Abstract
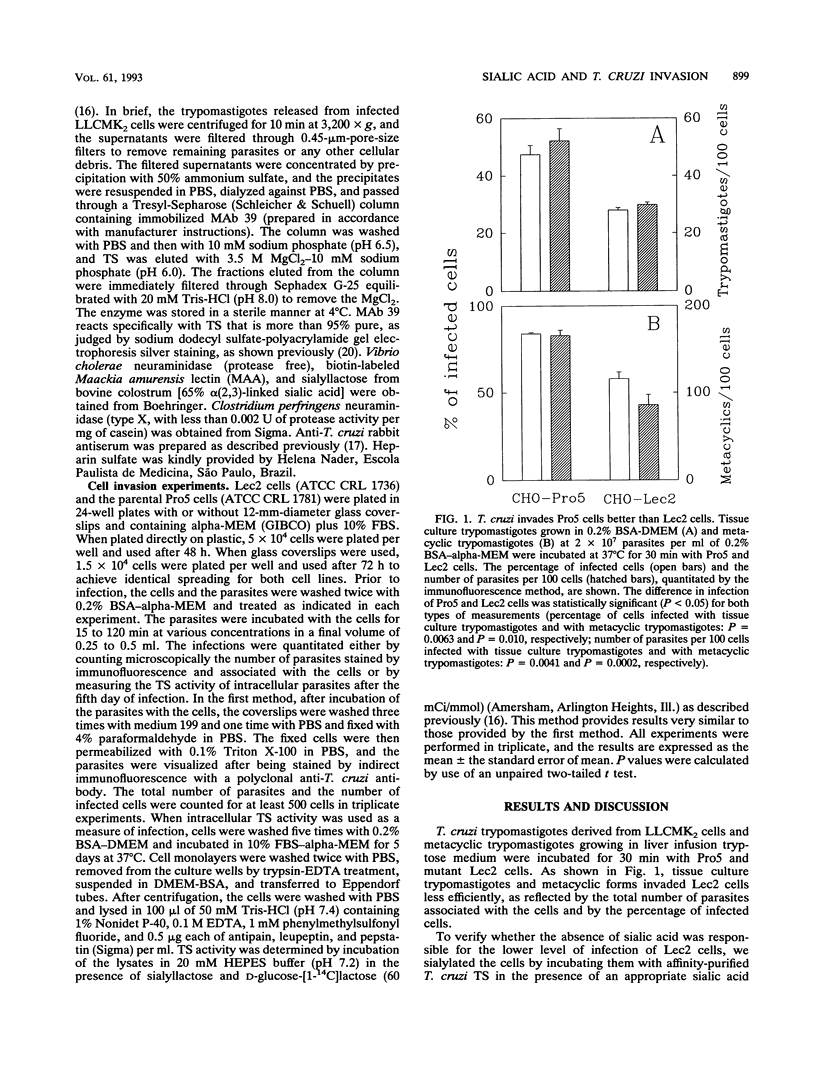
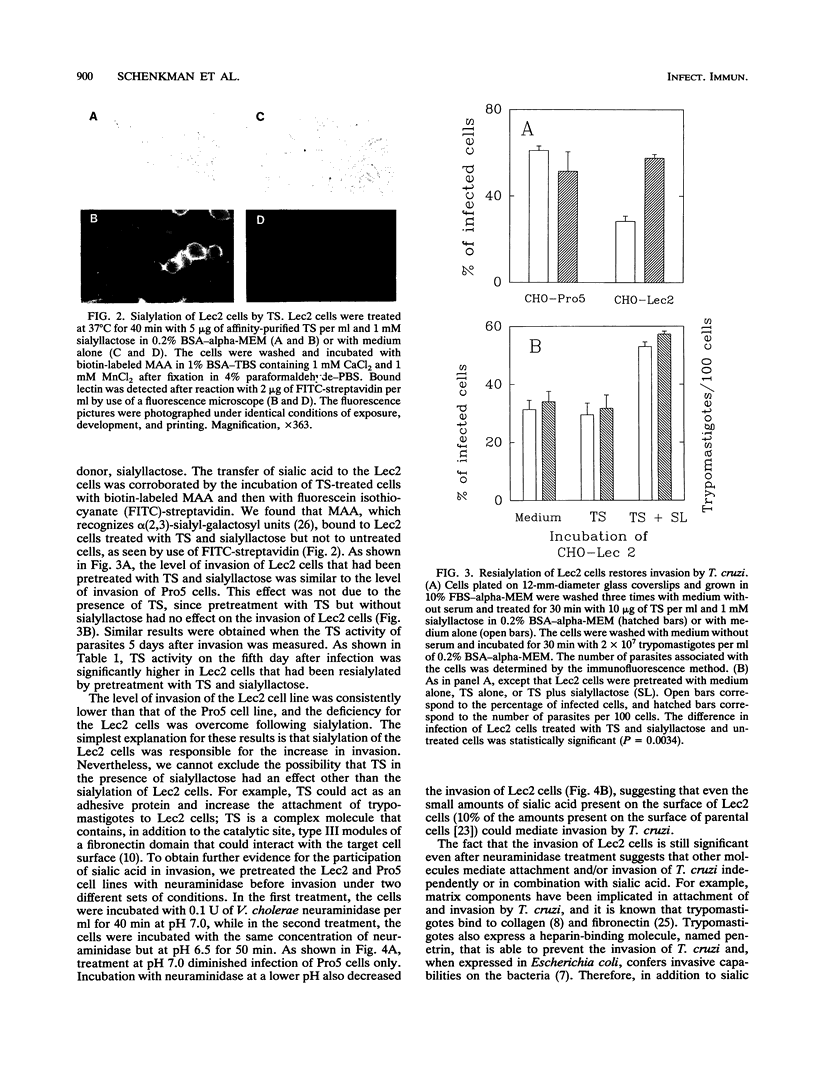
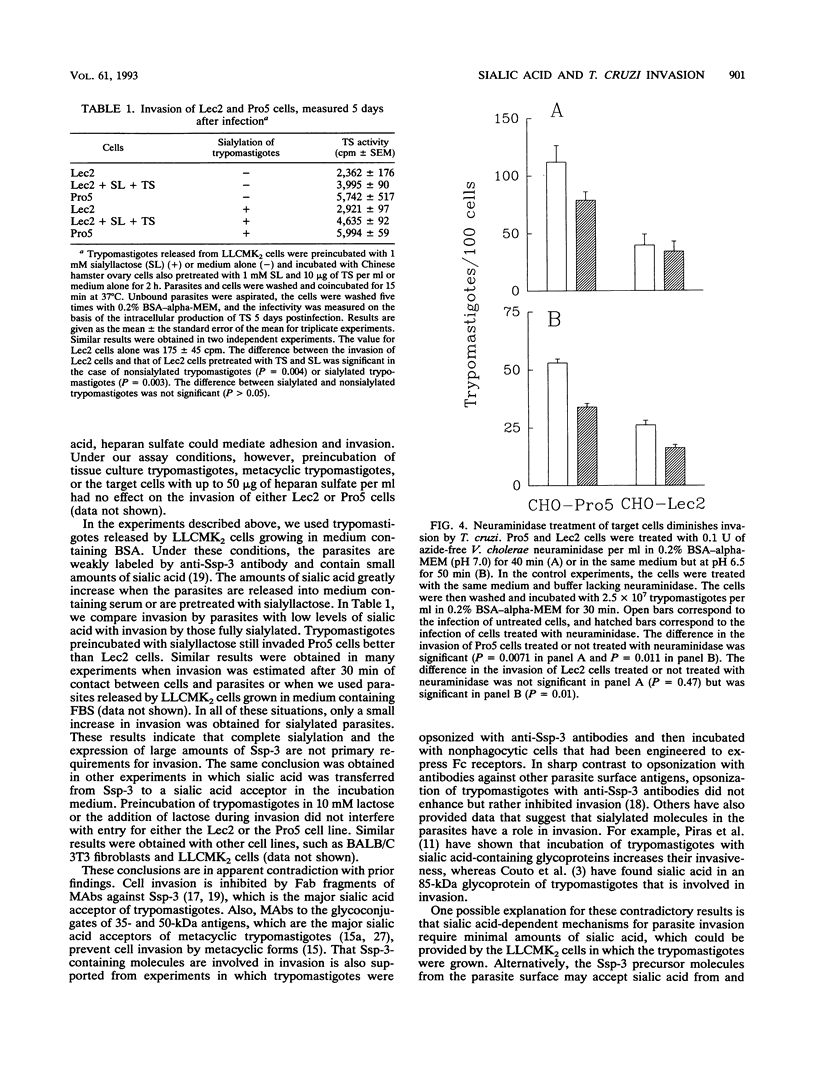
We have used a Chinese hamster ovary cell mutant (Lec2) that express much less sialic acid on the surface than the parental cell line (Pro5) to investigate whether sialic acid plays a role during cell invasion by Trypanosoma cruzi. Trypomastigotes derived from a tissue culture (corresponding to bloodstream trypomastigotes) and metacyclic trypomastigotes (corresponding to infective stages of the insect vector) invaded the Lec2 mutant less efficiently than the parental cell line. Invasion of the Lec2 mutant cells could be restored to the Pro5 level by resialylation of the mutant cells with T. cruzi trans-sialidase and sialyllactose. Conversely, pretreatment of the Pro5 parental cells with bacterial neuraminidase decreased invasion. These results indicate that sialic acid associated with the host cell contributes to invasion by T. cruzi.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. W., Hong K. S., Robbins E. S., Nussenzweig V. Stage-specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi. Exp Parasitol. 1987 Dec;64(3):474–484. doi: 10.1016/0014-4894(87)90062-2. [DOI] [PubMed] [Google Scholar]
- BRENER Z., CHIARI E. VARIA C OES MORFOL'OGICAS OBSERVADAS EM DIFERENTES AMOSTRAS DE TRYPANOSOMA CRUZI. Rev Inst Med Trop Sao Paulo. 1963 Sep-Oct;5:220–224. [PubMed] [Google Scholar]
- Couto A. S., Gonçalves M. F., Colli W., de Lederkremer R. M. The N-linked carbohydrate chain of the 85-kilodalton glycoprotein from Trypanosoma cruzi trypomastigotes contains sialyl, fucosyl and galactosyl (alpha 1-3)galactose units. Mol Biochem Parasitol. 1990 Feb;39(1):101–107. doi: 10.1016/0166-6851(90)90012-b. [DOI] [PubMed] [Google Scholar]
- Deutscher S. L., Nuwayhid N., Stanley P., Briles E. I., Hirschberg C. B. Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell. 1984 Dec;39(2 Pt 1):295–299. doi: 10.1016/0092-8674(84)90007-2. [DOI] [PubMed] [Google Scholar]
- Ortega-Barria E., Pereira M. E. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell. 1991 Oct 18;67(2):411–421. doi: 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- Ouaissi M. A., Cornette J., Afchain D., Capron A., Gras-Masse H., Tartar A. Trypanosoma cruzi infection inhibited by peptides modeled from a fibronectin cell attachment domain. Science. 1986 Oct 31;234(4776):603–607. doi: 10.1126/science.3094145. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Mejia J. S., Ortega-Barria E., Matzilevich D., Prioli R. P. The Trypanosoma cruzi neuraminidase contains sequences similar to bacterial neuraminidases, YWTD repeats of the low density lipoprotein receptor, and type III modules of fibronectin. J Exp Med. 1991 Jul 1;174(1):179–191. doi: 10.1084/jem.174.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras M. M., Henríquez D., Piras R. The effect of fetuin and other sialoglycoproteins on the in vitro penetration of Trypanosoma cruzi trypomastigotes into fibroblastic cells. Mol Biochem Parasitol. 1987 Jan 15;22(2-3):135–143. doi: 10.1016/0166-6851(87)90043-0. [DOI] [PubMed] [Google Scholar]
- Previato J. O., Andrade A. F., Pessolani M. C., Mendonça-Previato L. Incorporation of sialic acid into Trypanosoma cruzi macromolecules. A proposal for a new metabolic route. Mol Biochem Parasitol. 1985 Jun;16(1):85–96. doi: 10.1016/0166-6851(85)90051-9. [DOI] [PubMed] [Google Scholar]
- Prioli R. P., Mejia J. S., Pereira M. E. Monoclonal antibodies against Trypanosoma cruzi neuraminidase reveal enzyme polymorphism, recognize a subset of trypomastigotes, and enhance infection in vitro. J Immunol. 1990 Jun 1;144(11):4384–4391. [PubMed] [Google Scholar]
- Prioli R. P., Rosenberg I., Pereira M. E. High- and low-density lipoproteins enhance infection of Trypanosoma cruzi in vitro. Mol Biochem Parasitol. 1990 Jan 15;38(2):191–198. doi: 10.1016/0166-6851(90)90022-e. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Diaz C., Nussenzweig V. Attachment of Trypanosoma cruzi trypomastigotes to receptors at restricted cell surface domains. Exp Parasitol. 1991 Jan;72(1):76–86. doi: 10.1016/0014-4894(91)90123-e. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Jiang M. S., Hart G. W., Nussenzweig V. A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell. 1991 Jun 28;65(7):1117–1125. doi: 10.1016/0092-8674(91)90008-m. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Kurosaki T., Ravetch J. V., Nussenzweig V. Evidence for the participation of the Ssp-3 antigen in the invasion of nonphagocytic mammalian cells by Trypanosoma cruzi. J Exp Med. 1992 Jun 1;175(6):1635–1641. doi: 10.1084/jem.175.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman S., Pontes de Carvalho L., Nussenzweig V. Trypanosoma cruzi trans-sialidase and neuraminidase activities can be mediated by the same enzymes. J Exp Med. 1992 Feb 1;175(2):567–575. doi: 10.1084/jem.175.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman S., Robbins E. S., Nussenzweig V. Attachment of Trypanosoma cruzi to mammalian cells requires parasite energy, and invasion can be independent of the target cell cytoskeleton. Infect Immun. 1991 Feb;59(2):645–654. doi: 10.1128/iai.59.2.645-654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P., Siminovitch L. Complementation between mutants of CHO cells resistant to a variety of plant lectins. Somatic Cell Genet. 1977 Jul;3(4):391–405. doi: 10.1007/BF01542968. [DOI] [PubMed] [Google Scholar]
- Stanley P., Sudo T., Carver J. P. Differential involvement of cell surface sialic acid residues in wheat germ agglutinin binding to parental and wheat germ agglutinin-resistant Chinese hamster ovary cells. J Cell Biol. 1980 Apr;85(1):60–69. doi: 10.1083/jcb.85.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura H., Schenkman S., Nussenzweig V., Eichinger D. Only some members of a gene family in Trypanosoma cruzi encode proteins that express both trans-sialidase and neuraminidase activities. EMBO J. 1992 Nov;11(11):3837–3844. doi: 10.1002/j.1460-2075.1992.tb05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velge P., Ouaissi M. A., Cornette J., Afchain D., Capron A. Identification and isolation of Trypanosoma cruzi trypomastigote collagen-binding proteins: possible role in cell-parasite interaction. Parasitology. 1988 Oct;97(Pt 2):255–268. doi: 10.1017/s0031182000058467. [DOI] [PubMed] [Google Scholar]
- Wang W. C., Cummings R. D. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked alpha-2,3 to penultimate galactose residues. J Biol Chem. 1988 Apr 5;263(10):4576–4585. [PubMed] [Google Scholar]
- Yoshida N., Mortara R. A., Araguth M. F., Gonzalez J. C., Russo M. Metacyclic neutralizing effect of monoclonal antibody 10D8 directed to the 35- and 50-kilodalton surface glycoconjugates of Trypanosoma cruzi. Infect Immun. 1989 Jun;57(6):1663–1667. doi: 10.1128/iai.57.6.1663-1667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingales B., Carniol C., de Lederkremer R. M., Colli W. Direct sialic acid transfer from a protein donor to glycolipids of trypomastigote forms of Trypanosoma cruzi. Mol Biochem Parasitol. 1987 Nov;26(1-2):135–144. doi: 10.1016/0166-6851(87)90137-x. [DOI] [PubMed] [Google Scholar]
- Zingales B., Colli W. Trypanosoma cruzi: interaction with host cells. Curr Top Microbiol Immunol. 1985;117:129–152. doi: 10.1007/978-3-642-70538-0_7. [DOI] [PubMed] [Google Scholar]
- de Souza W. Cell biology of Trypanosoma cruzi. Int Rev Cytol. 1984;86:197–283. doi: 10.1016/s0074-7696(08)60180-1. [DOI] [PubMed] [Google Scholar]



