Abstract
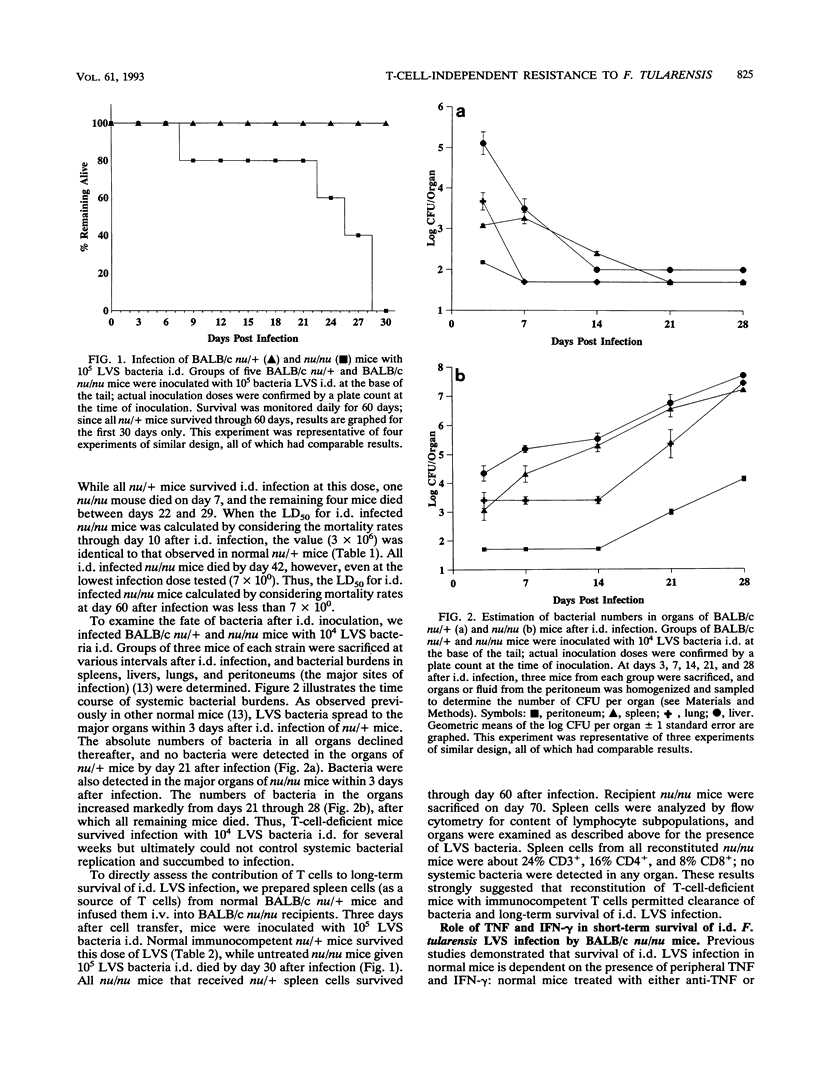
The intraperitoneal 50% lethal dose (LD50) for Francisella tularensis LVS in both normal control heterozygote BALB/c nu/+ mice and BALB/c nu/nu mice was 2 x 10(0). Both nu/+ and nu/nu mice given 10(7) LVS bacteria or more intradermally (i.d.) died, with a mean time to death of about 7 to 8 days. On the other hand, nu/+ mice given 10(6) LVS bacteria or less survived for more than 60 days and cleared systemic bacteria, while nu/nu mice given 10(6) LVS bacteria or less survived for more than 10 days but died between days 25 and 30. Thus, the short-term (i.e., < 10-day) i.d. LD50 of both nu/nu and nu/+ mice was 3 x 10(6), but the long-term (i.e., > 10-day) i.d. LD50 of nu/nu mice was less than 7 x 10(0). The short-term survival of i.d. infection was dependent on tumor necrosis factor and gamma interferon: treatment of nu/nu mice with anti-tumor necrosis factor or anti-gamma interferon at the time of i.d. infection resulted in death from infection 7 to 8 days later, whereas control infected nu/nu mice survived for 26 days. nu/nu mice infected with LVS i.d. generated LVS-specific serum antibodies, which were predominantly immunoglobulin M: titers peaked 7 days after i.d. infection but declined sharply by day 21, after which mice died. Surprisingly, nu/nu mice given 10(3) LVS bacteria i.d. became resistant to a lethal challenge (5,000 LD50s) of LVS intraperitoneally within 2 days after i.d. infection; nu/nu mice similarly infected with LVS i.d. and challenged with Salmonella typhimurium (10 LD50s) were not protected. nu/nu mice given nu/+ spleen cells intravenously as a source of mature T cells survived i.d. infection for more than 60 days and cleared bacteria. Taken together, these studies demonstrate that i.d. infection of nu/nu mice with LVS rapidly generates T-cell-independent, short-term, specific protective immunity against lethal challenge, but T lymphocytes are essential for long-term survival.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony L. S., Ghadirian E., Nestel F. P., Kongshavn P. A. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb Pathog. 1989 Dec;7(6):421–428. doi: 10.1016/0882-4010(89)90022-3. [DOI] [PubMed] [Google Scholar]
- Asarnow D. M., Goodman T., LeFrancois L., Allison J. P. Distinct antigen receptor repertoires of two classes of murine epithelium-associated T cells. Nature. 1989 Sep 7;341(6237):60–62. doi: 10.1038/341060a0. [DOI] [PubMed] [Google Scholar]
- Asarnow D. M., Kuziel W. A., Bonyhadi M., Tigelaar R. E., Tucker P. W., Allison J. P. Limited diversity of gamma delta antigen receptor genes of Thy-1+ dendritic epidermal cells. Cell. 1988 Dec 2;55(5):837–847. doi: 10.1016/0092-8674(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Bandeira A., Itohara S., Bonneville M., Burlen-Defranoux O., Mota-Santos T., Coutinho A., Tonegawa S. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-cell antigen receptor gamma delta. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. S. Immunization against tularemia: analysis of the effectiveness of live Francisella tularensis vaccine in prevention of laboratory-acquired tularemia. J Infect Dis. 1977 Jan;135(1):55–60. doi: 10.1093/infdis/135.1.55. [DOI] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F. Effects of purified anti-Lyt-2 mAb treatment on murine listeriosis: comparative roles of Lyt-2+ and L3T4+ cells in resistance to primary and secondary infection, delayed-type hypersensitivity and adoptive transfer of resistance. Immunology. 1990 Sep;71(1):107–112. [PMC free article] [PubMed] [Google Scholar]
- Dunn P. L., North R. J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991 Sep;59(9):2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins K. L., Leiby D. A., Winegar R. K., Nacy C. A., Fortier A. H. Rapid generation of specific protective immunity to Francisella tularensis. Infect Immun. 1992 Nov;60(11):4571–4577. doi: 10.1128/iai.60.11.4571-4577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Bockemühl J. Listeria monocytogenes infection in nude mice. Infect Immun. 1975 Aug;12(2):437–439. doi: 10.1128/iai.12.2.437-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier A. H., Slayter M. V., Ziemba R., Meltzer M. S., Nacy C. A. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect Immun. 1991 Sep;59(9):2922–2928. doi: 10.1128/iai.59.9.2922-2928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T., Lefrancois L. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. J Exp Med. 1989 Nov 1;170(5):1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T., Lefrançois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988 Jun 30;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Havran W. L., Poenie M., Tigelaar R. E., Tsien R. Y., Allison J. P. Phenotypic and functional analysis of gamma delta T cell receptor-positive murine dendritic epidermal clones. J Immunol. 1989 Mar 1;142(5):1422–1428. [PubMed] [Google Scholar]
- Hormaeche C. E., Mastroeni P., Arena A., Uddin J., Joysey H. S. T cells do not mediate the initial suppression of a Salmonella infection in the RES. Immunology. 1990 Jun;70(2):247–250. [PMC free article] [PubMed] [Google Scholar]
- Killar L. M., Eisenstein T. K. Immunity to Salmonella typhimurium infection in C3H/HeJ and C3H/HeNCrlBR mice: studies with an aromatic-dependent live S. typhimurium strain as a vaccine. Infect Immun. 1985 Mar;47(3):605–612. doi: 10.1128/iai.47.3.605-612.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishihara K., Yoshikai Y., Matsuzaki G., Mak T. W., Nomoto K. Functional alpha and beta T cell chain receptor messages can be detected in old but not in young athymic mice. Eur J Immunol. 1987 Apr;17(4):477–482. doi: 10.1002/eji.1830170407. [DOI] [PubMed] [Google Scholar]
- Kupper T. S. The activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responses. J Invest Dermatol. 1990 Jun;94(6 Suppl):146S–150S. doi: 10.1111/1523-1747.ep12876130. [DOI] [PubMed] [Google Scholar]
- Lawetzky A., Hünig T. Analysis of CD3 and antigen receptor expression on T cell subpopulations of aged athymic mice. Eur J Immunol. 1988 Mar;18(3):409–416. doi: 10.1002/eji.1830180314. [DOI] [PubMed] [Google Scholar]
- Leiby D. A., Fortier A. H., Crawford R. M., Schreiber R. D., Nacy C. A. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992 Jan;60(1):84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Lees R. K., Bron C., Sordat B., Miescher G. T cell antigen receptor expression in athymic (nu/nu) mice. Evidence for an oligoclonal beta chain repertoire. J Exp Med. 1987 Jul 1;166(1):195–209. doi: 10.1084/jem.166.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura M., Galanos C. Induction of hypersensitivity to endotoxin and tumor necrosis factor by sublethal infection with Salmonella typhimurium. Infect Immun. 1990 Apr;58(4):935–937. doi: 10.1128/iai.58.4.935-937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C., Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992 Feb;60(2):450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C. Role of CD4+ T cells and T-independent mechanisms in acquired resistance to Salmonella typhimurium infection. J Immunol. 1990 Aug 15;145(4):1265–1269. [PubMed] [Google Scholar]
- Newborg M. F., North R. J. On the mechanism of T cell-independent anti-Listeria resistance in nude mice. J Immunol. 1980 Feb;124(2):571–576. [PubMed] [Google Scholar]
- Nixon-Fulton J. L., Kuziel W. A., Santerse B., Bergstresser P. R., Tucker P. W., Tigelaar R. E. Thy-1+ epidermal cells in nude mice are distinct from their counterparts in thymus-bearing mice. A study of morphology, function, and T cell receptor expression. J Immunol. 1988 Sep 15;141(6):1897–1903. [PubMed] [Google Scholar]
- O'Brien A. D., Metcalf E. S. Control of early Salmonella typhimurium growth in innately Salmonella-resistant mice does not require functional T lymphocytes. J Immunol. 1982 Oct;129(4):1349–1351. [PubMed] [Google Scholar]
- Orme I. M. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988 May 15;140(10):3589–3593. [PubMed] [Google Scholar]
- Orme I. M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987 Jan 1;138(1):293–298. [PubMed] [Google Scholar]
- Raff M. C. Theta-bearing lymphocytes in nude mice. Nature. 1973 Dec 7;246(5432):350–351. doi: 10.1038/246350a0. [DOI] [PubMed] [Google Scholar]
- Schreiber R. D., Hicks L. J., Celada A., Buchmeier N. A., Gray P. W. Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity. J Immunol. 1985 Mar;134(3):1609–1618. [PubMed] [Google Scholar]
- Sheehan K. C., Ruddle N. H., Schreiber R. D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989 Jun 1;142(11):3884–3893. [PubMed] [Google Scholar]
- TIGERTT W. D. Soviet viable Pasteurella tularensis vaccines. A review of selected articles. Bacteriol Rev. 1962 Sep;26:354–373. doi: 10.1128/br.26.3.354-373.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigelaar R. E., Lewis J. M., Bergstresser P. R. TCR gamma/delta+ dendritic epidermal T cells as constituents of skin-associated lymphoid tissue. J Invest Dermatol. 1990 Jun;94(6 Suppl):58S–63S. doi: 10.1111/1523-1747.ep12875138. [DOI] [PubMed] [Google Scholar]
- Tärnvik A. Nature of protective immunity to Francisella tularensis. Rev Infect Dis. 1989 May-Jun;11(3):440–451. [PubMed] [Google Scholar]
- Wentworth P. A., Ziegler H. K. Induction of macrophage Ia expression by lipopolysaccharide and Listeria monocytogenes in congenitally athymic nude mice. J Immunol. 1987 May 15;138(10):3167–3173. [PubMed] [Google Scholar]
- Wherry J. C., Schreiber R. D., Unanue E. R. Regulation of gamma interferon production by natural killer cells in scid mice: roles of tumor necrosis factor and bacterial stimuli. Infect Immun. 1991 May;59(5):1709–1715. doi: 10.1128/iai.59.5.1709-1715.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M. Cell-mediated immune response to Salmonella typhimurium infection in mice: development of nonspecific bactericidal activity against Listeria monocytogenes. Infect Immun. 1976 Apr;13(4):1069–1073. doi: 10.1128/iai.13.4.1069-1073.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



