% of the noninfected control mice were found to be infertile.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barron A. L., Rank R. G., Moses E. B. Immune response in mice infected in the genital tract with mouse pneumonitis agent (Chlamydia trachomatis biovar). Infect Immun. 1984 Apr;44(1):82–85. doi: 10.1128/iai.44.1.82-85.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron A. L., White H. J., Rank R. G., Soloff B. L., Moses E. B. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J Infect Dis. 1981 Jan;143(1):63–66. doi: 10.1093/infdis/143.1.63. [DOI] [PubMed] [Google Scholar]
- Brunham R. C., Kuo C. C., Cles L., Holmes K. K. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect Immun. 1983 Mar;39(3):1491–1494. doi: 10.1128/iai.39.3.1491-1494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Peeling R., Maclean I., Kosseim M. L., Paraskevas M. Chlamydia trachomatis-associated ectopic pregnancy: serologic and histologic correlates. J Infect Dis. 1992 Jun;165(6):1076–1081. doi: 10.1093/infdis/165.6.1076. [DOI] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles A. M., Crosby H. A., Pearce J. H. Analysis of the human serological response to Chlamydia trachomatis 60-kDa proteins by two-dimensional electrophoresis and immunoblotting. FEMS Microbiol Lett. 1991 Jul 1;65(3):299–303. doi: 10.1016/0378-1097(91)90231-x. [DOI] [PubMed] [Google Scholar]
- Gump D. W., Gibson M., Ashikaga T. Evidence of prior pelvic inflammatory disease and its relationship to Chlamydia trachomatis antibody and intrauterine contraceptive device use in infertile women. Am J Obstet Gynecol. 1983 May 15;146(2):153–159. doi: 10.1016/0002-9378(83)91044-x. [DOI] [PubMed] [Google Scholar]
- Gupta R. S. Sequence and structural homology between a mouse T-complex protein TCP-1 and the 'chaperonin' family of bacterial (GroEL, 60-65 kDa heat shock antigen) and eukaryotic proteins. Biochem Int. 1990;20(4):833–841. [PubMed] [Google Scholar]
- Jones R. B., Ardery B. R., Hui S. L., Cleary R. E. Correlation between serum antichlamydial antibodies and tubal factor as a cause of infertility. Fertil Steril. 1982 Nov;38(5):553–558. doi: 10.1016/s0015-0282(16)46634-3. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989 Mar 1;169(3):663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton D. L., Halbert S. A., Kuo C. C., Wang S. P., Holmes K. K. Host response to primary Chlamydia trachomatis infection of the fallopian tube in pig-tailed monkeys. Fertil Steril. 1983 Dec;40(6):829–840. [PubMed] [Google Scholar]
- Patton D. L., Kuo C. C., Wang S. P., Halbert S. A. Distal tubal obstruction induced by repeated Chlamydia trachomatis salpingeal infections in pig-tailed macaques. J Infect Dis. 1987 Jun;155(6):1292–1299. doi: 10.1093/infdis/155.6.1292. [DOI] [PubMed] [Google Scholar]
- Patton D. L., Landers D. V., Schachter J. Experimental Chlamydia trachomatis salpingitis in mice: initial studies on the characterization of the leukocyte response to chlamydial infection. J Infect Dis. 1989 Jun;159(6):1105–1110. doi: 10.1093/infdis/159.6.1105. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Oda R., Tse P., Gastaldi C., Stone S. C., de la Maza L. M. Comparison of a single-antigen microimmunofluorescence assay and inclusion fluorescent-antibody assay for detecting chlamydial antibodies and correlation of the results with neutralizing ability. J Clin Microbiol. 1989 Feb;27(2):350–352. doi: 10.1128/jcm.27.2.350-352.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Zhong G. M., Carlson E., de la Maza L. M. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect Immun. 1988 Apr;56(4):885–891. doi: 10.1128/iai.56.4.885-891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey K. H., Newhall W. J., 5th, Rank R. G. Humoral immune response to chlamydial genital infection of mice with the agent of mouse pneumonitis. Infect Immun. 1989 Aug;57(8):2441–2446. doi: 10.1128/iai.57.8.2441-2446.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey K. H., Rank R. G. Resolution of chlamydial genital infection with antigen-specific T-lymphocyte lines. Infect Immun. 1991 Mar;59(3):925–931. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey K. H., Soderberg L. S., Rank R. G. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988 May;56(5):1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M. Heat shock proteins as antigens of bacterial and parasitic pathogens. Curr Top Microbiol Immunol. 1991;167:145–160. doi: 10.1007/978-3-642-75875-1_9. [DOI] [PubMed] [Google Scholar]
- Soong Y. K., Kao S. M., Lee C. J., Lee P. S., Pao C. C. Endocervical chlamydial deoxyribonucleic acid in infertile women. Fertil Steril. 1990 Nov;54(5):815–818. [PubMed] [Google Scholar]
- Swenson C. E., Donegan E., Schachter J. Chlamydia trachomatis-induced salpingitis in mice. J Infect Dis. 1983 Dec;148(6):1101–1107. doi: 10.1093/infdis/148.6.1101. [DOI] [PubMed] [Google Scholar]
- Swenson C. E., Schachter J. Infertility as a consequence of chlamydial infection of the upper genital tract in female mice. Sex Transm Dis. 1984 Apr-Jun;11(2):64–67. doi: 10.1097/00007435-198404000-00002. [DOI] [PubMed] [Google Scholar]
- Taylor H. R., Johnson S. L., Schachter J., Caldwell H. D., Prendergast R. A. Pathogenesis of trachoma: the stimulus for inflammation. J Immunol. 1987 May 1;138(9):3023–3027. [PubMed] [Google Scholar]
- Taylor H. R., Maclean I. W., Brunham R. C., Pal S., Whittum-Hudson J. Chlamydial heat shock proteins and trachoma. Infect Immun. 1990 Sep;58(9):3061–3063. doi: 10.1128/iai.58.9.3061-3063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuffrey M., Falder P., Gale J., Quinn R., Taylor-Robinson D. Infertility in mice infected genitally with a human strain of Chlamydia trachomatis. J Reprod Fertil. 1986 Sep;78(1):251–260. doi: 10.1530/jrf.0.0780251. [DOI] [PubMed] [Google Scholar]
- Wagar E. A., Schachter J., Bavoil P., Stephens R. S. Differential human serologic response to two 60,000 molecular weight Chlamydia trachomatis antigens. J Infect Dis. 1990 Oct;162(4):922–927. doi: 10.1093/infdis/162.4.922. [DOI] [PubMed] [Google Scholar]
- Watkins N. G., Hadlow W. J., Moos A. B., Caldwell H. D. Ocular delayed hypersensitivity: a pathogenetic mechanism of chlamydial-conjunctivitis in guinea pigs. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7480–7484. doi: 10.1073/pnas.83.19.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weström L., Mårdh P. A. Chlamydial salpingitis. Br Med Bull. 1983 Apr;39(2):145–150. doi: 10.1093/oxfordjournals.bmb.a071806. [DOI] [PubMed] [Google Scholar]