Abstract
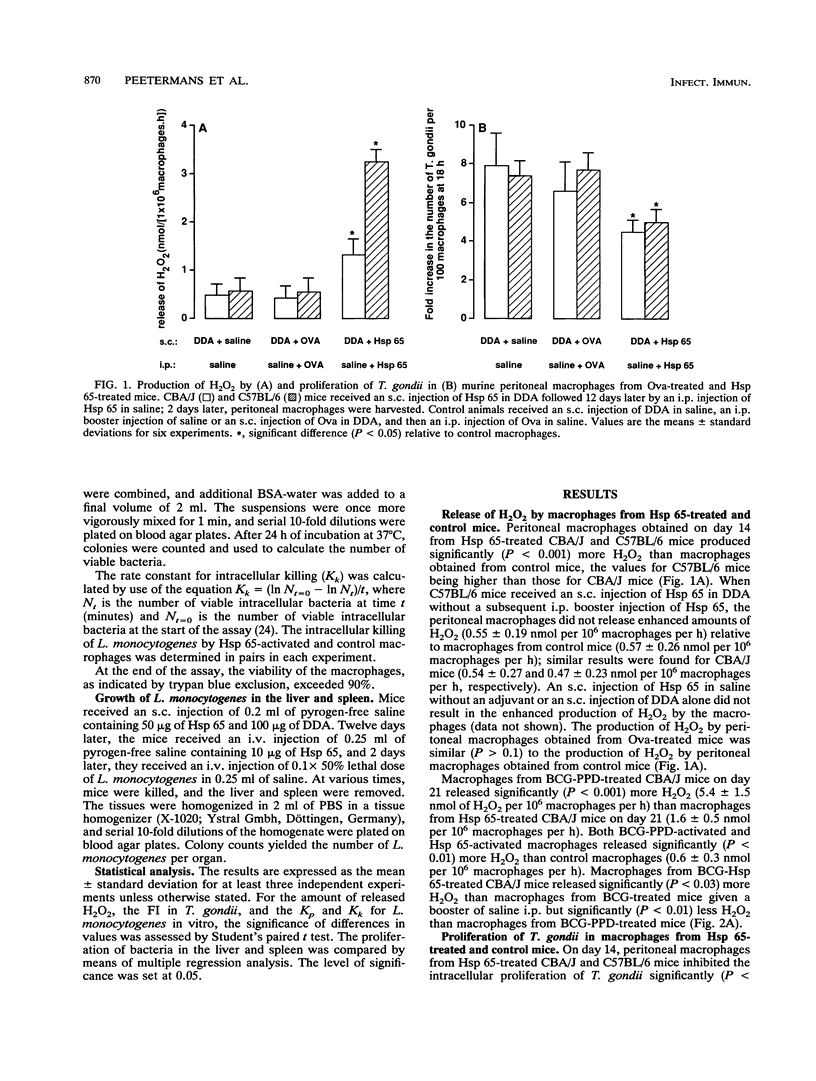
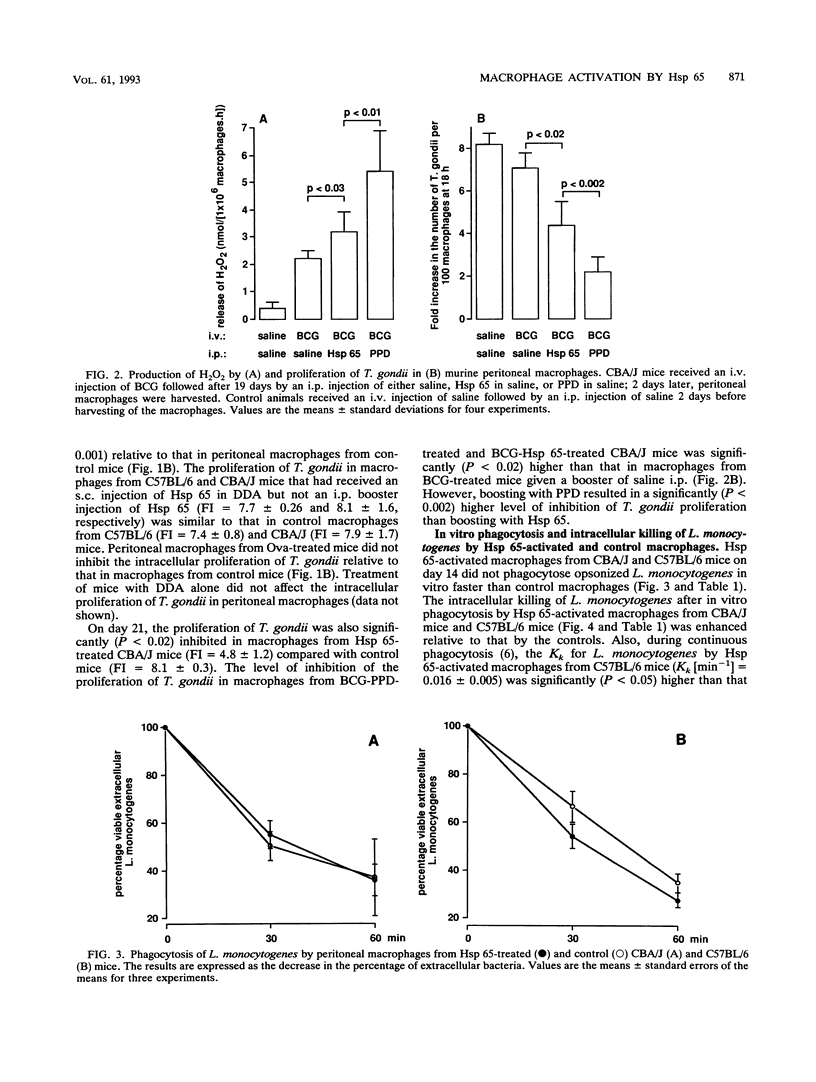
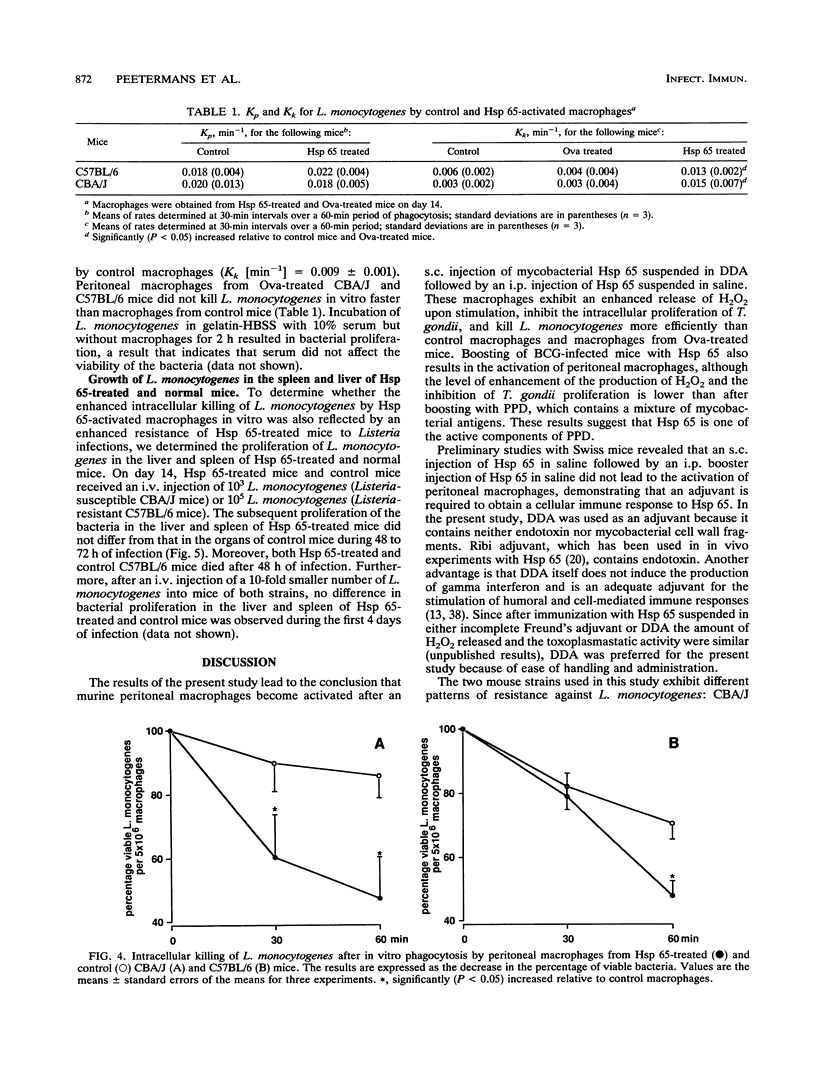
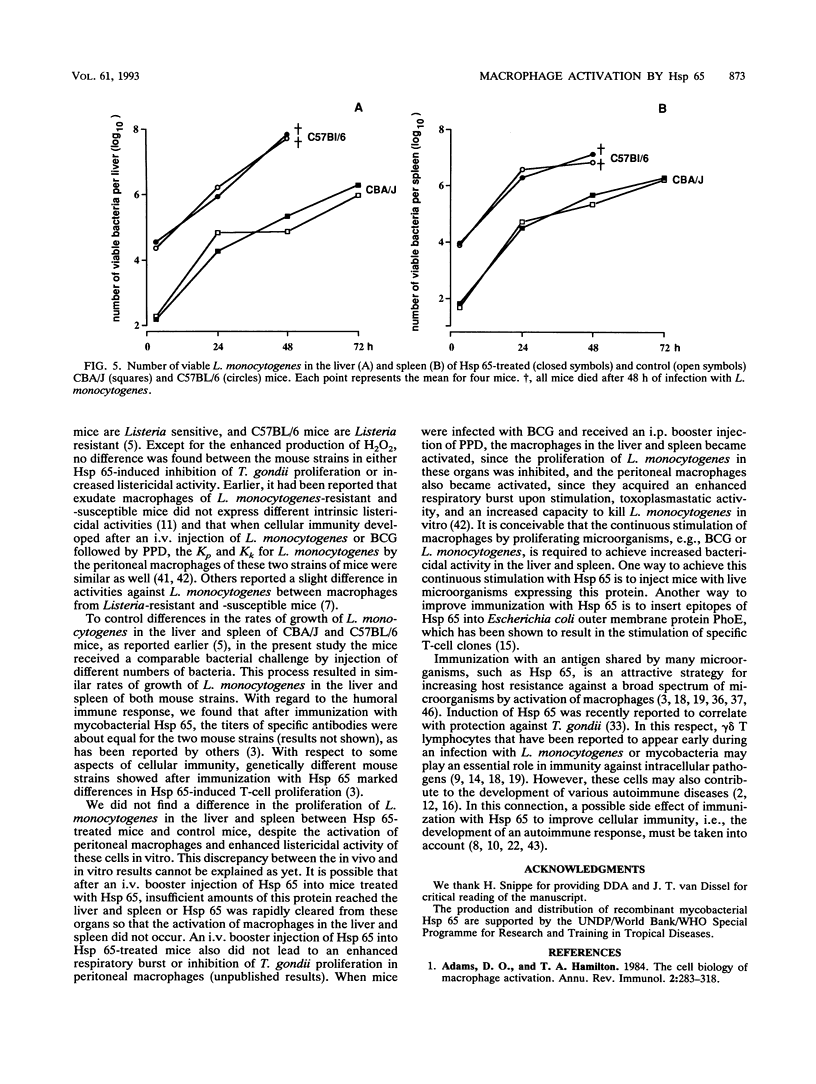
After an intraperitoneal (i.p.) injection of purified protein derivative, peritoneal macrophages from mice infected with Mycobacterium bovis bacillus Calmette-Guérin (BCG) show an enhanced respiratory burst, inhibit the intracellular proliferation of Toxoplasma gondii, and kill Listeria monocytogenes more efficiently than peritoneal macrophages from normal mice. One of the immunodominant antigens of Mycobacterium spp. is the 65-kDa heat shock protein (Hsp 65), and in the present study, we determined whether injection of this protein into mice leads to activation of their peritoneal macrophages. After an i.p. injection of Hsp 65, peritoneal macrophages from BCG-infected CBA/J mice also released more H2O2, inhibited the proliferation of T. gondii, and killed L. monocytogenes faster than peritoneal macrophages from normal mice, although Hsp 65 was less effective than purified protein derivative. When normal mice were injected with Hsp 65 suspended in saline after a booster injection with Hsp 65, their macrophages did not display enhanced antimicrobial activity, indicating that an adjuvant was required for a cellular immune response against Hsp 65. In the present study, the adjuvant dimethyl dioctadecylammonium bromide (DDA) was preferred because it contains no endotoxin or mycobacterial antigens and because it has been reported that DDA does not induce the production of gamma interferon. Peritoneal macrophages from C57BL/6 and CBA/J mice that had received a subcutaneous injection of Hsp 65 suspended in DDA followed by an i.p. booster injection of Hsp 65 suspended in saline were activated, as indicated by the enhanced production of H2O2, inhibition of the intracellular proliferation of T. gondii, and increased rate of intracellular killing of L. monocytogenes in vitro relative to that by resident peritoneal macrophages and peritoneal macrophages obtained from mice that had received ovalbumin instead of Hsp 65. The rate of phagocytosis of L. monocytogenes was not affected by Hsp 65 treatment. Despite the in vitro expression of enhanced microbicidal activity of peritoneal macrophages, no difference in the growth of L. monocytogenes in the liver and spleen between Hsp 65-treated and control mice was found.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Born W., Happ M. P., Dallas A., Reardon C., Kubo R., Shinnick T., Brennan P., O'Brien R. Recognition of heat shock proteins and gamma delta cell function. Immunol Today. 1990 Feb;11(2):40–43. doi: 10.1016/0167-5699(90)90015-2. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Lamb J. R., Cox J. H., Rothbard J. B., Mehlert A., Ivanyi J. Differential pattern of T cell recognition of the 65-kDa mycobacterial antigen following immunization with the whole protein or peptides. Eur J Immunol. 1989 Jul;19(7):1303–1310. doi: 10.1002/eji.1830190723. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Interactions between rabbit polymorphonuclear leucocytes and staphylococci. J Exp Med. 1959 Sep 1;110:419–443. doi: 10.1084/jem.110.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. doi: 10.1128/iai.19.3.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Canono B. P., Henson P. M., Campbell P. A. Genetically determined resistance to listeriosis is associated with increased accumulation of inflammatory neutrophils and macrophages which have enhanced listericidal activity. Immunology. 1985 Jul;55(3):511–518. [PMC free article] [PubMed] [Google Scholar]
- Elias D., Markovits D., Reshef T., van der Zee R., Cohen I. R. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1576–1580. doi: 10.1073/pnas.87.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follows G. A., Munk M. E., Gatrill A. J., Conradt P., Kaufmann S. H. Gamma interferon and interleukin 2, but not interleukin 4, are detectable in gamma/delta T-cell cultures after activation with bacteria. Infect Immun. 1992 Mar;60(3):1229–1231. doi: 10.1128/iai.60.3.1229-1231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston J. S., Life P. F., Jenner P. J., Colston M. J., Bacon P. A. Recognition of a mycobacteria-specific epitope in the 65-kD heat-shock protein by synovial fluid-derived T cell clones. J Exp Med. 1990 Mar 1;171(3):831–841. doi: 10.1084/jem.171.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais F., Morris-Hooke A., Tran T. A., Skamene E. Analysis of macrophage bactericidal function in genetically resistant and susceptible mice by using the temperature-sensitive mutant of Listeria monocytogenes. Infect Immun. 1986 Nov;54(2):315–321. doi: 10.1128/iai.54.2.315-321.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haregewoin A., Singh B., Gupta R. S., Finberg R. W. A mycobacterial heat-shock protein-responsive gamma delta T cell clone also responds to the homologous human heat-shock protein: a possible link between infection and autoimmunity. J Infect Dis. 1991 Jan;163(1):156–160. doi: 10.1093/infdis/163.1.156. [DOI] [PubMed] [Google Scholar]
- Hiromatsu K., Yoshikai Y., Matsuzaki G., Ohga S., Muramori K., Matsumoto K., Bluestone J. A., Nomoto K. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992 Jan 1;175(1):49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E. J., Agterberg M., Wagenaar J. P., Adriaanse H., Boog C. J., Van De Zee R., Van Embden J. D., Van Eden W., Tommassen J. Efficient recognition by rat T cell clones of an epitope of mycobacterial hsp 65 inserted in Escherichia coli outer membrane protein PhoE. Eur J Immunol. 1990 Dec;20(12):2763–2768. doi: 10.1002/eji.1830201234. [DOI] [PubMed] [Google Scholar]
- Inoue T., Yoshikai Y., Matsuzaki G., Nomoto K. Early appearing gamma/delta-bearing T cells during infection with Calmétte Guérin bacillus. J Immunol. 1991 Apr 15;146(8):2754–2762. [PubMed] [Google Scholar]
- Karnovsky M. L., Lazdins J. K. Biochemical criteria for activated macrophages. J Immunol. 1978 Sep;121(3):809–813. [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity against intracellular bacteria: biological effector functions and antigen specificity of T lymphocytes. Curr Top Microbiol Immunol. 1988;138:141–176. [PubMed] [Google Scholar]
- Kaufmann S. H., Schoel B., van Embden J. D., Koga T., Wand-Württenberger A., Munk M. E., Steinhoff U. Heat-shock protein 60: implications for pathogenesis of and protection against bacterial infections. Immunol Rev. 1991 Jun;121:67–90. doi: 10.1111/j.1600-065x.1991.tb00823.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Väth U., Thole J. E., Van Embden J. D., Emmrich F. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur J Immunol. 1987 Mar;17(3):351–357. doi: 10.1002/eji.1830170308. [DOI] [PubMed] [Google Scholar]
- Koga T., Wand-Württenberger A., DeBruyn J., Munk M. E., Schoel B., Kaufmann S. H. T cells against a bacterial heat shock protein recognize stressed macrophages. Science. 1989 Sep 8;245(4922):1112–1115. doi: 10.1126/science.2788923. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Bal V., Mendez-Samperio P., Mehlert A., So A., Rothbard J., Jindal S., Young R. A., Young D. B. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989;1(2):191–196. doi: 10.1093/intimm/1.2.191. [DOI] [PubMed] [Google Scholar]
- Langermans J. A., Nibbering P. H., van der Hulst M. E., van Furth R. Microbicidal activities of Salmonella typhimurium- and interferon-gamma-activated mouse peritoneal macrophages. Pathobiology. 1991;59(3):189–193. doi: 10.1159/000163642. [DOI] [PubMed] [Google Scholar]
- Langermans J. A., van der Hulst M. E., Nibbering P. H., van Furth R. Activation of mouse peritoneal macrophages during infection with Salmonella typhimurium does not result in enhanced intracellular killing. J Immunol. 1990 Jun 1;144(11):4340–4346. [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. W., Christman M. F., Jacobson F. S., Storz G., Ames B. N. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Interferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann Intern Med. 1988 Apr;108(4):595–608. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S., Leonard E. J., Wyler D. J. Intracellular replication and lymphokine-induced destruction of Leishmania tropica in C3H/HeN mouse macrophages. J Immunol. 1981 Dec;127(6):2381–2386. [PubMed] [Google Scholar]
- Nagasawa H., Oka M., Maeda K., Jian-Guo C., Hisaeda H., Ito Y., Good R. A., Himeno K. Induction of heat shock protein closely correlates with protection against Toxoplasma gondii infection. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3155–3158. doi: 10.1073/pnas.89.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbering P. H., Langermans J. A., van de Gevel J. S., van der Hulst M. B., van Furth R. Nitrite production by activated murine macrophages correlates with their toxoplasmastatic activity, Ia antigen expression, and production of H2O2. Immunobiology. 1991 Dec;184(1):93–105. doi: 10.1016/S0171-2985(11)80575-9. [DOI] [PubMed] [Google Scholar]
- Ruch W., Cooper P. H., Baggiolini M. Assay of H2O2 production by macrophages and neutrophils with homovanillic acid and horse-radish peroxidase. J Immunol Methods. 1983 Oct 28;63(3):347–357. doi: 10.1016/s0022-1759(83)80008-8. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M. Heat shock proteins as antigens of bacterial and parasitic pathogens. Curr Top Microbiol Immunol. 1991;167:145–160. doi: 10.1007/978-3-642-75875-1_9. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Sweetser D., Thole J., van Embden J., Young R. A. The etiologic agents of leprosy and tuberculosis share an immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect Immun. 1987 Aug;55(8):1932–1935. doi: 10.1128/iai.55.8.1932-1935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippe H., Belder M., Willers J. M. Dimethyl diotadecyl ammonium bromide as adjuvant for delayed hypersensitivity in mice. Immunology. 1977 Dec;33(6):931–936. [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Dauwerse H. G., Das P. K., Groothuis D. G., Schouls L. M., van Embden J. D. Cloning of Mycobacterium bovis BCG DNA and expression of antigens in Escherichia coli. Infect Immun. 1985 Dec;50(3):800–806. doi: 10.1128/iai.50.3.800-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A. Stress proteins and immunology. Annu Rev Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]
- van Dissel J. T., Leijh P. C., van Furth R. Differences in initial rate of intracellular killing of Salmonella typhimurium by resident peritoneal macrophages from various mouse strains. J Immunol. 1985 May;134(5):3404–3410. [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., van den Barselaar M. T., Sluiter W., Leijh P. C., van Furth R. Divergent changes in antimicrobial activity after immunologic activation of mouse peritoneal macrophages. J Immunol. 1987 Sep 1;139(5):1665–1672. [PubMed] [Google Scholar]
- van Eden W. Heat-shock proteins as immunogenic bacterial antigens with the potential to induce and regulate autoimmune arthritis. Immunol Rev. 1991 Jun;121:5–28. doi: 10.1111/j.1600-065x.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]



