Abstract
We report a patient who initially presented with an abdominal paraganglioma and subsequently metastatic papillary cell renal cancer. Genetic analysis revealed a 141 G>A (exon 2) Trp47X mutation within the succinate dehydrogenase B gene. Treatment with the novel multi-targeted tyrosine kinase inhibitor sunitinib resulted in a sustained partial response and reduced the level of the angiogenic marker PIGF.
Trial registration number:
a6181037
BACKGROUND
The genes encoding the Krebs cycle enzymes succinate and fumarate dehydrogenase are known tumour suppressers. Individuals with succinate dehydrogenase deficiency (SDH) are predisposed to paraganglioma, papillary renal cell carcinoma (RCC) and phaeochromocytoma. SDH results from a somatic “second hit” leading to the activation of hypoxia inducible factor (HIF); among other effects, this increases angiogenic signalling. Advances in the understanding of the biology of renal cell cancer has allowed the development of multi-targeted tyrosine kinase inhibitors (TKI) like sunitinib which inhibit aberrant tumourogenic molecular pathways.
This is the first report of successful treatment of a patient with metastatic papillary RCC associated with SDH B deficiency caused by a 141 G>A (exon 2) mutation using sunitinib. This case illustrates how an isolated genetic mutation can lead to the development of a clinical cancer syndrome and how targeting the downstream effects of that mutation can result in sustained clinical benefit for the patient.
CASE PRESENTATION
A 10-year-old girl presented with recurrent central abdominal pain. Clinical examination revealed a central abdominal mass. She underwent investigation and surgical removal of an isolated abdominal aortic paraganglioma. Over the following 8 years the patient remained well with no evidence of recurrence or new paraganglioma on clinical examination or abdominal ultrasound scanning. At her request, she was discharged from the clinic aged 18 years.
The patient re-presented, aged 26, with haematuria and abdominal pain. Computed tomography (CT) scanning of the abdomen revealed a mass within the left kidney. Laparoscopic surgical resection of the mass and a single enlarged lymph node was successful and histology revealed type II papillary renal cell carcinoma (RCC). Postoperative re-staging CT scans of the chest, abdomen and pelvis showed no evidence of metastatic disease and the patient commenced routine clinical follow-up.
The unusual combination of a paraganglioma and papillary RCC in a young patient suggested the possibility of an underlying genetic condition and the patient was referred for a genetics opinion. At assessment, the patient was noted to have no family history of paraganglioma or other related tumours. Clinical examination revealed one café au lait spot and no lymphadenopathy or evidence of carotid body tumours. Cardiovascular, respiratory, abdominal and neurological examinations were normal. DNA was extracted from peripheral blood leucocytes for polymerase chain reaction (PCR) amplification of the SDH genes. The PCR products were sequenced and compared to known sequence variations of the SDH genes. This confirmed the presence of a 141 G>A (exon 2) substitution within the SDH B gene resulting in a premature stop codon at codon 47.
Biochemical screening for latent phaeochromocytomas, pituitary function abnormalities and thyroid studies revealed no abnormalities. Magnetic resonance imaging (MRI) is superior to CT in detecting latent paraganglioma.1 Twelve months after the resection of the papillary RCC a screening MRI revealed a 3.5 cm mass between the urethra and anterior vaginal vault and a right supraclavicular lymph node mass. The pelvic mass had a high T2 signal which is typical for paraganglioma, unlike RCC which enhances poorly with MRI. MIBG scanning of the mass did not reveal any abnormality and urinary catecholamine screening was negative.
Fine needle aspirates (FNA) were taken of the supraclavicular lymph nodes. The patient was commenced on α- and β-blockers in preparation for removal of the pelvic mass which, given the MRI appearances, may have been a pelvic paraganglioma. Open surgical resection of the pelvic mass was successful. Histology of the lymph node FNA and pelvic mass revealed metastatic type II papillary RCC identical to the previously resected tumour.
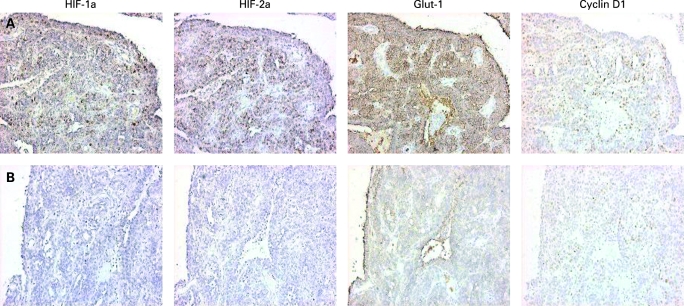
Immunohistochemical analysis was performed on tissue blocks from the pelvic RCC metastasis. Serial sections were stained with antibodies for HIF-1α, cyclin D1 (Neomarkers), HIF-2α (laboratory generated), carbonic anhydrase IX (J Pastorek) and glucose transporter-1 (DAKO). This showed heterogeneous HIF activation throughout the tumour (fig 1).
Figure 1. Immunohistochemistry for HIF-1a, HIF-2a, Glut-1 and cyclin D1 on the pelvic renal cell carcinoma metastasis.
Hypoxia inducible factor (HIF) expression was heterogeneous throughout the blocks examined. Row A shows a region with positive staining for both HIF-a isoforms and the HIF targets Glut-1 and Cyclin D1. Row B shows a region of the tumour with little HIF-a isoform expression and decreased expression of Glut-1 and Cyclin D1.
In the absence of significant symptoms and the palliative intent of treatment, the patient was initially observed. The patient progressed rapidly and within 3 months CT scans showed evidence of progression within the right supraclavicular lymph nodes, partial metastatic destruction of the right scapula and new splenic metastasis (fig 2).
Figure 2. Computed tomography (CT) image A shows metastatic involvement of the right scapular, periscapular tissue and deposit within right sided lymph nodes.
CT image B shows metastatic involvement of the right supraclavicular lymph nodes. CT images C and D show the lesions have responded after 24 weeks of treatment with sunitinib.
Although many investigators feel that immunotherapy has little activity in papillary RCC, the data are limited.2,3. It was decided to offer the patient first line treatment within the context of the RE04 trial (IL-2, 5-fluorouracil and interferon-α vs interferon-α alone). The patient consented and was randomised to the triple therapy arm of the REO4 trial (IL-2, 5-fluorouracil and interferon-α). Treatment was stopped after 28 days due to systemic toxicity (severe arthralgia and flu-like symptoms). After the treatment related toxicities had resolved, repeat CT scanning 6 weeks later showed further disease progression. The patient commenced second line treatment on the open access programme for the multi-targeted kinase inhibitor sunitinib.
Sunitinib was administered in continuous 6 week cycles: 50 mg of sunitinib daily for 28 days followed by 14 days off. Serum samples were collected before and after each sunitinib cycle, and enzyme linked immunosorbent assays (ELISAs, R&D systems) for placenta growth factor (PlGF), vascular endothelial growth factor (VEGF) and VEGF receptor 2 (R2) were performed.
Sunitinib was initially well tolerated with mild hand–foot reaction and oral stomatitis, which resolved during the 2 week rest period of each cycle. After two cycles the patient developed mild renal impairment and myelosuppression. Treatment was delayed and the sunitinib dose reduced to 37.5 mg for subsequent cycles. The patient returned to work after 5 months of treatment. After 9 months, the patient became hypothyroid and commenced thyroxine.
Repeat CT imaging after two cycles showed an excellent partial response to treatment with a reduction in size of the supraclavicular lymphadenopathy, periscapular and splenic deposits. CT scanning after four cycles of treatment confirmed a continuing response to treatment with the supraclavicular lymphadenopathy and periscapular lesions no longer seen on CT imaging and no new sites of disease identified (fig 2). At the time of writing, the patient continues on sunitinib and the partial response has been maintained for over 24 months.
INVESTIGATIONS
Family history, physical examination, 24-hour urine collection for measurement of metanephrines and catecholamines, CT imaging of the chest abdomen and pelvis, MRI imaging of the chest, abdomen and pelvis, tissue biopsy, germline mutation analysis of SDH, analysis of serum angiogenic markers, immuno-histochemical analysis of histological specimens.
TREATMENT
Papillary RCC is the second most common type of kidney cancer and makes up around 10–15% of all RCC. Around 35% of patients with papillary RCC have low grade tumours with the remaining 65% having high grade tumours. Low grade papillary RCC can be divided into subtypes I A, IB and type IIA using cellular morphology, Fuhrman grade and gene expression profiling. High grade type II B tumours (Fuhrman grade >3) are associated with SDH deficiency and have a poorer prognosis than other subtypes of papillary RCC.4 The highest chance of cure in early stage papillary RCC is offered by radical nephrectomy and lymph node dissection. Despite fully resected disease 20–30% of patients will subsequently relapse and require systemic treatment.
There is currently no systemic treatment of proven efficacy in papillary RCC and trials specifically addressing this issue are required. Renal tumours are intrinsically highly resistant to both chemotherapy and radiotherapy. Immunotherapy with either interferon-α or IL-2 have until recently been the only standard treatments available for metastatic disease and only have proven efficacy against clear cell carcinoma. Therapy with interferon-α or IL-2 is toxic, poorly tolerated and response rates are generally low, falling within the region of 10–20%.5 However the outlook for patients is improving, with sunitinib, sorafenib and temsirolimus gaining US Food and Drug Administration (FDA) approval for use in RCC in 2007.
Sunitinib maleate is a multi-targeted receptor TKI which effectively inhibits VEGFR1,2, 3, FLT-1, PDGFR, KIT, and FLT3. Sunitinib has shown significant activity against metastatic RCC in phase II studies with between 34–40% of patients having partial responses and progression-free survival of between 8.3 and 8.7 months.6,7 A recent phase III study, comparing sunitinib to interferon in the first line treatment of metastatic clear cell RCC, favoured sunitinib with a notably better response rate (31% vs 6%) and progression-free survival (11 vs 5 months) than interferon.8
A recent study of 41 patients with metastatic papillary RCC receiving sunitinib (13 patients) or sorafenib treatment (28 patients) showed a low overall partial response rate of 4.8% (2 of 41 patients).9 Both responders were treated with sunitinib and had response durations of 8 and 12 months. The sustained and significant treatment response to sunitinib seen in our patient is unusual and may reflect the unique tumour biology of the SDH associated cancer.
PIGF is a homologue of VEGF and is expressed at high levels in vascular tumours such as RCC and is a potential biomarker in RCC.10 A recent study of PIGF and VEGF and VEGF related proteins, in which the majority of patients had clear cell RCC, using the same sunitinib treatment regimen showed threefold or more rises in PIGF, VEGF and VEGF R2 levels after the first and subsequent cycles of treatment, indicating that Sunitinib has pharmacodynamic effects on PIGF and VEGF and VEGF R2.11
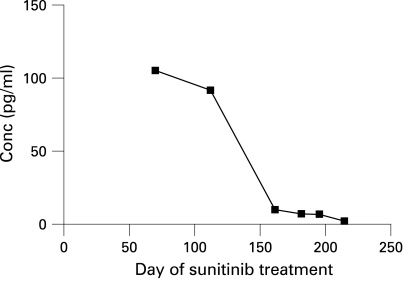
Our data revealed a consistent fall in serum PIGF levels with increasing time of drug treatment (fig 3). However, our serum samples were not sufficiently synchronised with the sunitinib cycles to allow comment on intra-cycle PIGF changes in this patient. The decrease in PIGF expression may have been due to down regulation of its regulatory pathways by sunitinib or may simply reflect decreasing tumour volume over time.
Figure 3. Plasma concentration of placental growth factor (PIGF).
The graph shows a gradual decline in PIGF with time after commencement of sunitinib treatment.
DISCUSSION
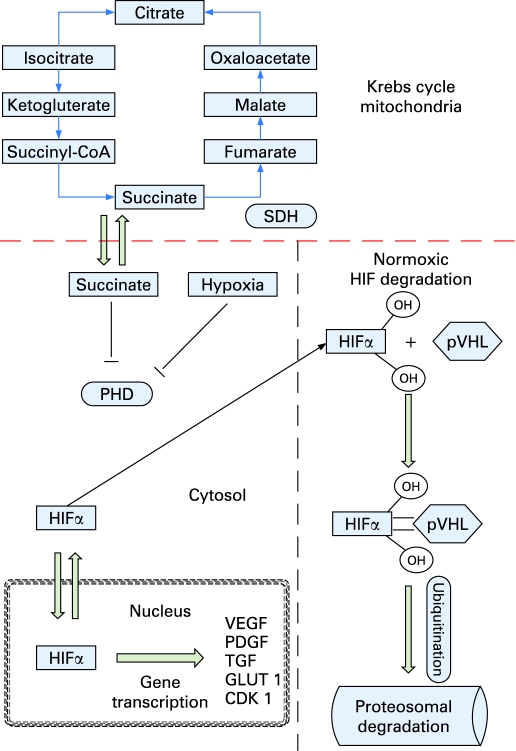
The enzyme SDH is found exclusively within mitochondria catalysing the oxidation of succinate to fumarate and the breakdown of acetyl-coenzyme A (CoA), leading to the production of ATP and water (fig 4). SDH is composed of 4 subunits, each encoded by a single gene. To date 98 mutations are recognised in SDH genes, in addition to 120 sequence variations and 22 polymorphisms.12
Figure 4. The Krebs cycle and how succinate and hypoxia can inhibit proyl-hydroxylases (PHD) which target hypoxia inducible factor (HIF) for proteosomal degradation by pVHL.
The accumulation of HIF leads to the transcription of angiogenic factors such as vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF) and transforming growth factor α (TGFα).
Subunit A (5p15) is a flavoprotein and biallelic deficiency leads to Leigh’s syndrome, ataxia and late onset optic atrophy. Subunit B (1p36) forms the catalytic domain and subunits C (1q21) and D (11q23) are anchor proteins. The SDHB, SDHC and SDHD are tumour suppressor genes; individuals with subunit B mutations are predisposed to developing type II papillary renal cell carcinoma, paraganglioma and phaeochromocytoma.13,14
The genetics analysis confirmed the presence of a 141 G>A (exon 2) substitution within the SDHB gene resulting in a stop codon at codon 47. This mutation has been described in a family with a history of phaeochromocytoma and paraganglioma in which the index case and her sister and paternal aunt carried the 141 G>A (exon 2) mutation. The same study found the 141 G>A (exon 2) substitution in an anonymous blood donor control in which no family history is available.15 At the time of writing no members of the patient’s family have been tested for presence of the 141 G>A (exon 2) mutation.
SDH deficiency results from a somatic “second hit” leading to activation of HIF; among other effects, this increases angiogenic signalling. HIF is a heterodimeric transcription factor which acts as a master regulator in the cellular adaptation to hypoxia. HIF expression leads to the transcription of many angiogenic, survival and proliferation factors including VEGF, platelet derived growth factor and transforming growth factor α.
HIF is continuously produced by cells and in normoxia undergoes rapid hydroxylation by prolyl hydroxylases (PHD). PHD hydroxylates HIF at prolyl residues generating binding sites for the von Hippel–Lindau protein (pVHL) complex which ubiquitinates HIF and targets it for proteosomal degradation (fig 4). The HIF hydroxylation reaction converts α-ketoglutarate and oxygen into carbon dioxide and succinate. Low cellular oxygen tension leads to inhibition of PHD, stabilisation of HIF and upregulation of its downstream target genes.16 In vitro studies have shown that high levels of intracellular succinate, such as those seen in SDH, cause product inhibition of PHD.17,18 This may lead to the stabilisation of HIF under normoxic conditions and the development of pseudohypoxia which favours the development of vascular tumours such as RCC and paraganglioma.
The immunohistochemistry analysis (fig 1) shows that both HIF-isoforms and GLUT 1 and cyclin D1 are dysregulated in parts of the tumour, showing that HIF is not constitutively active throughout the tumour. The differential HIF dysregulation seen in this tumour suggests that hypoxia may still be partially regulating HIF transcription. However, succinate accumulation in areas of high metabolic demand may also be playing a major role in inhibiting HIF breakdown and therefore be promoting tumourigenesis.
This is the first report of successful treatment of a patient with metastatic papillary RCC associated with SDH B deficiency caused by a 141 G>A (exon 2) mutation using sunitinib. This case illustrates how an isolated genetic mutation can lead to the development of a clinical cancer syndrome and how targeting the downstream effects of that mutation can result in clinical benefit for the patient.
LEARNING POINTS
SDH is associated with papillary renal cell carcinoma, paraganglioma and phaeochromocytoma.
Treatment with sunitinib should be considered for SDH associated papillary renal cell carcinoma.
Abnormalities in angiogenic signalling pathways are common in cancer and an important therapeutic target.
Rare disorders can provide unusual insights into the interactions between common metabolic pathways.
Cancers with specific genetic mutations may respond unusually well to selected targeted therapies.
Footnotes
Competing interests: Mark Tuthill, none to declare; Ravi Barod, none to declare; Linda Pyle, none to declare; Terry Cook, none to declare; Shern Chew, none to declare; Patrick Maxwell, ReOx Ltd: Director, consultant, equity holder; Martin Gore, Pfizer: honoraria, advisory board member, research support; Tim Eisen, Pfizer: honoraria, advisory board member, research support.
Patient consent: Patient/guardian consent was obtained for publication
REFERENCES
- 1.Sahdev A, Sohaib A, Monson JP, et al. CT and MR imaging of unusual locations of extra-adrenal paragangliomas (pheochromocytomas). Eur Radiol 2005; 15: 85–92 [DOI] [PubMed] [Google Scholar]
- 2.Herrmann E, Brinkmann OA, Bode ME, et al. Histologic subtype of metastatic renal cell carcinoma predicts response to combined immunochemotherapy with interleukin 2, interferon alpha and 5-fluorouracil. Eur Urol 2007; 51: 1625–32 [DOI] [PubMed] [Google Scholar]
- 3.Ronnen EA, Kondagunta GV, Ishill N, et al. Treatment outcome for metastatic papillary renal cell carcinoma patients. Cancer 2006; 107: 2617–21 [DOI] [PubMed] [Google Scholar]
- 4.Yang XJ, Tan MH, Kim HL, et al. A molecular classification of papillary renal cell carcinoma. Cancer Res 2005; 65: 5628–37 [DOI] [PubMed] [Google Scholar]
- 5.Negrier S, Escudier B, Lasset C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. N Engl J Med 1998; 338: 1272–8 [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multi targeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 2006; 24: 16–24 [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Rini BI, Bukowski RM, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA 2006; 295: 2516–24 [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007; 356: 115–24 [DOI] [PubMed] [Google Scholar]
- 9.Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol 2008; 26: 127–31 [DOI] [PubMed] [Google Scholar]
- 10.Takahashi A, Sasaki H, Kim SJ, et al. Markedly increased amounts of messenger RNAs for vascular endothelial growth factor and placenta growth factor in renal cell carcinoma associated with angiogenesis. Cancer Res 1994; 54: 4233–7 [PubMed] [Google Scholar]
- 11.Deprimo SE, Bello CL, Smeraglia J, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med 2007; 5: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayley JP, Devilee P, Taschner PE. The SDH mutation database: an online resource for succinate dehydrogenase sequence variants involved in pheochromocytoma, paraganglioma and mitochondrial complex II deficiency. (http://www.biomedcentral.com/1471-2350/6/39) (Accessed 18 February 2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neumann HP, Pawlu C, Peczkowska M, et al. European-American Paraganglioma Study Group Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA 2004; 292: 943–51 [DOI] [PubMed] [Google Scholar]
- 14.Vanharanta S, Buchta M, McWhinney SR, et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet 2004; 74: 153–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayley JP, van Minderhout I, Weiss MM, et al. Mutation analysis of SDHB and SDHC: novel germline mutations in sporadic head and neck paraganglioma and familial paraganglioma and/or pheochromocytoma. BMC Med Genet 2006; 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 2004; 5: 343–354 [DOI] [PubMed] [Google Scholar]
- 17.Pollard PJ, Briere JJ, Alam NA, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1 alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet 2005; 14: 2231–9 [DOI] [PubMed] [Google Scholar]
- 18.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 2005; 7: 77–85 [DOI] [PubMed] [Google Scholar]