Abstract
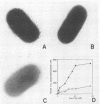
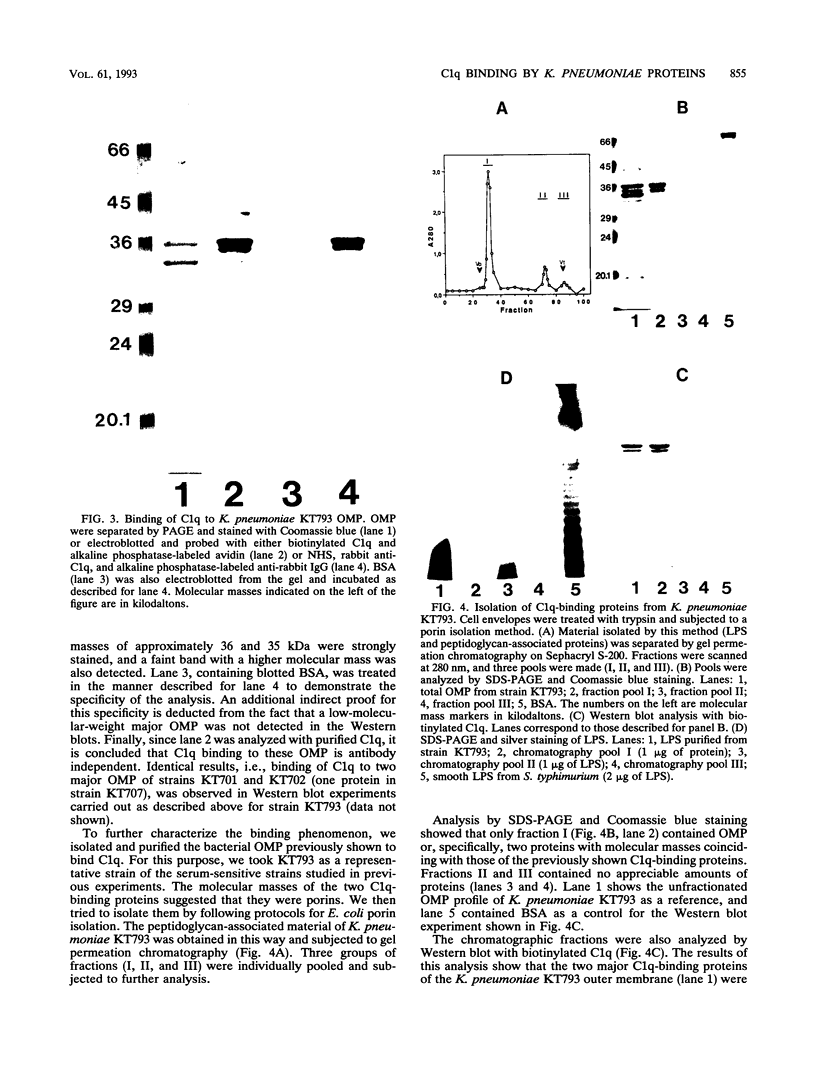
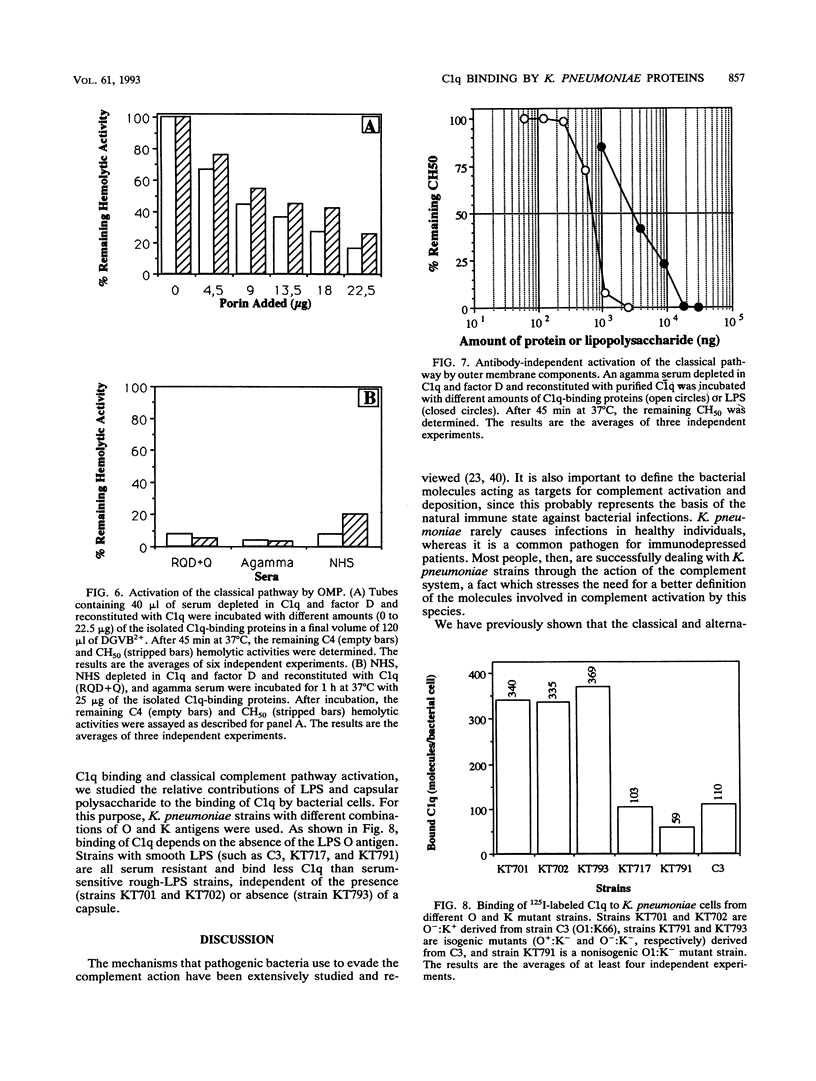
The mechanisms of killing of Klebsiella pneumoniae serum-sensitive strains in nonimmune serum by the complement classical pathway have been studied. The bacterial cell surface components that bind C1q more efficiently were identified as two major outer membrane proteins, presumably the porins of this bacterial species. These two outer membrane proteins were isolated from a representative serum-sensitive strain. We have demonstrated that in their purified form, they bind C1q and activate the classical pathway in an antibody-independent manner, with the subsequent consumption of C4 and reduction of the serum total hemolytic activity. Activation of the classical pathway has been observed in human nonimmune serum and agammaglobulinemic serum (both depleted in factor D). Binding of C1q to other components of the bacterial outer membrane, in particular the rough lipopolysaccharide, could not be demonstrated. Activation of the classical pathway by this lipopolysaccharide was also much less efficient than activation by the two outer membrane proteins. The antibody-independent binding of C1q to serum-sensitive strains was independent of the presence of capsular polysaccharide, while strains possessing lipopolysaccharide O antigen bind less C1q and are resistant to complement-mediated killing.
Full text
PDF





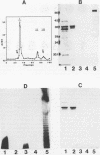
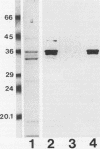
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcolea J. M., Antón L. C., Marqués G., Sánchez-Corral P., Vivanco F. Formation of covalent complexes between the fourth component of human complement and IgG immune aggregates. Complement. 1987;4(1):21–32. doi: 10.1159/000463004. [DOI] [PubMed] [Google Scholar]
- Antón L. C., Alcolea J. M., Sánchez-Corral P., Marqués G., Sánchez A., Vivanco F. C3 binds covalently to the C gamma 3 domain of IgG immune aggregates during complement activation by the alternative pathway. Biochem J. 1989 Feb 1;257(3):831–838. doi: 10.1042/bj2570831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert B., Chesne S., Arlaud G. J., Colomb M. G. Antibody-independent interaction between the first component of human complement, C1, and the outer membrane of Escherichia coli D31 m4. Biochem J. 1985 Dec 1;232(2):513–519. doi: 10.1042/bj2320513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio E., Antón L. C., Marqués G., Sánchez A., Vivanco F. Formation of covalently linked C3-C3 dimers on IgG immune aggregates. Eur J Immunol. 1991 Feb;21(2):343–349. doi: 10.1002/eji.1830210215. [DOI] [PubMed] [Google Scholar]
- Benedí V. J., Ciurana B., Tomás J. M. Isolation and characterization of Klebsiella pneumoniae unencapsulated mutants. J Clin Microbiol. 1989 Jan;27(1):82–87. doi: 10.1128/jcm.27.1.82-87.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benge G. R. Bactericidal activity of human serum against strains of Klebsiella from different sources. J Med Microbiol. 1988 Sep;27(1):11–15. doi: 10.1099/00222615-27-1-11. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Elting L. S., Rodriquez S., Hernandez M. Klebsiella bacteremia. A 10-year review in a cancer institution. Cancer. 1989 Dec 1;64(11):2368–2376. doi: 10.1002/1097-0142(19891201)64:11<2368::aid-cncr2820641129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Bryan C. S., Reynolds K. L., Brenner E. R. Analysis of 1,186 episodes of gram-negative bacteremia in non-university hospitals: the effects of antimicrobial therapy. Rev Infect Dis. 1983 Jul-Aug;5(4):629–638. doi: 10.1093/clinids/5.4.629. [DOI] [PubMed] [Google Scholar]
- Ciurana B., Tomás J. M. Role of lipopolysaccharide and complement in susceptibility of Klebsiella pneumoniae to nonimmune serum. Infect Immun. 1987 Nov;55(11):2741–2746. doi: 10.1128/iai.55.11.2741-2746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R., Morrison D. C. Binding and activation of the first component of human complement by the lipid A region of lipopolysaccharides. J Immunol. 1978 Jun;120(6):1862–1868. [PubMed] [Google Scholar]
- Cooper N. R. The classical complement pathway: activation and regulation of the first complement component. Adv Immunol. 1985;37:151–216. doi: 10.1016/s0065-2776(08)60340-5. [DOI] [PubMed] [Google Scholar]
- Figueroa J. E., Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991 Jul;4(3):359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaither T. A., Alling D. W., Frank M. M. A new one-step method for the functional assay of the fourth component (C4) of human and guinea pig complement. J Immunol. 1974 Aug;113(2):574–583. [PubMed] [Google Scholar]
- Galdiero F., Tufano M. A., Sommese L., Folgore A., Tedesco F. Activation of complement system by porins extracted from Salmonella typhimurium. Infect Immun. 1984 Nov;46(2):559–563. doi: 10.1128/iai.46.2.559-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García de la Torre M., Romero-Vivas J., Martínez-Beltrán J., Guerrero A., Meseguer M., Bouza E. Klebsiella bacteremia: an analysis of 100 episodes. Rev Infect Dis. 1985 Mar-Apr;7(2):143–150. doi: 10.1093/clinids/7.2.143. [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B., Bossone S., Erdei A., Reid K. B. Reversible biotinylation of C1q with a cleavable biotinyl derivative. Application in C1q receptor (C1qR) purification. J Immunol Methods. 1988 Jun 13;110(2):251–260. doi: 10.1016/0022-1759(88)90111-1. [DOI] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanteur D., Lakey J. H., Pattus F. The bacterial porin superfamily: sequence alignment and structure prediction. Mol Microbiol. 1991 Sep;5(9):2153–2164. doi: 10.1111/j.1365-2958.1991.tb02145.x. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Brown E. J., Frank M. M. Complement and bacteria: chemistry and biology in host defense. Annu Rev Immunol. 1984;2:461–491. doi: 10.1146/annurev.iy.02.040184.002333. [DOI] [PubMed] [Google Scholar]
- Joiner K. A. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- Kolb W. P., Kolb L. M., Podack E. R. C1q: isolation from human serum in high yield by affinity chromatography and development of a highly sensitive hemolytic assay. J Immunol. 1979 May;122(5):2103–2111. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Latsch M., Möllerfeld J., Ringsdorf H., Loos M. Studies on the interaction of C1q, a subcomponent of the first component of complement, with porins from Salmonella minnesota incorporated into artificial membranes. FEBS Lett. 1990 Dec 10;276(1-2):201–204. doi: 10.1016/0014-5793(90)80542-q. [DOI] [PubMed] [Google Scholar]
- Liberti P. A., Bausch D. M., Baillie R. D. The incorporation of high pH euglobulin precipitation in the isolation of C1q. J Immunol Methods. 1981;40(2):243–245. doi: 10.1016/0022-1759(81)90071-5. [DOI] [PubMed] [Google Scholar]
- Loos M., Bitter-Suermann D., Dierich M. Interaction of the first (C1), the second (C2) and the fourth (C4) component of complement with different preparations of bacterial lipopolysaccharides and with lipid A. J Immunol. 1974 Mar;112(3):935–940. [PubMed] [Google Scholar]
- Loos M., Clas F. Antibody-independent killing of gram-negative bacteria via the classical pathway of complement. Immunol Lett. 1987 Feb;14(3):203–208. doi: 10.1016/0165-2478(87)90102-7. [DOI] [PubMed] [Google Scholar]
- Loos M., Wellek B., Thesen R., Opferkuch W. Antibody-independent interaction of the first component of complement with Gram-negative bacteria. Infect Immun. 1978 Oct;22(1):5–9. doi: 10.1128/iai.22.1.5-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino S., Camprubí S., Albertí S., Benedí V. J., Tomás J. M. Mechanisms of Klebsiella pneumoniae resistance to complement-mediated killing. Infect Immun. 1992 Jun;60(6):2529–2535. doi: 10.1128/iai.60.6.2529-2535.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Nikaido H. Proteins forming large channels from bacterial and mitochondrial outer membranes: porins and phage lambda receptor protein. Methods Enzymol. 1983;97:85–100. doi: 10.1016/0076-6879(83)97122-7. [DOI] [PubMed] [Google Scholar]
- Pluschke G., Mayden J., Achtman M., Levine R. P. Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing. Infect Immun. 1983 Dec;42(3):907–913. doi: 10.1128/iai.42.3.907-913.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts I. S., Saunders F. K., Boulnois G. J. Bacterial capsules and interactions with complement and phagocytes. Biochem Soc Trans. 1989 Jun;17(3):462–464. doi: 10.1042/bst0170462. [DOI] [PubMed] [Google Scholar]
- Stemmer F., Loos M. Evidence for direct binding of the first component of complement, C1, to outer membrane proteins from Salmonella minnesota. Curr Top Microbiol Immunol. 1985;121:73–84. doi: 10.1007/978-3-642-45604-6_4. [DOI] [PubMed] [Google Scholar]
- Sánchez-Corral P., Antón L. C., Alcolea J. M., Marqués G., Sánchez A., Vivanco F. Proteolytic activity of the different fragments of factor B on the third component of complement (C3). Involvement of the N-terminal domain of Bb in magnesium binding. Mol Immunol. 1990 Sep;27(9):891–900. doi: 10.1016/0161-5890(90)90156-t. [DOI] [PubMed] [Google Scholar]
- Sánchez-Corral P., Antón L. C., Alcolea J. M., Marqués G., Sánchez A., Vivanco F. Separation of active and inactive forms of the third component of human complement, C3, by fast protein liquid chromatography (FPLC). J Immunol Methods. 1989 Aug 15;122(1):105–113. doi: 10.1016/0022-1759(89)90340-2. [DOI] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenner A. J., Lesavre P. H., Cooper N. R. Purification and radiolabeling of human C1q. J Immunol. 1981 Aug;127(2):648–653. [PubMed] [Google Scholar]
- Tomás J. M., Benedí V. J., Ciurana B., Jofre J. Role of capsule and O antigen in resistance of Klebsiella pneumoniae to serum bactericidal activity. Infect Immun. 1986 Oct;54(1):85–89. doi: 10.1128/iai.54.1.85-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Tsai C. M. The analysis of lipopolysaccharide (endotoxin) in meningococcal polysaccharide vaccines by silver staining following SDS-polyacrylamide gel electrophoresis. J Biol Stand. 1986 Jan;14(1):25–33. doi: 10.1016/s0092-1157(86)80006-3. [DOI] [PubMed] [Google Scholar]
- Vivanco-Martínez F., Bragado R., Albar J. P., Juarez C., Ortíz-Masllorens F. Chemical modification of carboxyl groups in human Fc gamma fragments: structural role and effect on the complement fixation. Mol Immunol. 1980 Mar;17(3):327–336. doi: 10.1016/0161-5890(80)90053-x. [DOI] [PubMed] [Google Scholar]
- Vukajlovich S. W., Hoffman J., Morrison D. C. Activation of human serum complement by bacterial lipopolysaccharides: structural requirements for antibody independent activation of the classical and alternative pathways. Mol Immunol. 1987 Apr;24(4):319–331. doi: 10.1016/0161-5890(87)90173-8. [DOI] [PubMed] [Google Scholar]
- Williams P., Lambert P. A., Brown M. R., Jones R. J. The role of the O and K antigens in determining the resistance of Klebsiella aerogenes to serum killing and phagocytosis. J Gen Microbiol. 1983 Jul;129(7):2181–2191. doi: 10.1099/00221287-129-7-2181. [DOI] [PubMed] [Google Scholar]
- Yin E. T., Galanos C., Kinsky S., Bradshaw R. A., Wessler S., Lüderitz O., Sarmiento M. E. Picogram-sensitive assay for endotoxin: gelation of Limulus polyphemus blood cell lysate induced by purified lipopolysaccharides and lipid A from Gram-negative bacteria. Biochim Biophys Acta. 1972 Jan 28;261(1):284–289. doi: 10.1016/0304-4165(72)90340-6. [DOI] [PubMed] [Google Scholar]