Abstract
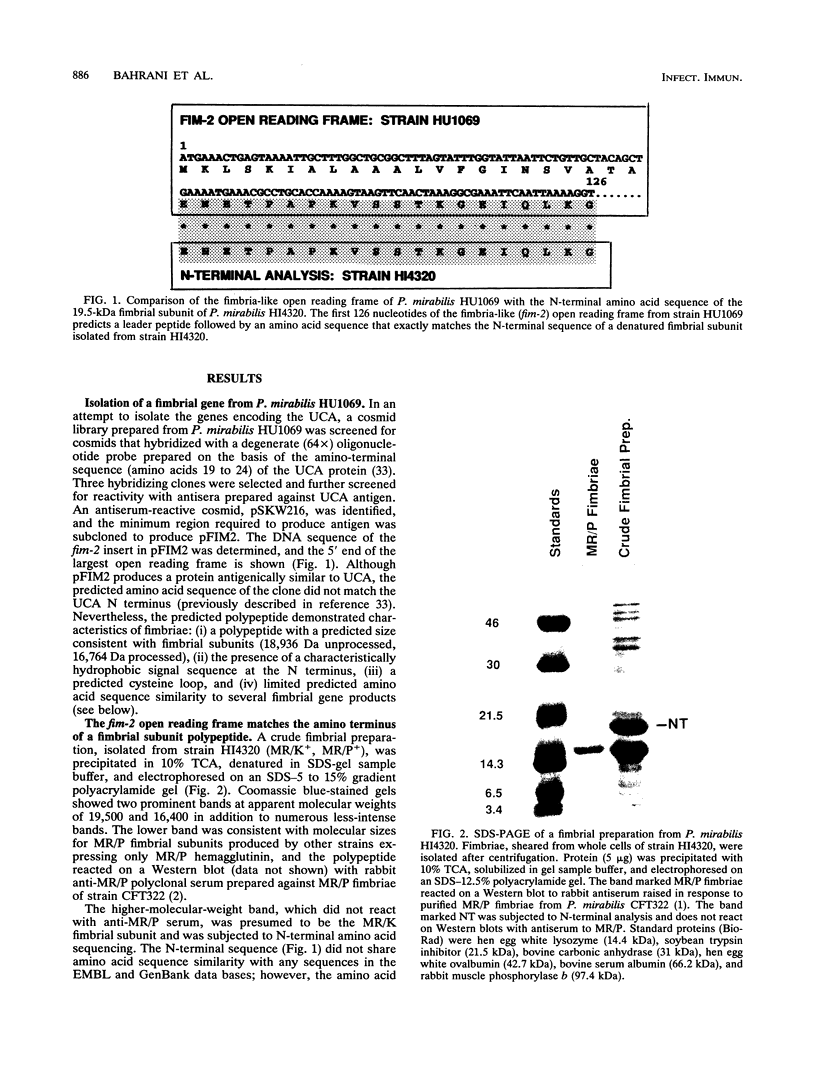
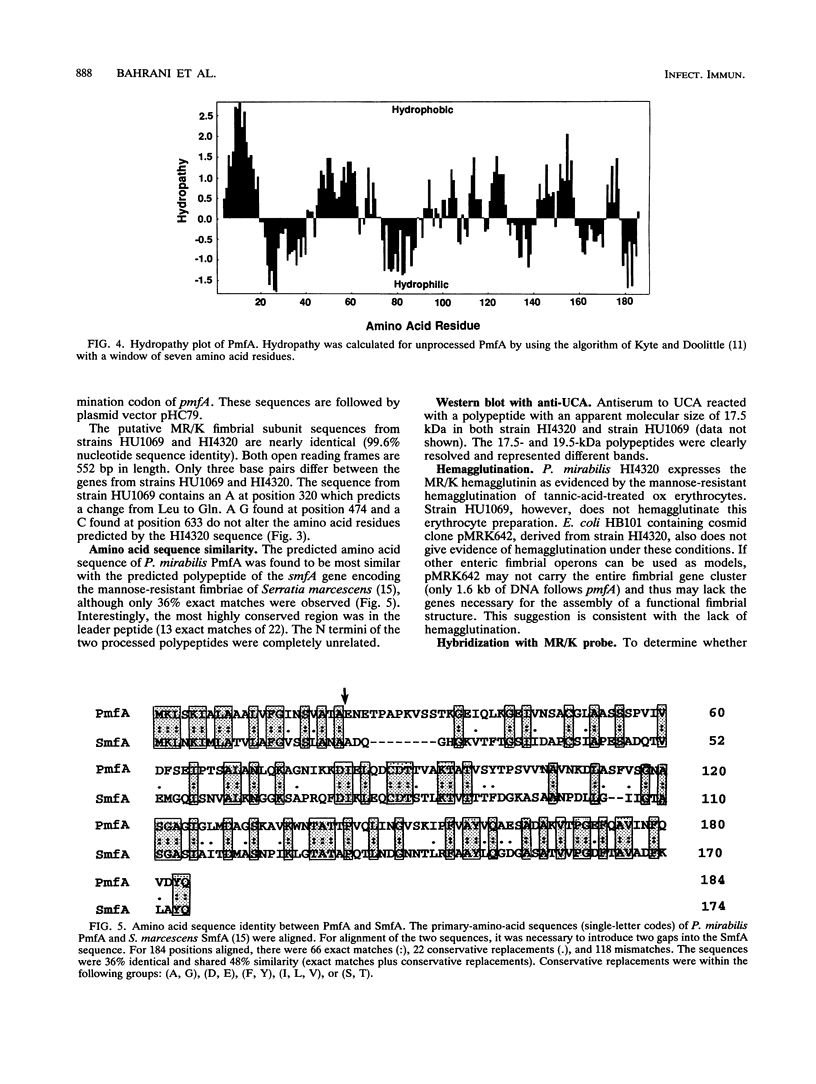
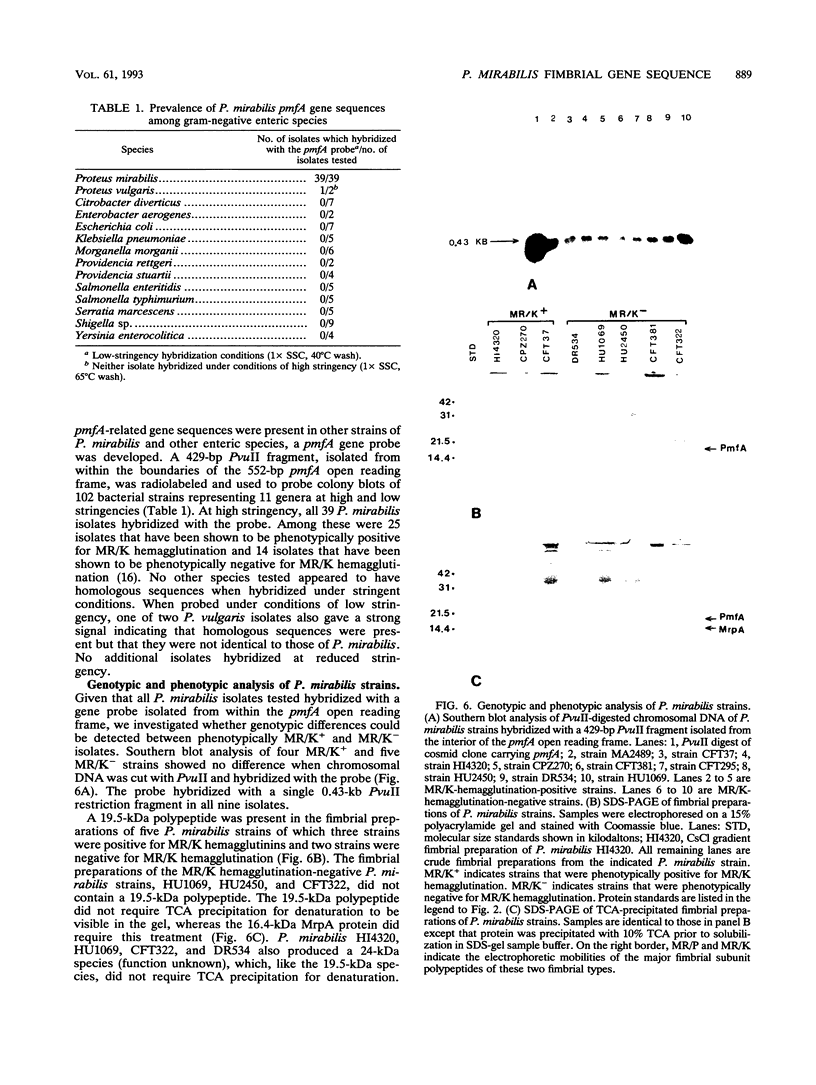
Proteus mirabilis, a common cause of urinary tract infection in hospitalized and catheterized patients, produces mannose-resistant/klebsiella-like (MR/K) and mannose-resistant/proteus-like (MR/P) hemagglutinins. The gene encoding the major structural subunit of a fimbria, possibly MR/K, was identified in two strains. A degenerate oligonucleotide probe based on the N terminus of the Proteus uroepithelial cell adhesin and antiserum raised against the denatured polypeptide were used to screen a cosmid gene bank of strain HU1069. A cosmid clone that reacted with the probe and antiserum was identified, and a fimbria-like open reading frame was determined by nucleotide sequencing. The predicted N-terminal amino acid sequence of the processed polypeptide, ENETPAPKVSSTKGEIQLKG (residues 23 to 42), did not match the uroepithelial cell adhesin N terminus but, rather, matched exactly the N-terminal amino acid sequence of a polypeptide with an apparent molecular size of 19.5 kDa isolated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of a fimbrial preparation from strain HI4320 expressing MR/K hemagglutinin. By using an oligonucleotide from the HU1069 open reading frame, the fimbrial gene was isolated and sequenced from a cosmid gene bank clone of strain HI4320. A 552-bp open reading frame predicts a 184-amino-acid polypeptide including a 22-amino-acid hydrophobic leader sequence. The unprocessed polypeptide is predicted to be 18,921 Da; the processed polypeptide is predicted to be 16,749 Da. The predicted amino acid sequence of the polypeptide encoded by the gene, designated pmfA, displayed 36% exact matches with the mannose-resistant fimbrial subunit encoded by smfA of Serratia marcescens but only 15% exact matches with the predicted sequence encoded by mrkA of Klebsiella pneumoniae.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahrani F. K., Johnson D. E., Robbins D., Mobley H. L. Proteus mirabilis flagella and MR/P fimbriae: isolation, purification, N-terminal analysis, and serum antibody response following experimental urinary tract infection. Infect Immun. 1991 Oct;59(10):3574–3580. doi: 10.1128/iai.59.10.3574-3580.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrani F. K., Mobley H. L. Proteus mirabilis MR/P fimbriae: molecular cloning, expression, and nucleotide sequence of the major fimbrial subunit gene. J Bacteriol. 1993 Jan;175(2):457–464. doi: 10.1128/jb.175.2.457-464.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Larsson P., Lomberg H. Attachment of Proteus mirabilis to human urinary sediment epithelial cells in vitro is different from that of Escherichia coli. Infect Immun. 1980 Mar;27(3):804–807. doi: 10.1128/iai.27.3.804-807.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairley K. F., Bond A. G., Brown R. B., Habersberger P. Simple test to determine the site of urinary-tract infection. Lancet. 1967 Aug 26;2(7513):427–428. doi: 10.1016/s0140-6736(67)90849-5. [DOI] [PubMed] [Google Scholar]
- Gerlach G. F., Allen B. L., Clegg S. Molecular characterization of the type 3 (MR/K) fimbriae of Klebsiella pneumoniae. J Bacteriol. 1988 Aug;170(8):3547–3553. doi: 10.1128/jb.170.8.3547-3553.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach G. F., Clegg S., Allen B. L. Identification and characterization of the genes encoding the type 3 and type 1 fimbrial adhesins of Klebsiella pneumoniae. J Bacteriol. 1989 Mar;171(3):1262–1270. doi: 10.1128/jb.171.3.1262-1270.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976 Mar;13(5):346–350. [PubMed] [Google Scholar]
- Korhonen T. K., Tarkka E., Ranta H., Haahtela K. Type 3 fimbriae of Klebsiella sp.: molecular characterization and role in bacterial adhesion to plant roots. J Bacteriol. 1983 Aug;155(2):860–865. doi: 10.1128/jb.155.2.860-865.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mizunoe Y., Nakabeppu Y., Sekiguchi M., Kawabata S., Moriya T., Amako K. Cloning and sequence of the gene encoding the major structural component of mannose-resistant fimbriae of Serratia marcescens. J Bacteriol. 1988 Aug;170(8):3567–3574. doi: 10.1128/jb.170.8.3567-3574.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R. Hemagglutinin, urease, and hemolysin production by Proteus mirabilis from clinical sources. J Infect Dis. 1990 Mar;161(3):525–530. doi: 10.1093/infdis/161.3.525. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Chippendale G. R., Tenney J. H., Mayrer A. R., Crisp L. J., Penner J. L., Warren J. W. MR/K hemagglutination of Providencia stuartii correlates with adherence to catheters and with persistence in catheter-associated bacteriuria. J Infect Dis. 1988 Feb;157(2):264–271. doi: 10.1093/infdis/157.2.264. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Warren J. W. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987 Nov;25(11):2216–2217. doi: 10.1128/jcm.25.11.2216-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Adegbola R. A. Antigenic relationships among type-3 fimbriae of Enterobacteriaceae revealed by immunoelectronmicroscopy. J Med Microbiol. 1985 Aug;20(1):113–121. doi: 10.1099/00222615-20-1-113. [DOI] [PubMed] [Google Scholar]
- Old D. C., Adegbola R. A. Haemagglutinins and fimbriae of Morganella, Proteus and Providencia. J Med Microbiol. 1982 Nov;15(4):551–564. doi: 10.1099/00222615-15-4-551. [DOI] [PubMed] [Google Scholar]
- Sareneva T., Holthöfer H., Korhonen T. K. Tissue-binding affinity of Proteus mirabilis fimbriae in the human urinary tract. Infect Immun. 1990 Oct;58(10):3330–3336. doi: 10.1128/iai.58.10.3330-3336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoia D., Martinetto P., Achino A., Pugliese A. Adhesion of Proteus species to various cell types. Eur J Clin Microbiol. 1983 Dec;2(6):571–576. doi: 10.1007/BF02016568. [DOI] [PubMed] [Google Scholar]
- Senior B. W. The special affinity of particular types of Proteus mirabilis for the urinary tract. J Med Microbiol. 1979 Feb;12(1):1–8. doi: 10.1099/00222615-12-1-1. [DOI] [PubMed] [Google Scholar]
- Setia U., Serventi I., Lorenz P. Bacteremia in a long-term care facility. Spectrum and mortality. Arch Intern Med. 1984 Aug;144(8):1633–1635. [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Ofek I. Influence of pili on the virulence of Proteus mirabilis in experimental hematogenous pyelonephritis. J Infect Dis. 1978 Nov;138(5):664–667. doi: 10.1093/infdis/138.5.664. [DOI] [PubMed] [Google Scholar]
- Smeets F., Gower P. E. The site of infection in 133 patients with bacteriuria. Clin Nephrol. 1973 Sep-Oct;1(5):290–296. [PubMed] [Google Scholar]
- Tarkkanen A. M., Allen B. L., Westerlund B., Holthöfer H., Kuusela P., Risteli L., Clegg S., Korhonen T. K. Type V collagen as the target for type-3 fimbriae, enterobacterial adherence organelles. Mol Microbiol. 1990 Aug;4(8):1353–1361. doi: 10.1111/j.1365-2958.1990.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. W., Tenney J. H., Hoopes J. M., Muncie H. L., Anthony W. C. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982 Dec;146(6):719–723. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]
- Wray S. K., Hull S. I., Cook R. G., Barrish J., Hull R. A. Identification and characterization of a uroepithelial cell adhesin from a uropathogenic isolate of Proteus mirabilis. Infect Immun. 1986 Oct;54(1):43–49. doi: 10.1128/iai.54.1.43-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]





