Abstract
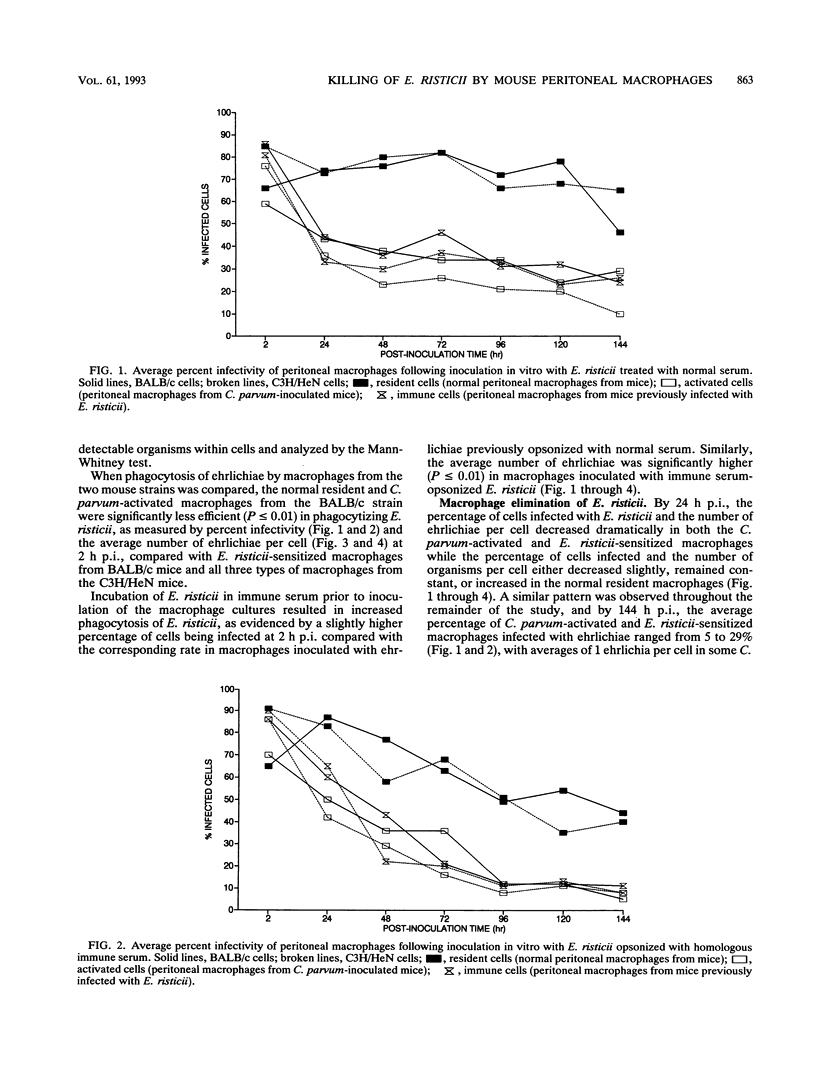
Normal resident murine peritoneal macrophages inoculated in vitro with Ehrlichia risticii readily phagocytized the organism but were unable to suppress ehrlichial replication as determined by indirect fluorescent-antibody staining of the inoculated cells. In contrast, macrophages from Corynebacterium parvum-inoculated and E. risticii-recovered mice rapidly eliminated the ehrlichiae. Macrophages from E. risticii-recovered mice were as effective as the C. parvum-activated cells in phagocytizing and eliminating the organism. Opsonization of E. risticii with homologous antiserum prior to inoculation of macrophage cultures resulted in enhancement of phagocytosis and greater suppression of E. risticii replication in all macrophage groups. These findings indicate that the pathogenesis of E. risticii infection centers on the ability of the organism to enter and replicate within the macrophage with avoidance of macrophage antimicrobial effects. An immune response results in macrophage activation with enhancement of the macrophage's ability to eliminate E. risticii. Opsonization of E. risticii with anti-E. risticii serum renders E. risticii more susceptible to macrophage destruction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. VI. Differential opsonizing and neutralizing action of human typhus rickettsia-specific cytophilic antibodies in cultures of human macrophages. Infect Immun. 1976 Oct;14(4):1071–1076. doi: 10.1128/iai.14.4.1071-1076.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Inhibition of Chlamydia psittaci in oxidatively active thioglycolate-elicited macrophages: distinction between lymphokine-mediated oxygen-dependent and oxygen-independent macrophage activation. Infect Immun. 1983 May;40(2):464–471. doi: 10.1128/iai.40.2.464-471.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science. 1976 Aug 20;193(4254):678–680. doi: 10.1126/science.948742. [DOI] [PubMed] [Google Scholar]
- Coombs G. H. Proteinases of Leishmania mexicana and other flagellate protozoa. Parasitology. 1982 Feb;84(1):149–155. doi: 10.1017/s003118200005174x. [DOI] [PubMed] [Google Scholar]
- Dawson J. E., Abeygunawardena I., Holland C. J., Buese M. M., Ristic M. Susceptibility of cats to infection with Ehrlichia risticii, causative agent of equine monocytic ehrlichiosis. Am J Vet Res. 1988 Dec;49(12):2096–2100. [PubMed] [Google Scholar]
- Eissenberg L. G., Goldman W. E. Histoplasma capsulatum fails to trigger release of superoxide from macrophages. Infect Immun. 1987 Jan;55(1):29–34. doi: 10.1128/iai.55.1.29-34.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-On J., Bradley D. J., Freeman J. C. Leishmania donovani: action of excreted factor on hydrolytic enzyme activity of macrophages from mice with genetically different resistance to infection. Exp Parasitol. 1980 Apr;49(2):167–174. doi: 10.1016/0014-4894(80)90114-9. [DOI] [PubMed] [Google Scholar]
- FOX J. P. The long persistence of Rickettsia orientalis in the blood and tissues of infected animals. J Immunol. 1948 Jun;59(2):109–114. [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci U S A. 1981 May;78(5):3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschlag M. R., Suntharalingam K., Fikrig S. The effect of Chlamydia trachomatis on luminol-dependent chemiluminescence of human polymorphonuclear leukocytes: requirements for opsonization. J Infect Dis. 1985 Jun;151(6):1045–1051. doi: 10.1093/infdis/151.6.1045. [DOI] [PubMed] [Google Scholar]
- Holland C. J., Ristic M., Cole A. I., Johnson P., Baker G., Goetz T. Isolation, experimental transmission, and characterization of causative agent of Potomac horse fever. Science. 1985 Feb 1;227(4686):522–524. doi: 10.1126/science.3880925. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Maxfield F. R. Legionella pneumophila inhibits acidification of its phagosome in human monocytes. J Cell Biol. 1984 Dec;99(6):1936–1943. doi: 10.1083/jcb.99.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Yeh S., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. I. Mechanism of entry and intracellular fate of the parasite. J Exp Med. 1972 Nov 1;136(5):1157–1172. doi: 10.1084/jem.136.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. M., Mohanakumar T. Expression of a new cell surface antigen on activated murine macrophages. J Exp Med. 1977 Nov 1;146(5):1461–1466. doi: 10.1084/jem.146.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. M. Membrane damage by complement. Johns Hopkins Med J. 1981 Jun;148(6):243–258. [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S. Macrophages in resistance to rickettsial infection: macrophage activation in vitro for killing of Rickettsia tsutsugamushi. J Immunol. 1979 Dec;123(6):2544–2549. [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976 Jun 1;143(6):1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Rikihisa Y. Inhibition of Ehrlichia risticii infection in murine peritoneal macrophages by gamma interferon, a calcium ionophore, and concanavalin A. Infect Immun. 1991 Oct;59(10):3418–3423. doi: 10.1128/iai.59.10.3418-3423.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Calderone R. A. Inhibition of specific amino acid uptake in Candida albicans by lysosomal extracts from rabbit alveolar macrophages. Infect Immun. 1978 Aug;21(2):506–513. doi: 10.1128/iai.21.2.506-513.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: tumoricidal activity by macrophages from C3H/HeJ mice requires at least two activation stimuli. Cell Immunol. 1978 Nov;41(1):35–51. doi: 10.1016/s0008-8749(78)80026-4. [DOI] [PubMed] [Google Scholar]
- Scribner D. J., Fahrney D. Neutrophil receptors for IgG and complement: their roles in the attachment and ingestion phases of phagocytosis. J Immunol. 1976 Apr;116(4):892–897. [PubMed] [Google Scholar]
- Sibley L. D., Franzblau S. G., Krahenbuhl J. L. Intracellular fate of Mycobacterium leprae in normal and activated mouse macrophages. Infect Immun. 1987 Mar;55(3):680–685. doi: 10.1128/iai.55.3.680-685.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells M. Y., Rikihisa Y. Lack of lysosomal fusion with phagosomes containing Ehrlichia risticii in P388D1 cells: abrogation of inhibition with oxytetracycline. Infect Immun. 1988 Dec;56(12):3209–3215. doi: 10.1128/iai.56.12.3209-3215.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Levine R. P. How complement kills E. coli. I. Location of the lethal lesion. J Immunol. 1981 Sep;127(3):1146–1151. [PubMed] [Google Scholar]
- Wyrick P. B., Brownridge E. A., Ivins B. E. Interaction of Chlamydia psittaci with mouse peritoneal macrophages. Infect Immun. 1978 Mar;19(3):1061–1067. doi: 10.1128/iai.19.3.1061-1067.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



