Abstract
Major causes of morbidity in intravenous drug users are infections. In infective endocarditis, the tricuspid valve is mainly involved. Masses can cause septic embolisms and, in rare cases, they are associated with mycotic aneurysms of pulmonary arteries that lead to severe haemorrhage.
We report the case of a young woman with a history of intravenous drug abuse and prolonged infective tricuspid valve endocarditis. Initially, echocardiography showed large masses on the anterior leaflet of the tricuspid valve and severe tricuspid regurgitation; blood cultures revealed staphylococcus and streptococcus species. Eight months after initial diagnosis, she presented with severe haemoptysis and fever. CT revealed a ruptured mycotic aneurysm of the right pulmonary artery. Lobectomy was performed immediately.
Postoperatively, the patient fully recovered. After continued antibiotic treatment, follow-up examinations showed negative echocardiographic findings and blood cultures results.
Background
Major causes of morbidity and admissions to hospital in intravenous drug users are infections. In intravenous drug users with infective endocarditis, the right heart is involved in most cases—mainly the tricuspid valve. Masses on the tricuspid valve can cause multiple septic pulmonary embolisms, which might lead to various complications like pneumonia, pulmonary infarction, pulmonary abscess, pleural effusion, empyema and even pneumothorax. In rare cases, rupture of a mycotic aneurysm of a pulmonary artery can cause fatal pulmonary haemorrhage. As the development of mycotic aneurysms is a rare complication, the correct diagnosis is often made at a late stage; therefore, treatment is delayed. Since the mortality of ruptured mycotic aneurysms is high, consequences may be fatal. We present the case of a young woman with a history of former intravenous drug abuse and a prolonged infective tricuspid endocarditis who was admitted with haemoptysis due to a mycotic pulmonary artery aneurysm.
Case presentation
A 26-year-old woman with a former history of intravenous drug use attending a buprenorphine substitution programme presented with fever and arthralgia of several joints to our university hospital. Her temperature was 39.0 °C with a respiratory rate of 21 breaths/min and a pulse rate of 122 bpm.
Investigations
At admission, she had wheezing and crackles over both lungs and a systolic murmur in the right fourth intercostal space. Laboratory results showed an elevated C reactive protein (CRP) of 15.93 mg/dl (normal range < 0.5), an anaemia with a haemoglobin of 9.0 g/dl (12.0–16.0) and thrombocytopenia of 55 000/µl (150 000–450 000). Serologic tests for HIV and hepatitis C virus were negative.
CT scan demonstrated bilateral pulmonary infiltrations due to bronchopneumonia as well as hepatosplenomegaly.
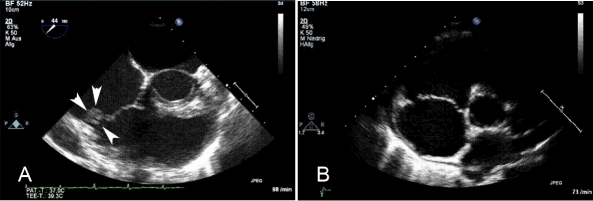
Initial echocardiography showed a mass of 12×7 mm attached to the anterior leaflet of the tricuspid valve, with prolapse into the right atrium and causing a moderate-to-severe tricuspid regurgitation. In the subsequent time, tricuspid regurgitation worsened (figure 1A).
Figure 1.
(A) The transoesophageal echocardiography shows a mass (12×7 mm in size) located at the anterior leaflet of the tricuspid valve prior to antibiotic treatment. (B) After an antibiotic treatment with several antibiotic regimes for 18 months, including a 4-week intravenous treatment with vancomycin, no masses could be detected any more in the final follow-up examination (transthoracal ((B) and transoesophageal echocardiography).
Treatment
In conclusion, the diagnosis ‘endocarditis of the tricuspid valve’ was made.
Initially penicillin G was administered intravenously and, due to bronchopneumonia, tazobactam/piperacillin and clarithromycin were added. Three sets of blood cultures revealed Staphylococcus aureus. Accordingly, antibiotic treatment was changed to gentamycin and tazobactam/piperacillin and was continued intravenously for 7 days. Thereafter, flucloxacillin was recommended orally for 4 weeks.
Outcome and follow-up
During the subsequent year the patient again presented with recurrent septic pulmonary embolisms, pneumonias and fever up to 40 °C. Repeated blood cultures were positive for various staphylococcus and streptococcus species including S aureus, Streptococcus gordonii, S sanguis and S viridians. The patient was managed conservatively with antibiotics according to current guidelines; tricuspid valve replacement was not performed.
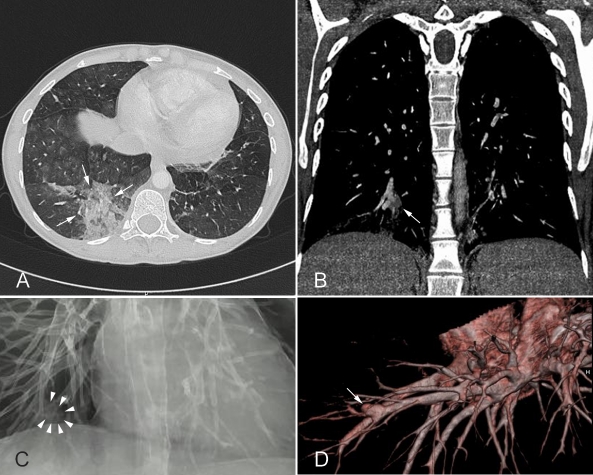
Eight months after the first presentation, the patient suddenly developed massive haemoptysis. At admission, haemoglobin was low (7.4 g/dl). CT scan revealed a large partially ruptured mycotic aneurysm with a diameter of 6–7 mm with partial thrombosis of the right pulmonary artery as well as signs of previous haemorrhage (figure 2).
Figure 2.
(A) CT scan of the thorax shows a haemorrhage in the right lower lobe. (B–D) Show the source of the bleeding—an aneurysm of a segmental pulmonary artery of the right lower lobe.
As the mycotic aneurysm was progressive during conservative management, an interventional treatment with embolisation or covered stenting did not seem to be the best therapeutic option since it would not prevent the further growth or rupture of the aneurysm. Bronchoscopy revealed diffuse bleeding from the right lower lung lobe, which was temporarily controlled by subcutaneous application of antitussiva and bronchial tamponade. As the bleeding could not be treated sufficiently, the patient underwent an urgent lobectomy of the right lower lung lobe to prevent a fatal haemorrhage even though there was an elevated surgery risk due to septic status and severe tricuspid regurgitation.
The patient was positioned in a 45 °C angle to elevate the right sight of the chest and an antero-lateral thoracotomy was chosen with incision at the fifth intercostal space. Deflation of the right lung was only performed when absolutely necessary as haemodynamic insufficiency occurred with significant decrease of oxygen saturation and considerable increase of the central venous pressure. The bronchial stump was covered with a combined flap of pleural tissue and intercostal muscle; additionally it was sealed with fibrin glue containing antibiotics.
Surgery of the tricuspid valve was not performed since lobectomy to stem the bleeding required a lateral thoracotomy. However, tricuspid valve intervention would have included sternotomy. Furthermore, perioperative morbidity and mortality would have been higher due to septic complications and perioperative bleeding during extracorporal circulation.
Histopathology confirmed a mycotic aneurysm with partial thrombosis of the pulmonary artery and inflammatory infiltration affecting the bronchus wall resulting in massive recurrent bleeding into the lung parenchyma (figure 3).
Figure 3.
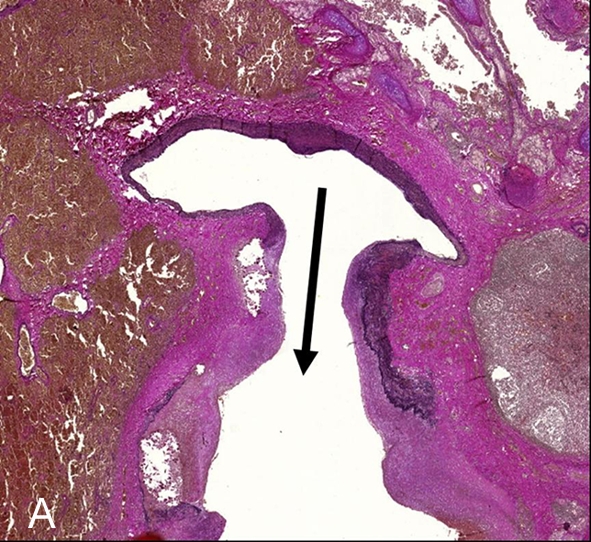
Elastica-van Giesson-staining (1×). This special staining to detect collagen (red) and elastic fibres (black) highlights vascular architecture with extravascular erythrocytes in the surrounding lung parenchyma (brown). The arrow base is located within the normal artery part while the arrow head marks the ectatic vessel wall with degradation of fibres and smooth muscle cells by granulocytes indicating mycotic genesis of the aneurysm.
After surgery was performed, the patient fully recovered and cefazolin was intravenously administered for another 6 weeks. Repeated echocardiography showed that the mass on the anterior leaflet of the tricuspid valve was regressive but still detectable. Oral treatment with ciprofloxacin was administered for another 6 weeks.
Five months later the patient again presented with an elevated CRP of 8 mg/dl and S viridians was revealed in blood cultures. The patient refused to be admitted for administration of intravenous antibiotics; therefore, we started with oral antibiotics. After 6 weeks of oral treatment with amoxicillin, blood cultures were still positive for S viridans and the structure on the anterior leaflet of the tricuspid valve was still detected in echocardiography.
Therefore, the patient was admitted to the hospital to receive vancomycin intravenously for 4 weeks. At the end of treatment echocardiography did not reveal any masses at the tricuspid valve any more (figure 1B). The patient was discharged. Further follow-up examinations showed negative results in blood cultures, no signs of infection, no masses but persistent moderate-to-severe tricuspid valve regurgitation.
Discussion
Infective endocarditis in intravenous drug users most commonly affects the tricuspide valve. More than 90% of cases of infective endocarditis in intravenous drug users are caused by staphylococcus or streptococcus species. When drug treatment and surgery is applied, intravenous drug users with infective endocarditis have an in-hospital survival rate of 91%.1
Pulmonary artery aneurysms often are associated with infective endocarditis or pneumonia and reveal a high mortality.2 3 Mycotic aneurysms and pseudoaneurysms of the pulmonary artery are a rare but severe complication especially in intravenous drug users with infective endocarditis of the right heart. Mortality from mycotic pulmonary artery aneurysms is high and has been estimated over 50%.4
Pathophysiologically, the dilatation of the vessel wall causes the aneurysm. In contrast, a pseudoaneurysm results from the destruction of the two inner layers of the vessel or sometimes even the destruction of the entire vessel wall. Haemorrhage is often prevented from fatal bleeding because of the surrounding thrombus, but there is a high risk of rupture of the aneurysms after complete destruction of the vessel wall, especially when the surrounding thrombus lyses. Therefore, aneurysms and pseudoaneurysms have a high risk of rupture with fatal bleeding.
Pulmonary artery aneurysms can also result from congenital causes like deficiency of the vessel wall, valvular and postvalvular stenosis, increased flow due to left to right shunt, pulmonary arterial hypertension with chronic pulmonary embolisms, septic microemboli to the vasa vasorum, an infective focus, an iatrogenic catheter trauma and inflammatory erosion secondary to infections such as haematogenous seeding, vasculitis or trauma with direct contamination.5
In cases of aneurysms caused by septic embolism, S aureus is often detected in blood cultures.4 6 However, other microorganisms, including Mycobacterium tuberculosis, Actinomyces and Treponema pallidum can cause these aneurysms. Mycotic pulmonary aneurysms have been described in conjunction with endocarditis in injecting drug users.4 Early diagnosis and subsequent surgical or interventional treatment is the main goal as there is a high risk of rupture.2 4
Our patient was admitted to hospital with haemoptysis and lobectomy was performed immediately. Lobectomy or, in rare cases, pneumonectomy is the standard treatment option even if it is associated with high perioperative morbidity.7 However, the optimum management of a case like that is not defined in current international guidelines. Few cases have been described that were managed conservatively.8 Alternative treatment options include transcatheter embolisation with detachable balloons, coils, stent grafts and vascular plugs, which are less invasive than the surgical treatment.9 However, coil embolisation can only be performed if the aneurysm can be technically reached by catheterisation.7 10 11 12
In this case, life-threatening haemoptysis occurred presumably due to rupture of the pseudoaneurysm. Due to the location of the aneurysm in the right lower lobe surgical intervention involved lobectomy as standard procedure. As the patient was in a good clinical condition, surgery was performed immediately. To avoid pulmonary amputation, aneurysmectomy or occlusion of the arterial defect by direct suture can be applied.
However, these techniques are associated with a high mortality. Massive haemoptysis was observed after suture line rupture and the penetration to the adjacent bronchi.
In our case, lobectomy and pulmonary artery plasty was performed for complete resection of the damaged pulmonary artery wall. This procedure was safe and simple and was the definitive treatment for pulmonary artery aneurysm.13
Reconstruction or replacement of the tricuspid valve with severe regurgitation was not performed mainly because a sternotomy would not have allowed control of the bleeding in the right lung quickly. Performing both, the tricuspid intervention and the lobectomy would have caused a highly elevated morbidity and mortality risk regarding septic complications and perioperative bleeding during extracorporal circulation. Following the strategy of surgical treatment of the ruptured mycotic aneurysm and the conservative management of the tricuspid endocarditis, the perioperative risk for the patient was reduced significantly. Follow-up examinations showed a good clinical outcome and, up to now, tricupide valve reconstruction or replacement was not necessary.
It is important to recognise the development of mycotic aneurysms in intravenous drug users who present with infective endocarditis and sudden haemoptysis or who have a history of infective endocarditis and suspicious findings in the CT or radiograph. This typical complication can be life threatening, but various therapeutic options exist when detection is early. Individual treatment plans must be developed.
Learning points.
Infective endocarditis of the right heart can cause septic embolism with severe pulmonary complications.
Mycotic aneurysms are associated with infective endocarditis and high mortality as their rupture can lead to fatal haemorrhage.
Early diagnosis and immediate treatment significantly reduces the morality rate.
Treatment must be planned individually, including interventional treatment and surgery.
Reconstruction or replacement of the involved valve is not mandatory.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Mathew J, Addai T, Anand A, et al. Clinical features, site of involvement, bacteriologic findings, and outcome of infective endocarditis in intravenous drug users. Arch Intern Med 1995;155:1641–8 [PubMed] [Google Scholar]
- 2.Kido T, Nakata Y, Aoki K, et al. Infective endocarditis of the tricuspid valve in a non-drug user. Jpn J Med 1991;30:154–6 [DOI] [PubMed] [Google Scholar]
- 3.Dransfield MT, Johnson JE. A mycotic pulmonary artery aneurysm presenting as an endobronchial mass. Chest 2003;124:1610–12 [DOI] [PubMed] [Google Scholar]
- 4.Benveniste O, Bruneel F, Bédos JP, et al. Ruptured mycotic pulmonary artery aneurysm: an unusual complication of right-sided endocarditis. Scand J Infect Dis 1998;30:626–9 [DOI] [PubMed] [Google Scholar]
- 5.Nguyen ET, Silva CI, Seely JM, et al. Pulmonary artery aneurysms and pseudoaneurysms in adults: findings at CT and radiography. AJR Am J Roentgenol 2007;188:W126–34 [DOI] [PubMed] [Google Scholar]
- 6.Fowler VG, Sheld WM, Bayer AS. Endocarditis and intravascular infections. In: Mandell GI, Bennett JE, Dolin R, eds. Principles and practice of infectious diseases. London: Churchill Livingstone, 2004;1005–22 [Google Scholar]
- 7.Ghaye B, Trotteur G, Dondelinger RF. Multiple pulmonary artery pseudoaneurysms: intrasaccular embolization. Eur Radiol 1997;7:176–9 [DOI] [PubMed] [Google Scholar]
- 8.McLean L, Sharma S, Maycher B. Mycotic pulmonary arterial aneurysms in an intravenous drug user. Can Respir J 1998;5:307–11 [DOI] [PubMed] [Google Scholar]
- 9.Chou MC, Liang HL, Pan HB, et al. Percutaneous stent-graft repair of a mycotic pulmonary artery pseudoaneurysm. Cardiovasc Intervent Radiol 2006;29:890–2 [DOI] [PubMed] [Google Scholar]
- 10.Deshmukh H, Rathod K, Garg A, et al. Ruptured mycotic pulmonary artery pseudoaneurysm in an infant: transcatheter embolization and CT assessment. Cardiovasc Intervent Radiol 2003;26:485–7 [DOI] [PubMed] [Google Scholar]
- 11.Uzun O, Erkan L, Akpolat I, et al. Pulmonary involvement in Behçet's disease. Respiration 2008;75:310–21 [DOI] [PubMed] [Google Scholar]
- 12.Tan LK, Powrie DJ, Rowland R, et al. Fever and haemoptysis in an injecting drug user. Eur Respir J 2007;29:1061–3 [DOI] [PubMed] [Google Scholar]
- 13.Makoto T, Ryoji Y, Ryu N, et al. Successful surgical treatment of pulmonary artery aneurysm in Behc ¸et's syndrome. Interact Cardiovasc Thorac Surg 2009;8:390–2 [DOI] [PubMed] [Google Scholar]