Abstract
A 62-year-old man diagnosed with a stage I lung adenocarcinoma was treated by an upper right lobectomy. Eighteen months later an elevation of carcinoembryoinc antigen (CEA) was detected, and CT tomography revealed a stage IV disease. Chemotherapy including cisplatin (Platinol) and docetaxel (Taxotere) was administered. He presented 12 days after receiving an intravenous infusion because he noticed a burning sensation, erythema and blisters at the site of the last infusion and proximal to that area. On physical examination he had a 9×4.5 cm swollen area of erythema and multiple blisters. The diagnosis of delayed and distant docetaxel extravasation was made. The treatment consisted of normal saline washes, topical hydrocortisone and antibiotic-based ointment which produced relief of the symptoms. This reaction resolved over the next 6 weeks, leaving two areas of brownish pigmentation of the skin as the only sequelae.
BACKGROUND
Accidental extravasation of various chemical compounds and drugs can cause tissue injury. It is an unusual complication but it can be potentially severe and cause skin necrosis or ulcerations.1 Although several treatment modalities have been suggested to limit the skin damage caused by certain agents, prevention is the mainstay to avoid tissue injury, so it is very important that doctors and nurses are aware of this possible complication in order to prevent it.
This report illustrates the severe consequences of accidental extravasations, and so reminds us how important it is to perform the procedure of intravenous injection of any drug carefully.
CASE PRESENTATION
In March 2008, a 62-year-old white man came under our care with an erythematous and oedematous area with multiple blisters on his right antecubital fossa. He had been diagnosed with a stage I well-differentiated lung adenocarcinoma 18 months before. He had undergone an upper right lobectomy and then he was followed periodically. In October 2007 an asymptomatic elevation of the tumour marker carcinoembryonic antigen (CEA) was detected: 1.430 ng/ml (normal value <5 ng/ml). CT scans revealed the presence of multiple splenic nodules and mediastinal adenopathies denoting an already advanced stage of the disease. Based on these data we administered a combination palliative chemotherapy protocol including cisplatin 75 mg/m2 on day 1 (Platinol; Bristol-Meyers Squibb, Princeton, New Jersey, USA) as a dilution of <0.04 mg/dl followed by docetaxel 75 mg/m2 on day 1 (Taxotere; Aventis Pharma, Bridgewater, New Jersey, USA) every 3 weeks. Because one of the cytotoxic agents is a vesicant and the infusion of both of them is prolonged, he was offered the placement of a subcutaneous device but he refused it. Therefore, he was treated with six cycles administered by peripheral venous access. Routine laboratory tests were obtained 3 or 4 days before each course of chemotherapy because the patient lived far from the Hospital.
On March 19 he came to receive the last cycle. Venous acces was difficult in his left arm so an intravenous line was inserted in his right median antebrachial vein and drugs were administered through a pump as a slow infusion without evidence of acute extravasation during administration or immediately after it.
Ten days later he noticed a burning sensation and itching on the right antecubital fossa and on the site of the last injection. A few hours after those symptoms appeared he developed two erythematous and swollen areas in the same locations and so he presented to the local Emergency Department where he was given a prescription of non-steroidal anti-inflammatory drugs. He was diagnosed as having an atypical dermatitis. Over the next 2 days, the erythema and swelling grew in size, the burning and dryness sensation worsened and he noted the appearance of multiple blisters, so he presented to us 12 days after the intravenous infusion had taken place. On physical examination he had a 9×4.5 cm swollen area of erythema and multiple blisters on the right antecubital fossa and another isolated and small erythematous lesion with a blister located over the site of the last venipuncture (figs 1–4).
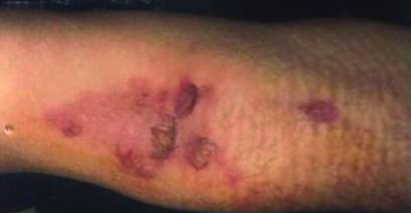
Figure 1.
Twelve days after infusion of chemotheraphy.
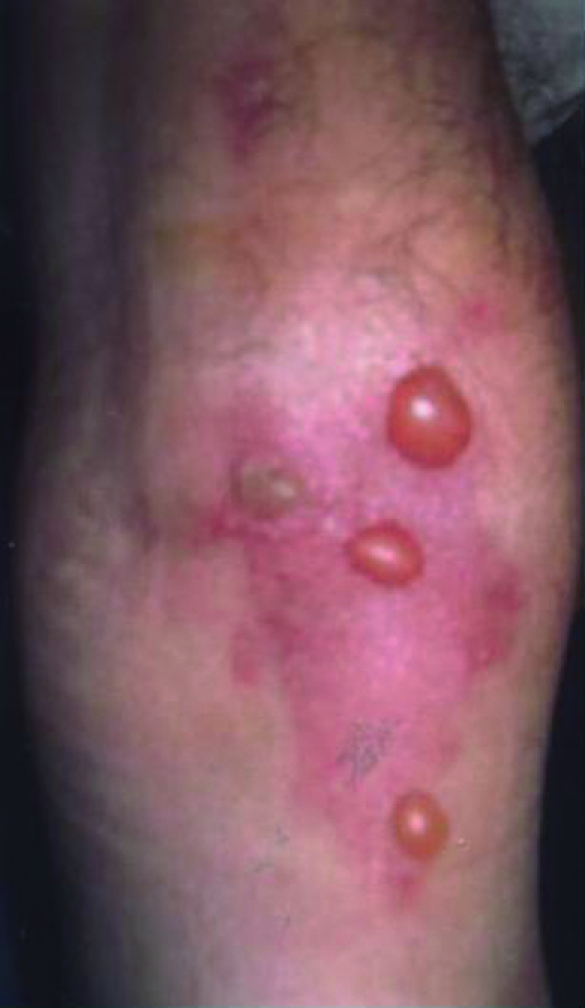
Figure 4.
Two months after docetaxel infusion.
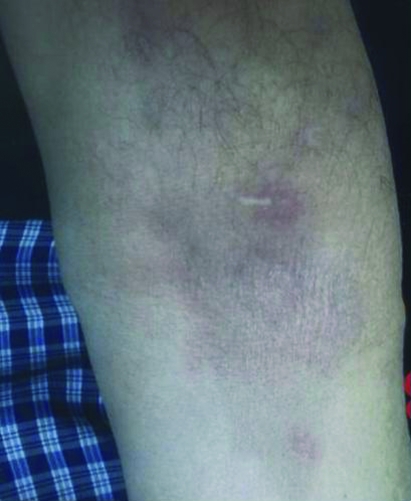
Figure 2.
Three weeks after docetaxel infusion.
Figure 3.
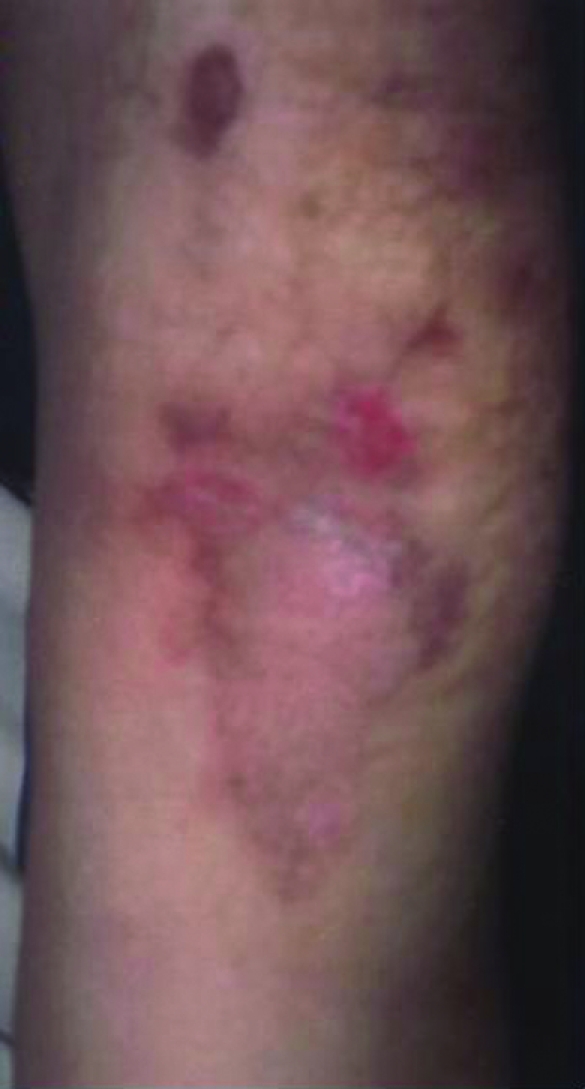
Five weeks after infusion. Two areas of brownish pigmentations as the sequelae.
It was not painful to the touch and the range of motion was correct. He had no swollen lymph nodes on the axilla and he had no fever. Based on these data the diagnosis of delayed and distant extravasation was suggested.
TREATMENT
The lesions were treated with local washes with normal saline followed by three times daily topical applications of hydrocortisone and antibiotic-based ointment which produced relief of symptoms. No plastic surgical intervention was necessary.
OUTCOME AND FOLLOW-UP
This reaction resolved over the next 6 weeks, leaving two areas of brownish pigmentation of the skin as the only sequelae.
DISCUSSION
Although it is an unusual problem, accidental extravasation of various types of drugs (as anticancer therapy) and chemical compounds is a feared complication that may lead to progressive tissue damage.1 Leakage of these substances can cause severe skin necrosis and ulcerations which could require extensive surgical intervention and leave functional impairment as sequelae.2
Chemotherapeutic agents are well-recognised causes of these lesions, especially vesicant types. Other substances include calcium bicarbonate, contrast materials, propofol, etc.
Docetaxel is a cytotoxic agent from the taxane family that inhibits microtubule depolymerisation and promotes tubulin assembly.3 Several types of cutaneous toxicity, such as acral erythrodysestesia or fixed drug eruption, have been described,4 but few cases of tissue damage secondary to its extravasation have been reported.5
Docetaxel has been defined as a potential low vesicant agent6 that may cause erythema, blistering and pain at the site of injection, with usually complete recovery after conservative management.
Berghammer et al7 and El Saghir et al8 reported another two cases, both of them with early symptoms of extravasation, but the first one with late lesions as dermatitis and brown discoloration. In our case, the symptoms developed several days after the infusion without any symptom of acute extravasation. Patel et al9 described in the literature two cases similar to our report but with mitomycin C. Its ability to cause delayed and remote tissue injury after intravenous administration is known. In neither patient did these authors observe evidence of acute extravasation during or immediately after administration, as in our case. However, within 48 h, one patient reported erythema, burning and pain in the hand contralateral to the administration site, and the second one developed three distinct ulcerated lesions on her forearm within 6 weeks of receiving the agent. The lesions were all located at sites of previous venipunctures.
Amano et al1 have described another case with arginine monohydrochloride, which is an agent used to examine the cause of growth retardation in children. Its extravasation causes early symptoms too but also delayed lesions, with necrotic changes and skin ulceration. These authors therefore observed that this agent may cause skin damage more slowly than some chemotherapeutic agents, and this reaction is seen in our case too, because the development of tissue damage was delayed.
Although many cases have been reported in the literature, no definitive recommendations for treating docetaxel extravasation were found except the early application of local cold compresses that could reduce the tissue inflammation and tissue necrosis. It is currently unknown whether other agents such as dimethylsulfoxide (DMSO) can change the course of tissue injury.10
Many authors have proposed isotonic saline, and local hypothermia could restrict the inflammation and tissue necrosis induced by the extravasation of docetaxel, and it was observed that repetitive topical application of DMSO beyond the day of extravasation had no additional benefit.11
This report is all the more relevant because to our knowledge this is the first case documented in the literature about a multiple, distant and delayed tissue injury after two indolent extravasations of docetaxel occurring at the site of injection and the site of previous recent venipuncture in the same arm to obtain samples for analysis. Many cases with delayed lesions have been described with this agent,12 but none secondary to two indolent extravasations.
This case report is a good illustration of an unusual but dreaded complication with intravenous infusions which are the most frequent way to administer drugs in medicine.
Because of the morbidity associated with extravasation and the lack of definitive treatment to prevent tissue damage, clinical physicians should be aware of these possible complications so that they can be prevented.13
LEARNING POINTS
Avoid the chemotherapy infusions in the same arm from which recent samples for analysis have been obtained.
Instruct your patients to look after the venipuncture areas and to consult with a specialist if they note a burning sensation or itching.
Clinical physicians should be aware of extravasation complications so that they can be prevented.
Acknowledgments
To Dr Fidel Asensio Fierro, my master in medicine and my best friend
Footnotes
Competing interests: none.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Amano H, Nagai Y, Kowase T, et al. Cutaneous necrosis induced by extravasation of arginine monohydrochloride. Acta Derm Venereol 2008; 88: 310–1 [DOI] [PubMed] [Google Scholar]
- 2.Bowlby HA, Elanjian SI. Necrosis caused by extravasation of arginine hydrochloride. Ann Pharmacother 1992; 26: 263–4 [DOI] [PubMed] [Google Scholar]
- 3.Raley J, Geisler JP, Buekers TE, et al. Docetaxel extravasation causing significant delayed tissue injury. Gynecol Oncol 2000; 78: 259–60 [DOI] [PubMed] [Google Scholar]
- 4.Payne AS, James WD, Weiss RB. Dermatologic toxicity of chemotherapeutic agents. Semin Oncol 2006; 33: 86–97 [DOI] [PubMed] [Google Scholar]
- 5.Ener RA, Meglathery SB, Styler M. Extravasation of systemic hemato-oncological therapies. Ann Oncol 2004; 15: 858–62 [DOI] [PubMed] [Google Scholar]
- 6.Schrijvers DL: Extravasation: a dreaded complication of chemotherapy. Ann Oncol 2003; 14(suppl 3): S26–30 [DOI] [PubMed] [Google Scholar]
- 7.Berghammer P, Pöhnl R, Baur M, et al. Docetaxel extravasation. Support Care Cancer 2001; 9: 131–4 [DOI] [PubMed] [Google Scholar]
- 8.El Saghir NS, Otrock ZK. Docetaxel extravasation into the normal breast during breast cancer treatment. Anticancer Drugs 2004; 15: 401–4 [DOI] [PubMed] [Google Scholar]
- 9.Patel JS, Krusa M. Distant and delayed mytomicin C extravasation. Pharmacotherapy 1999; 19: 1002–5 [DOI] [PubMed] [Google Scholar]
- 10.Berghammer P, Pöhnl R, Baur M, et al. Docetaxel extravasation. Support Care Cancer 2001; 9: 131–4 [DOI] [PubMed] [Google Scholar]
- 11.Goolsby TV, Lombardo FA. Extravasation of chemotherapeutic agents: prevention and treatment. Semin Oncol 2006; 33: 139–43 [DOI] [PubMed] [Google Scholar]
- 12.Ascherman JA, Knowles SL, Attkiss K. Docetaxel (taxotere) extravasations: a report of five cases with treatment recommendations. Ann Plast Surg 2000; 45: 438–41 [DOI] [PubMed] [Google Scholar]
- 13.Raley J, Geisler JP, Buekers TE, et al. Docetaxel extravasation causing significant delayed tissue injury. Gynecol Oncol 2000; 78: 259–60 [DOI] [PubMed] [Google Scholar]