Abstract
Thymolipomas are rare tumours located in the anterior mediastinum. Sometimes these tumours may be combined with thymomas or lymphomas. We present a unique case of a thymic carcinoid arising within a thymolipoma. A 68-year-old patient presented with chronic chest and neck pain, which was initially thought to be caused by coronary artery disease. A chest x-ray, exercise tolerance test and coronary angiography were unremarkable. The following CT scan of the neck and chest showed a small tumour in the anterior mediastinum. A robotic-assisted thymectomy was performed and histological examination revealed a neuroendocrine tumour of the thymus within a thymolipoma. The patient was discharged 3 days after surgery in good general condition.
Background
Thymolipomas are rare benign tumours of the thymus, accounting for 2–9% of all thymic neoplasms. They occur most frequently in young adults and have no sex predilection.
The tumour is characterised by a pattern of slow, encapsulated growth. Association with myasthenia gravis, Graves’ disease, aplastic anaemia and other haematological disorders has been reported. To date, there are no reports of local recurrence after surgical resection of a thymolipoma.
Thymic carcinoid is also a scarce primary malignant thymic neoplasm, accounting for approximately 2–4% of all anterior thoracic malignancies, occurs over a wide patient age range (median age, 43 years) and has a male predilection of 3:1. One-third of these tumours are functionally active, with the remaining two-thirds being asymptomatic. Symptoms include chest pain, weight loss, cutaneous flushing, asthma-like symptoms and endocrinological disorders such as Cushing syndrome (33–40% of cases) or multiple endocrine neoplasia, specifically type 1 (19–25%) or ectopic adrenocorticotropic hormone syndrome. A life-threatening carcinoid crisis (2%) presenting with intense flushing, diarrhoea, tachycardia, hypertension or hypotension can occur spontaneously or as a response to stress caused by, for example, anaesthesia, surgical manipulation or chemotherapy.
According to the WHO classification, four main subtypes can be distinguished: typical carcinoid, atypical carcinoid, non-small cell neuroendocrine carcinoma and small cell neuroendocrine carcinoma. These entities can be distinguished on the basis of morphology, mitosis count and the presence or absence of necrosis.1
Although the simultaneous occurrence of various subtypes of thymomas has been well documented (AB/B2, B2/B3, B3/thymic carcinomas),1 combinations of thymolipomas, thymomas or thymic carcinomas and neuroendocrine tumours are extremely rare,2 and a thymolipoma combined with a thymic carcinoid has not been previously reported.
Case presentation
A 68-year-old man with a past history of coronary artery disease experienced angina pectoris, and chest and neck pain. A chest x-ray, exercise tolerance test and coronary angiography were unremarkable. In December 2007, an enhanced chest and neck CT scan revealed a small tumour in the anterior mediastinum approximately 1.5 cm in diameter. Pre-operatively, a CT scan of the rest of body did not show any other primary tumours or enlarged lymph nodes. In January 2008, the patient underwent robotic-assisted thymectomy. The resected specimens measured 9×6×3 cm. Within these specimens an encapsulated area of a maximum diameter of 6 cm consisting of mature fat was found with a 1.5 cm firm, circumscribed nodule at one pole showing microcystic features. Histological and immunohistochemical examination confirmed the diagnosis of a thymic carcinoid arising within a thymolipoma.
Investigations
Tissues were fixed in 4% phosphate buffered formaldehyde, dehydrated and wax-embedded. Sections 3 µm thick were routinely stained with haematoxylin and eosin for histological examination.
For immunohistochemical staining the following antibodies were used: AE1+3 (Chemicon International, Temecula, California, USA), CD 56, parathyroid hormone (Novocastra Laboratories, Newcastle upon Tyne, UK), chromogranin A, synaptophysin and Ki-67 (Dakocytomation, Glostrup, Denmark). The staining was visualised by the EnVision System (Dakocytomation) according to the manufacturer's instruction.
For electron microscopy, samples from paraffin-embedded material were treated as described previously by Steiner et al.3 Subsequently the samples were investigated using a Zeiss EM 109 electron microscope and micrographs taken on ILFORD PAN F 50 negative film.
Histological examination of the 1.5 cm tumour revealed a microcystic pattern with trabecular architecture (figure 1A), sometimes interrupted by spindle shaped cells, without invasion into lymph or blood vessels. The stroma was richly vascularised. Immunohistochemically, the tumour was positive for the cytokeratin marker AE1+3 (figure 1B) as well as for neuroendocrine markers CD 56, chromogranin A and synaptophysin (figure 1C) and negative for the human parathyroid hormone and the proliferation marker Ki-67. The neuroendocrine differentiation was also demonstrated by electron microscopy (figure 1D), which showed numerous endocrine particles. No necrosis and no mitotic activity were observed within the tumour mass. The small tumour was delineated by a fibrous capsule and surrounded by a thymolipoma (measuring 6 cm) consisting of mature fat without atypia or atrophic thymic tissue (figure 1E,F).
Figure 1.
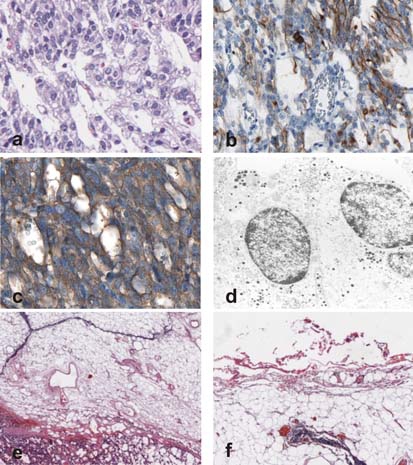
(A) High power view of the carcinoid tumour showing a trabecular-like growth pattern (haematoxylin and eosin, original magnification×40). (B) Positivity of the tumour cells for the cytokeratin marker AE1+3 (immunoperoxidase with methylene blue counterstain, original magnification×40). (C) Immunohistochemical investigation with the synaptophysin marker (immunoperoxidase with methylene blue counterstain, original magnification×40). (D) Electron microscopy, showing numerous endocrine particles (original magnification×3000). (E) The thymolipoma adjunct to the neuroendocrine tumour, showing a strand-like disposition of thymic tissue (haematoxylin and eosin, original magnification×10). (F) Small fibrous capsule of the thymolipoma (haematoxylin and eosin, original magnification×10).
Outcome and follow-up
The post-operative course was uneventful, and the patient was discharged 3 days after surgery. A total body somatostatin receptor scintigraphy 1 month later showed no further tumours. No adjuvant therapy was administered. At the 2-year follow-up the patient was free of tumour recurrence.
Discussion
This is the first reported case of a thymic carcinoid within a thymolipoma. Neuroendocrine tumours of the thymus are sometimes combined with different subtypes of thymomas and thymic carcinomas. In general, thymic carcinoid has a poor prognosis due to a high rate of recurrence and metastasis, but new therapeutic strategies including ocreotide based therapies may improve survival.4 Nevertheless, because of the rare occurrence of combined tumours of the thymus, data on therapy strategies and long-term follow-up are lacking.
Several combined thymoma tumours have been reported. Chen investigated a total of 200 thymomas1 and found several cases of mixed type thymomas (AB/B2, B2/B3 and B3/thymic carcinoma). In a large series of 228 patients reported by Ströbel et al, 21% of cases showed combinations of type B1, B2 and B3 thymoma components and thymic carcinoma features in various proportions, with combinations of type B2 and B3 thymomas being by far the most common.5 Similar results were found in a study by Engel et al.6 Type A thymomas also seem to arise in combination with thymic carcinomas, but reported cases are rare.7–9 Perhaps some type A thymomas with spindle cell differentiation and type B3 features10 represent cases with tumour progression, however, these special cases of combined type A thymomas and thymic carcinomas need further investigation.
In the medical literature, only two cases of thymic tumours within a thymolipoma have been described. Recently, the occurrence of a type B2 thymoma and an undifferentiated thymic carcinoma within a thymolipoma arising in a 36-year-old woman has been reported.11 Another thymolipoma occurred in a 67-year-old, otherwise healthy female patient who was incidentally found to have an anterior mediastinal mass on radiographic examination. Microscopical examination showed an encapsulated type B thymoma within a thymolipoma.12
Additionally, thymic tumours can not only be associated with autoimmune disorders but also with leukaemias or lymphomas, especially T cell lymphomas.13 One case described by Khoury et al14 was composed of a type B thymoma and infiltrates of chronic lymphatic leukaemia, while another case reported by Pillai et al15 showed an association between a thymolipoma and classical Hodgkin lymphoma.
The question of whether combined tumours arise by different genetic pathways or whether, for example in cases of thymic carcinomas, a neuroendocrine component progresses via further genetic alterations into a neuroendocrine tumour, needs to be clarified. The latter possibility appears likely considering that thymic carcinomas often show scattered groups of neuroendocrine cells.4 In further support for such a mechanism Marx et al4 reported a case of combined B3 and large cell neuroendocrine carcinoma that shared a large number of genetic alterations.
In conclusion, when dealing with tumours of the thymus, one should always be aware of the possibility of combined tumours, the most frequent being mixed type thymomas.
Learning points.
-
▶
Combined tumours of the thymus are rare, the most frequent being mixed type thymomas.
-
▶
Symptoms of thymic carcinoid include chest pain, weight loss, cutaneous flushing, asthma-like symptoms and endocrinological disorders.
-
▶
Histological and immunohistochemical investigation of excised tumour specimens is required for confirmation of the diagnosis and successful treatment.
Footnotes
Competing interests None.
Patient consent Obtained.
References
- 1.Chen G, Marx A, Wen-Hu C, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer 2002;95:420–9 [DOI] [PubMed] [Google Scholar]
- 2.Müller-Hermelink HK, Stroebel P, Zettl A, et al. Combined thymic epithelial tumours. In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, eds. World Health Organization Classification of Tumours. Pathology and genetics of tumors of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004:196–7 [Google Scholar]
- 3.Steiner HJ, Peringer P, Moser P, et al. Improved ultrastructural resolution by a new re-embedding procedure for formaldehyde-fixed paraffin-embedded tissues. Histopathology 2009;54:775–7 [DOI] [PubMed] [Google Scholar]
- 4.Marx A, Shimosato Y, Kuo TT, et al. Thymic neuroendocrine tumours. In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, eds. World Health Organization Classification of Tumours. Pathology and genetics of tumors of the Lung, Pleura, Thymus and Heart. Lyon, France: IARC Press; 2004:188–95 [Google Scholar]
- 5.Ströbel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol 2004;22:1501–9 [DOI] [PubMed] [Google Scholar]
- 6.Engel P, Marx A, Müller-Hermelink HK. Thymic tumours in Denmark. A retrospective study of 213 cases from 1970-1993. Pathol Res Pract 1999;195:565–70 [DOI] [PubMed] [Google Scholar]
- 7.Suster S, Moran CA. Primary thymic epithelial neoplasms showing combined features of thymoma and thymic carcinoma. A clinicopathologic study of 22 cases. Am J Surg Pathol 1996;20:1469–80 [DOI] [PubMed] [Google Scholar]
- 8.Matsuno Y, Morozumi N, Hirohashi S, et al. Papillary carcinoma of the thymus: report of four cases of a new microscopic type of thymic carcinoma. Am J Surg Pathol 1998;22:873–80 [DOI] [PubMed] [Google Scholar]
- 9.Kuo TT, Chan JK. Thymic carcinoma arising in thymoma is associated with alterations in immunohistochemical profile. Am J Surg Pathol 1998;22:1474–81 [DOI] [PubMed] [Google Scholar]
- 10.Rosai J, Sobin LH, eds. Histological typing of tumors of the thymus. World Health Organization. International histological classification of tumours. Heidelberg: Springer; 1999 [Google Scholar]
- 11.Haddad H, Joudeh A, El-Taani H, et al. Thymoma and thymic carcinoma arising in a thymolipoma: report of a unique case. Int J Surg Pathol 2009;17:55–9 [DOI] [PubMed] [Google Scholar]
- 12.Argani P, de Chiocca IC, Rosai J. Thymoma arising with a thymolipoma. Histopathology 1998;32:573–4 [PubMed] [Google Scholar]
- 13.Rovera F, Billo P, Capella C, et al. Concurrent epithelial thymoma and T-cell lymphoblastic lymphoma. Interact Cardiovasc Thorac Surg 2003;2:537–40 [DOI] [PubMed] [Google Scholar]
- 14.Khoury JD, Amin HM, Jorgensen JL, et al. Composite thymoma and chronic lymphocytic leukemia/small lymphocytic lymphoma involving the anterior mediastinum. Arch Pathol Lab Med 2003;127:E76–9 [DOI] [PubMed] [Google Scholar]
- 15.Pillai R, Yeoh N, Addis B, et al. Thymolipoma in association with Hodgkin's disease. J Thorac Cardiovasc Surg 1985;90:306–8 [PubMed] [Google Scholar]


