Abstract
Introduction:
Smoking behaviors, including heaviness of smoking and smoking cessation, are known to be under a degree of genetic influence. The enzyme catechol O-methyltransferase (COMT) is of relevance in studies of smoking behavior and smoking cessation due to its presence in dopaminergic brain regions. While the COMT gene is therefore one of the more promising candidate genes for smoking behavior, some inconsistencies have begun to emerge.
Methods:
We explored whether the rs4680 A (Met) allele of the COMT gene predicts increased heaviness of smoking and reduced likelihood of smoking cessation in a large population-based cohort of pregnant women. We further conducted a meta-analysis of published data from community samples investigating the association of this polymorphism with heaviness of smoking and smoking status.
Results:
In our primary sample, the A (Met) allele was associated with increased heaviness of smoking before pregnancy but not with the odds of continuing to smoke in pregnancy either in the first trimester or in the third trimester. Meta-analysis also indicated modest evidence of association of the A (Met) allele with increased heaviness of smoking but not with persistent smoking.
Conclusions:
Our data suggest a weak association between COMT genotype and heaviness of smoking, which is supported by our meta-analysis. However, it should be noted that the strength of evidence for this association was modest. Neither our primary data nor our meta-analysis support an association between COMT genotype and smoking cessation. Therefore, COMT remains a plausible candidate gene for smoking behavior phenotypes, in particular, heaviness of smoking.
Introduction
Most smokers report a desire and intention to quit, but while nearly half attempt to quit in any given year, only 2%–3% actually succeed (Twigg, Moon, Szatkowski, & Iggulden, 2009). The majority of quit attempts fail within days (Hughes, 2003), even with treatment, so that better treatment strategies are needed. Smoking behaviors, including heaviness of smoking and smoking cessation, are known to be under a degree of genetic influence (Munafo, Clark, Johnstone, Murphy, & Walton, 2004), and elucidating the genetic predictors of smoking behaviors may help to develop new pharmacotherapies for smoking cessation or identify subgroups for whom more intensive support may be necessary.
The enzyme catechol O-methyltransferase (COMT) is of relevance in studies of smoking behavior and smoking cessation due to its presence in dopaminergic brain regions. Its role is to degrade and inactivate neuronally released dopamine (Akil et al., 2003; Chen et al., 2004). The Val108/158Met polymorphism (rs4680) is located in exon 3 of the COMT gene and is a G > A (G1947A) transition that results in the substitution of a valine (G; Val) by a methionine (A; Met; Jonsson et al., 1999) at codon 108/158 for S-COMT/MB-COMT, respectively (Lachman et al., 1996). The A (Met) allele results in a threefold to fourfold reduction in COMT enzyme activity, which is hypothesized to result in relatively greater dopamine activity (Shield, Thomae, Eckloff, Wieben, & Weinshilboum, 2004). The chromosomal region (22q12) on which COMT is located has shown linkage with heavy smoking behavior (Saccone et al., 2007), and a number of studies have investigated the association between COMT rs4680 genotype and smoking behavior. Two studies have reported higher tobacco dependence among individuals carrying the A (Met) allele (Beuten, Payne, Ma, & Li, 2006; Guo et al., 2007), while another has reported an association between the A (Met) allele and increased smoking following exposure to an acute stressor (Amstadter et al., 2009). However, one study has reported a higher frequency of the G (Val) allele among smokers compared with nonsmokers (Nedic et al., 2010), while another has reported an association of the G (Val) allele with persistent smoking among light smokers (Shiels et al., 2008). Finally, one study failed to observe an association between COMT rs4680 genotype and heaviness of smoking (McKinney et al., 2000).
We recently reported evidence for a moderating effect of COMT rs4680 genotype on the relative efficacy of nicotine replacement therapy (NRT) transdermal patch compared with placebo (Johnstone et al., 2007). NRT produced relatively greater benefit compared with placebo in producing abstinence in individuals with the COMT AA (Met/Met) genotype compared with those with either the AG (Met/Val) or the GG (Val/Val) genotype. We subsequently replicated this association of the A (Met) allele with improved response to NRT in an open-label trial of the NRT transdermal patch (Munafo, Johnstone, Guo, Murphy, & Aveyard, 2008). Other studies have also investigated the role of the COMT gene in response to smoking cessation pharmacotherapy. Colilla et al. (2005) found that women who were homozygous for the low-activity A (Met) allele were more likely than those with the high-activity G (Val) allele to be abstinent from smoking at the end of a period of NRT, while Berrettini et al. (2007) found that a COMT haplotype of two single-nucleotide polymorphisms (including Val108/158Met) was associated with greater likelihood of abstinence in individuals treated with bupropion. However, Ton et al. (2007) reported no association of COMT genotype with cessation in almost 600 women taking part in a trial of D,L-fenfluramine for smoking cessation. Two recent investigations have shown an association between COMT genotype and smoking behavior in women only (Beuten et al., 2006; Colilla et al., 2005), although we did not observe sex differences in the association of COMT genotype with response to NRT (Johnstone et al., 2007).
However, Omidvar et al. (2009) reported data from more than 5,000 individuals indicating that, in elderly smokers, a reduced likelihood of cessation is associated with A (Met) allele carriers, while Breitling et al. (2009) did not observe an association between the COMT rs4680 polymorphism and cessation in a cohort of more than 1,400 heavy smokers, of whom more than 900 achieved abstinence, and David et al. (2002) did not observe an association with smoking status in more than 500 current smokers and ex-smokers. It is notable that in all these studies, smokers were drawn from community-based samples and were not explicitly recruited to be treatment seeking, unlike the studies of smokers participating in clinical trials described above. Given that the majority of cessation attempts do not include the use of behavioral support of pharmacotherapy (Chapman & MacKenzie, 2010), it is likely that most cessation attempts in these community-based smokers were spontaneous and unassisted.
An endemic difficulty in the search for genetic variants associated with complex behavioral phenotypes is the lack of robust replication (Colhoun, McKeigue, & Davey Smith, 2003; Ioannidis, Ntzani, Trikalinos, & Contopoulos-Ioannidis, 2001): Initially promising findings are frequently followed by failures to replicate or opposite findings. Consistent with this pattern, while COMT has emerged as one of the more promising candidate genes for smoking behavior, some inconsistencies have begun to emerge. Clearly, further research, ideally in large prospective cohorts, is needed to investigate whether COMT rs4680 genotype predicts smoking behavior. We therefore explored whether the COMT rs4680 A (Met) allele predicts increased heaviness of smoking and persistent smoking during pregnancy in a large population-based cohort of pregnant women, given the considerable proportion of women who stop smoking during pregnancy (Munafo, Heron, & Araya, 2008) due to the health and social pressures to do so.
Methods
Participants
We studied pregnant women of European ancestry from the Avon Longitudinal Study of Parents and Children (ALSPAC; Golding, Pembrey, & Jones, 2001), a prospective study that recruited pregnant women from Bristol, UK, with expected delivery dates between April 1991 and December 1992. All women gave informed consent, and ethical approval for the study was obtained from the ALSPAC Law and Ethics Committee and the Local Research Ethics Committees.
Data Collection
Cigarette smoking behavior of women before and during pregnancy was determined from questionnaires. A questionnaire was administered in the 18th gestational week asking about lifetime, prepregnancy, and first-trimester smoking behavior (whether or not the woman smoked and, for smokers, the quantity of cigarettes per day) and another in the 32nd week asking about current smoking behavior. At each timepoint, the data on smoking quantity were categorized into 1–9, 10–19, and 20+ cigarettes/day. Data on known covariates of smoking behavior (Lu, Tong, & Oldenburg, 2001) were also collected via questionnaire, including age, age started smoking, socioeconomic position (Szreter, 1984), educational level, parity, and partner's smoking status.
Genotyping
The COMT rs4680 polymorphism was genotyped in participants using standard methods. Genotyping was performed by KBiosciences (Hoddesdon, UK; www.kbioscience.co.uk), using their own system of fluorescence-based competitive allele-specific polymerase chain reaction (KASPar). The genotyping call rate was >95%. The percentage of duplicate samples included for genotyping was 9%. Concordance between duplicate samples was >99%. There was no evidence of deviation from Hardy–Weinberg equilibrium (p = .43).
Statistical Methods
We selected women of European ancestry on whom data on COMT rs4680 genotype and cigarette smoking immediately prior to pregnancy were available. We assumed an additive model of genetic action based on prior evidence that the rs4680 polymorphism is codominant (Weinshilboum, Otterness, & Szumlanski, 1999). This, combined with the roughly equal allele frequencies, increases the statistical power of this approach.
First, we assessed the association between the prepregnancy, first trimester and third trimester smoking quantity (cigarettes per day), and the rs4680 polymorphism by performing linear regression of smoking quantity level on number of A (Met) alleles. We also dichotomized smoking quantity to reflect “light” (1–9 cigarettes/day) and “heavy” (10+ cigarettes/day) smoking. We assessed the association between this variable and the number of A (Met) alleles using logistic regression. We repeated these analyses including known covariates of smoking behavior (age, age started smoking, socioeconomic position, educational level, parity, and partner's smoking status).
Second, we assessed the association between persistent smoking in the first trimester and third trimester and the rs4680 polymorphism. Participants were classified, using data collected on first trimester smoking, as “stopped smoking” or “continued to smoke.” A similar dichotomous variable was created using data on third trimester smoking assessed at 32 weeks. We performed logistic regression to assess the association between each dichotomized variable and number of A (Met) alleles. We repeated these analyses including known covariates of behavior and heaviness of smoking prior to pregnancy.
We also used bootstrapping methods to derive the regression error for the logistic regression models nonparametrically. For each model, we drew 10,000 samples with replacement using the R boot library (www.r-project.org) in order to create a sampling distribution of the statistic of interest. Bootstrapped regression estimates, their errors, and 95% CIs (corresponding to the 2.5th and 97.5th percentiles) were derived on the logit scale and subsequently transformed into odds ratios (ORs). Bootstrap p values (pempirical) were based on Wald tests.
Third, given the risk of chance findings in genetic association studies and in an attempt to resolve the discrepancy between studies of the COMT rs4680 polymorphism and both heaviness of smoking (light vs. heavy smokers, as defined above) and persistent smoking (current smokers vs. ex-smokers), we combined our data with those from previous studies in community samples (Breitling et al., 2009; David et al., 2002; Guo et al., 2007; Omidvar et al., 2009; Shiels et al., 2008). We used our prepregnancy heaviness of smoking and first trimester persistent smoking data as described above. Data were initially analyzed within a fixed effects framework and individual study allelic ORs pooled using inverse variance methods to generate a pooled OR and 95% CI. A fixed effects framework assumes that the association between genotype and phenotype is constant across studies, and between-study variation is considered to be due to chance or random variation. This assumption was checked using a χ2 test of goodness of fit for homogeneity. The p value of the pooled OR was determined using a Z test and the percentage of total variation across studies due to heterogeneity quantified using the I2 statistic. Conventionally, values of 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively (Higgins, Thompson, Deeks, & Altman, 2003). Where there was evidence of association in the presence of moderate to high between-study heterogeneity, a random effects framework was employed, with ORs pooled using DerSimonian and Laird methods. A random effects framework assumes that between-study variation is due to both chance or random variation and an individual study effect. Random effects models are more conservative than fixed effects models and generate a wider CI. We tested for small study bias, such as may arise from publication bias, using Egger's test (Egger, Davey Smith, Schneider, & Minder, 1997).
Results
Characteristics of Participants
There were n = 6,227 pregnant women of European ancestry on whom data on COMT rs4680 genotype and smoking status immediately prior to pregnancy were available. A total of n = 2,001 women reported smoking immediately prior to pregnancy, of whom n = 547 reported not smoking in the first trimester and n = 849 reported not smoking in the third trimester. Basic sample characteristics are presented in Table 1.
Table 1.
Characteristics of Participants
| All women (n = 6,227),n (%) | Smokers(n = 2,001), n (%) | Quit 18 week (n = 547), n (%) | Quit 22 week (n = 849), n (%) | |
| Age (years) | ||||
| <20 | 209 (3) | 143 (7) | 29 (5) | 63 (7) |
| 20–29 | 3,582 (58) | 1,285 (64) | 340 (62) | 535 (63) |
| >30 | 2,436 (39) | 573 (29) | 178 (33) | 251 (30) |
| Age started smoking (years) | ||||
| <16 | n/a | 746 (38) | 156 (29) | 275 (33) |
| 16–19 | n/a | 1048 (53) | 304 (56) | 457 (54) |
| 20+ | n/a | 186 (9) | 80 (15) | 110 (13) |
| Socioeconomic statusa | ||||
| I/II | 1,905 (35) | 442 (26) | 146 (30) | 218 (30) |
| III | 2,935 (54) | 938 (56) | 265 (55) | 405 (55) |
| IV/V | 624 (11) | 293 (18) | 70 (15) | 114 (15) |
| Educational levelb | ||||
| CSE/vocational | 1,786 (29) | 832 (42) | 174 (32) | 296 (35) |
| O-level | 2,217 (36) | 704 (35) | 206 (38) | 300 (35) |
| A-level/degree | 2,199 (35) | 457 (23) | 166 (30) | 251 (30) |
| Parityc | ||||
| 0 | 2,827 (46) | 986 (50) | 318 (59) | 509 (61) |
| 1 | 2,168 (35) | 593 (30) | 149 (28) | 207 (25) |
| 2+ | 1,160 (19) | 389 (20) | 70 (13) | 120 (14) |
| Partner smoking | ||||
| Yes | 2,168 (36) | 1,165 (62) | 267 (50) | 422 (52) |
| No | 3,839 (64) | 719 (38) | 263 (50) | 385 (48) |
Note. A-level = Advanced Level; CSE = Certificate of Secondary Education; n/a = not applicable; O-level = Ordinary Level.
Data on socioeconomic status based upon the Registrar General's 1980 (Szreter, 1984) classification (I, II, III Non-Manual, III Manual, IV, and V, where I represents professional and V unskilled manual).
Educational data ranked according to level of attainment (lowest: CSE/vocational and highest: A-level/degree), with O-level qualifications typically taken at 16 years and A-level qualifications typically taken at 18 years.
Parity indicates the number of times the participant had given birth.
Using data on all participants, the A (Met) allele was associated with smoking status prior to pregnancy (OR = 1.11, 95% CI = 1.03–1.20, p = .007), with the A (Met) allele more common among smokers than among nonsmokers. However, when known covariates of smoking behavior were included, no association was observed (OR = 1.03, 95% CI = 0.91–1.17, p = .65). The association of COMT rs4680 genotype with these covariates is presented in Table 2.
Table 2.
COMT rs4680 Genotype and Covariates of Smoking Behavior
| GG (Val/Val) | GA (Val/Met) | AA (Met/Met) | p valuea | |
| Age (years) | ||||
| <20 | 39 (19) | 106 (51) | 64 (30) | .083 |
| 20–29 | 831 (23) | 1,779 (50) | 972 (27) | |
| >30 | 590 (24) | 1,207 (50) | 639 (26) | |
| Age started smoking (years) | ||||
| <16 | 213 (20) | 533 (51) | 309 (29) | .255 |
| 16–19 | 386 (23) | 847 (50) | 447 (27) | |
| 20+ | 67 (22) | 148 (48) | 91 (30) | |
| Socioeconomic statusb | ||||
| I/II | 461 (24) | 957 (50) | 487 (26) | .026 |
| III | 704 (24) | 1,451 (49) | 780 (27) | |
| IV/V | 132 (21) | 298 (48) | 194 (31) | |
| Educational levelc | ||||
| CSE/vocational | 389 (22) | 888 (50) | 509 (28) | .010 |
| O-level | 518 (23) | 1,106 (50) | 593 (27) | |
| A-level/degree | 546 (25) | 1,087 (49) | 566 (26) | |
| Parityd | ||||
| 0 | 684 (24) | 1,384 (49) | 759 (26) | .812 |
| 1 | 472 (22) | 1,107 (51) | 589 (27) | |
| 2+ | 284 (24) | 566 (49) | 310 (27) | |
| Partner smoking | ||||
| Yes | 474 (22) | 1,070 (49) | 624 (29) | .003 |
| No | 943 (25) | 1,902 (49) | 994 (26) | |
Note. Analyses restricted to pregnant women of European ancestry on whom data on smoking status immediately prior to pregnancy were available (n = 6,227).
Linear association chi-square test.
Data on socioeconomic status based upon the Registrar General's 1980 (Szreter, 1984) classification (I, II, III Non-Manual, III Manual, IV, and V, where I represents professional and V unskilled manual).
Educational data ranked according to level of attainment (lowest: CSE/vocational and highest: A-level/degree), with O-level qualifications typically taken at 16 years and A-level qualifications typically taken at 18 years.
Parity indicates the number of times the participant had given birth.
Heaviness of Smoking
Among smokers on whom heaviness of smoking data were available (n = 1,132–1,963), logistic regression analyses indicated that the A (Met) allele was associated with increased heaviness of smoking (dichotomized as 1–9 cigarettes/day vs. 10+ cigarettes/day) before pregnancy (OR = 1.23, 95% CI = 1.06–1.42, p = .005, pempirical = .004), during the first trimester (OR = 1.24, 95% CI = 1.09–1.41, p = .001, pempirical = .001), with marginal evidence of association during the third trimester (OR = 1.16, 95% CI = 0.98–1.38, p = .080, pempirical = .081), most likely due to reduced numbers of smokers and lower power during this period. Linear regression analyses produced similar findings. When known covariates of smoking behavior were included, these results were not altered substantially. These results are presented in Table 3.
Table 3.
COMT rs4680 Genotype and Heaviness of Smoking
| Prepregnancy | First trimester | Third trimester | ||
| Logistic regression | ||||
| Unadjusted | OR (95% CI) | 1.23 (1.06–1.42) | 1.24 (1.09–1.41) | 1.16 (0.98–1.38) |
| Adjusted | OR (95% CI) | 1.20 (1.02–1.42) | 1.23 (1.06–1.43) | 1.08 (0.88–1.32) |
| Linear regression | ||||
| Unadjusted | B (95% CI) | +0.05 (+0.00 to +0.10) | +0.07 (+0.02 to +0.13) | +0.07 (+0.01 to +0.13) |
| Adjusted | B (95% CI) | +0.05 (−0.00 to +0.11) | +0.06 (+0.00 to +0.12) | +0.04 (−0.03 to+0.11) |
Note. Adjusted estimates include correction for age, age started smoking, socioeconomic status, educational level, parity, and partner smoking. OR = odds ratio.
Persistent Smoking in Pregnancy
Among those who reported smoking prior to pregnancy on whom smoking status data during pregnancy were available (n = 1,998), we did not observe an association between the A (Met) allele and the odds of continuing to smoke in pregnancy either in the first trimester (OR = 1.07, 95% CI = 0.93–1.23, p = .37, pempirical = .37) or in the third trimester (OR = 0.97, 95% CI = 0.86–1.11, p = .67, pempirical = .68). When known covariates of smoking behavior and heaviness of smoking prior to pregnancy were included, these results were not altered substantially. These results are presented in Table 4.
Table 4.
COMT rs4680 Genotype and Smoking Cessation
| GG (Val/Val) | GA (Val/Met) | AA (Met/Met) | |
| First trimester | |||
| Smoking, n (%) | 295 (70) | 746 (73) | 410 (73) |
| Quit, n (%) | 126 (30) | 269 (27) | 152 (27) |
| Third trimester | |||
| Smoking, n (%) | 244 (58) | 587 (58) | 318 (57) |
| Quit, n (%) | 177 (42) | 429 (42) | 243 (43) |
Note. Analyses restricted to women who reported smoking cigarettes immediately prior to pregnancy (n = 2,001).
Meta-Analysis
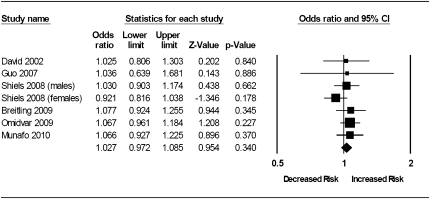
Meta-analysis of individual study allelic ORs, within a fixed effects framework (Munafo & Flint, 2004), indicated some evidence of association of the A (Met) allele with increased heaviness of smoking (k = 14, n = 13,312, OR = 1.07, 95% CI = 1.01–1.13, p = .035). There was low between-study heterogeneity (I2 = 17%, χ2 [13] = 15.60, p = .27). These results are presented in Figure 1 and were not altered substantially when dominant and recessive models of genetic action were tested. Egger's test did not indicate any evidence of small study bias, t(12) = 0.54, p = .60.
Figure 1.
Meta-analysis of COMT rs4680 genotype and heaviness of smoking. Fixed effects meta-analysis of COMT rs4680 genotype and heaviness of smoking indicates some evidence of association of the A (Met) allele with increased heaviness of smoking (bottom row). Data from the primary sample in the current study are included as present study.
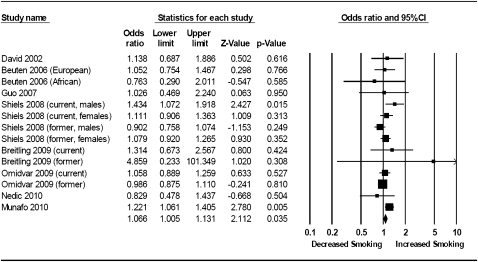
A similar meta-analysis of individual study allelic ORs did not indicate any evidence of association of the A (Met) allele with persistent smoking (k = 7, n = 11,469, OR = 1.03, 95% CI = 0.97–1.09, p = .34). There was no between-study heterogeneity (I2 = 0%, χ2 [6] = 4.41, p = .64). These results are presented in Figure 2 and were not altered substantially when we used our data on third trimester smoking status or when dominant and recessive models of genetic action were tested. Egger's test did not indicate any evidence of small study bias, t(12) = 0.14, p = .90.
Figure 2.
Meta-analysis of COMT rs4680 genotype and persistent smoking. Fixed effects meta-analysis of COMT rs4680 genotype and persistent smoking indicates no evidence of association of the A (Met) allele with persistent smoking (bottom row). Data from the primary sample in the current study are included as Munafo (2010).
Power Analysis
The results of our meta-analysis indicated that, in order to detect any effect of COMT rs4680 on persistent smoking or heaviness of smoking, a primary sample in excess of n = 25,000 would be required to achieve 80% power at an alpha level of .05. Therefore, although we detected evidence of association in our primary sample, further replication in a larger sample would be desirable. Power analyses were conducted using G*Power 3.1 (Faul, Erdfelder, Lang, & Buchner, 2007) and assumed a minor G (Val) allele frequency of 47% consistent with our meta-analysis.
Discussion
Our data suggest a weak association between COMT genotype and heaviness of smoking, which survived correction for age, age started smoking, socioeconomic position, educational level, parity, and partner's smoking status. This finding is supported by our meta-analysis, which indicated a small effect equivalent to <1% phenotypic variance, consistent with the growing consensus that single gene effects on complex phenotypes are likely to be very small (Clarke, Flint, Attwood, & Munafo, 2010). However, it should be noted that the strength of evidence for this association was modest, and the observed effect size was reduced in our meta-analysis compared with our primary sample. Furthermore, these effects did not reach genomewide significance and would not survive correction for multiple comparisons based on the two primary phenotypes we investigated. Therefore, COMT remains a plausible candidate gene for smoking behavior phenotypes, in particular, heaviness of smoking (and, by extension, tobacco dependence), but any effect is likely to be small, and further research is necessary to establish conclusively whether it is genuine.
Neither our primary data nor our meta-analysis support an association between COMT genotype and the likelihood of stopping smoking, which is at odds with previous reports of an association with smoking cessation among treatment-seeking smokers (Johnstone et al., 2007; Munafo, Johnstone, et al., 2008). One possibility suggested by these data is that the effects of COMT on smoking cessation may differ as a function of whether the cessation attempt is aided or unaided and, in particular, whether NRTs are used, given evidence of a moderating effect of COMT on response to NRT (Johnstone et al., 2007; Munafo, Johnstone, et al., 2008). A difficulty with existing data from clinical trials is that these tend to be smaller than those from community-based studies, so that while it is possible that effects of COMT differ between these populations (possibly as a function of medication use), it is also possible that the difference is due to the higher risk of false positives in smaller samples. This possibility will need to be explored in future studies of treatment-seeking smokers.
Converging evidence for a role of COMT in smoking behavior comes from neuroimaging studies. Brody et al. (2006) reported that COMT genotype moderated the effect of cigarette smoking on dopamine (DA) release, with the Val (G) allele associated with greater DA release following smoking. COMT is of particular interest given the relatively prominent role of the COMT enzyme in DA degradation in the prefrontal cortex, given the relative lack of dopamine transporters in this region. However, functionally, the Met (A) allele also appears to result in increased levels of tonic DA and reciprocal reductions in phasic DA released in subcortical regions (Bilder, Volavka, Lachman, & Grace, 2004). Therefore, Val (G) allele carriers with higher COMT enzyme activity may have decreased tonic intrasynaptic DA levels, leading to increased smoking-induced phasic DA release (Brody et al., 2006). A recent review (Contin et al., 2004) supports this possibility by suggesting that Val (G) allele carriers may have lower tonic extraneuronal DA and higher phasic DA subcortically compared with Met (A) allele carriers. As a result, Met (A) allele carriers may smoke more heavily in order to obtain equivalent levels of phasic DA release.
There are some limitations to our study that should be considered when interpreting these results. First, smoking status in the ALSPAC sample was not biochemically verified. However, this is offset by the relatively large sample size and prospective nature of data collection. In addition, there are no reasons to believe that misreporting would differ by genotype, so the likelihood of systematic bias is low. Second, COMT genotype was associated with socioeconomic position and educational attainment in our sample. There is no reason to believe that this reflects anything other than a chance finding, given the extensive evidence that in general, genetic variants are not related to such factors (Davey-Smith et al., 2007)—and particularly because this association is in the opposite direction to that which has been reported for general intelligence (Barnett, Scoriels, & Munafo, 2008). However, we adjusted our analyses for covariates related to smoking behavior, including socioeconomic position and educational attainment, and our findings with respect to heaviness of smoking were robust to these adjustments. Third, we did not collect data on concurrent medication use in our sample and, in particular, whether participants were using any smoking cessation pharmacotherapies to assist them in stopping. However, at the time of data collection, only nicotine replacement products were available for smoking cessation in the United Kingdom, and these were not available over the counter and not licensed for prescription to pregnant women. In addition, bupropion is not licensed as an antidepressant in the United Kingdom. It is therefore highly unlikely that many (if any) of the participants in our sample were using medications with effects on smoking cessation or heaviness of smoking. Fourth, association between the rs4680 variant and smoking behavior has not been reported in recent large genomewide association (GWA) studies of smoking phenotypes, including heaviness of smoking (Liu et al., 2010; Thorgeirsson et al., 2010; Tobacco-and-Genetics-Consortium, 2010), despite the variant being included on relevant GWA arrays. One possible reason is that the effect of the rs4680 variant is too small to appear among the top hits followed up in these studies. Another possibility is simply that our results represent a chance finding, given the small observed effect size and relatively modest sample available for analysis, even in our meta-analysis. This is a particular problem, given the history of nonreplication in genetic association studies (Davey-Smith et al., 2007; Munafo, 2009), and therefore, further replication in a larger independent sample would be desirable.
In conclusion, our data suggest weak evidence of association of the COMT rs4680 polymorphism with heaviness of smoking but not smoking cessation or persistent smoking. While the results of our meta-analysis did not indicate substantial between-study heterogeneity or the presence of small study bias, the lack of convergent evidence from recent GWA studies somewhat undermines confidence in these results. Nevertheless, COMT appears to remain a candidate gene for smoking behavior, warranting further investigation.
Funding
The UK Medical Research Council (74882), the Wellcome Trust (076467), and the University of Bristol provide core support for ALSPAC. This research was specifically funded by the Wellcome Trust (086684). RMF is funded by a Sir Henry Wellcome Postdoctoral Fellowship (085541). GDS works in a centre (CAiTE) that is supported by the UK Medical Research Council (G0600705) and the University of Bristol.
Declaration of Interests
None declared.
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. We are also grateful to the study authors who released their data in a format that enabled their inclusion in our meta-analysis. This publication is the work of the authors, and Marcus Munafò will serve as guarantor for the contents of this paper.
References
- Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. Journal of Neuroscience. 2003;23:2008–2013. doi: 10.1523/JNEUROSCI.23-06-02008.2003. Retrieved from http://www.jneurosci.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Nugent NR, Koenen KC, Ruggiero KJ, Acierno R, Galea S, et al. Association between COMT, PTSD, and increased smoking following hurricane exposure in an epidemiologic sample. Psychiatry. 2009;72:360–369. doi: 10.1521/psyc.2009.72.4.360. doi:10.1521/psyc.2009.72.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett JH, Scoriels L, Munafo MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biological Psychiatry. 2008;64:137–144. doi: 10.1016/j.biopsych.2008.01.005. doi:10.1016/j.biopsych.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Berrettini WH, Wileyto EP, Epstein L, Restine S, Hawk L, Shields P, et al. Catechol-O-methyltransferase (COMT) gene variants predict response to bupropion therapy for tobacco dependence. Biological Psychiatry. 2007;61:111–118. doi: 10.1016/j.biopsych.2006.04.030. doi:10.1016/j.biopsych.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Beuten J, Payne TJ, Ma JZ, Li MD. Significant association of catechol-O-methyltransferase (COMT) haplotypes with nicotine dependence in male and female smokers of two ethnic populations. Neuropsychopharmacology. 2006;31:675–684. doi: 10.1038/sj.npp.1300997. doi:10.1038/sj.npp.1300997. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: Relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. doi:10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- Breitling LP, Dahmen N, Illig T, Rujescu D, Nitz B, Raum E, et al. Variants in COMT and spontaneous smoking cessation: Retrospective cohort analysis of 925 cessation events. Pharmacogenetics and Genomics. 2009;19:657–659. doi: 10.1097/FPC.0b013e32832fabf3. doi:10.1097/FPC.0b013e32832fabf3. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S, et al. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Archives of General Psychiatry. 2006;63:808–816. doi: 10.1001/archpsyc.63.7.808. doi:10.1001/archpsyc.63.7.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S, MacKenzie R. The global research neglect of unassisted smoking cessation: Causes and consequences. PLoS Medicine. 2010;7:e1000216. doi: 10.1371/journal.pmed.1000216. doi:10.1371/journal.pmed.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. American Journal of Human Genetics. 2004;75:807–821. doi: 10.1086/425589. doi:10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke H, Flint J, Attwood AS, Munafo MR. Association of the 5-HTTLPR genotype and unipolar depression: A meta-analysis. Psychological Medicine. 2010;40:1767–1778. doi: 10.1017/S0033291710000516. doi:10.1017/S0033291710000516. [DOI] [PubMed] [Google Scholar]
- Colhoun HM, McKeigue PM, Davey Smith G. Problems of reporting genetic associations with complex outcomes. Lancet. 2003;361:865–872. doi: 10.1016/s0140-6736(03)12715-8. doi:10.1016/S0140-6736(03)12715-8. [DOI] [PubMed] [Google Scholar]
- Colilla S, Lerman C, Shields PG, Jepson C, Rukstalis M, Berlin J, et al. Association of catechol-O-methyltransferase with smoking cessation in two independent studies of women. Pharmacogenetics and Genomics. 2005;15:393–398. doi: 10.1097/01213011-200506000-00004. doi:10.1097/01213011-200506000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contin M, Martinelli P, Mochi M, Albani F, Riva R, Scaglione C, et al. Dopamine transporter gene polymorphism, spect imaging, and levodopa response in patients with Parkinson disease. Clinical Neuropharmacology. 2004;27:111–115. doi: 10.1097/00002826-200405000-00004. doi:10.1097/00002826-200405000-00004. [DOI] [PubMed] [Google Scholar]
- Davey-Smith G, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S. Clustered environments and randomized genes: A fundamental distinction between conventional and genetic epidemiology. PLoS Medicine. 2007;4:e352. doi: 10.1371/journal.pmed.0040352. doi:10.1371/journal.pmed.0040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Johnstone E, Griffiths SE, Murphy M, Yudkin P, Mant D, et al. No association between functional catechol O-methyl transferase 1947A>G polymorphism and smoking initiation, persistent smoking or smoking cessation. Pharmacogenetics. 2002;12:265–268. doi: 10.1097/00008571-200204000-00011. Retrieved from http://journals.lww.com/jpharmacogenetics/pages/default.aspx. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. Retrieved from http://www.bmj.com/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. Retrieved from http://brm.psychonomic-journals.org/ [DOI] [PubMed] [Google Scholar]
- Golding J, Pembrey M, Jones R. ALSPAC—The Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatric and Perinatal Epidemiology. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. doi:10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- Guo S, Chen da F, Zhou DF, Sun HQ, Wu GY, Haile CN, et al. Association of functional catechol O-methyl transferase (COMT) Val108Met polymorphism with smoking severity and age of smoking initiation in Chinese male smokers. Psychopharmacology (Berlin) 2007;190:449–456. doi: 10.1007/s00213-006-0628-4. doi:10.1007/s00213-006-0628-4. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. doi:10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Motivating and helping smokers to stop smoking. Journal of General Internal Medicine. 2003;18:1053–1057. doi: 10.1111/j.1525-1497.2003.20640.x. doi:10.1111/j.1525-1497.2003.20640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nature Genetics. 2001;29:306–309. doi: 10.1038/ng749. doi:10.1038/ng749. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Elliot KM, David SP, Murphy MF, Walton RT, Munafo MR. Association of COMT Val108/158Met genotype with smoking cessation in a nicotine replacement therapy randomized trial. Cancer Epidemiology Biomarkers and Prevention. 2007;16:1065–1069. doi: 10.1158/1055-9965.EPI-06-0936. doi:10.1158/1055-9965.EPI-06-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Molecular Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. doi:10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. doi:10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nature Genetics. 2010;42:436–440. doi: 10.1038/ng.572. doi:10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Tong S, Oldenburg B. Determinants of smoking and cessation during and after pregnancy. Health Promotion International. 2001;16:355–365. doi: 10.1093/heapro/16.4.355. doi:10.1093/heapro/16.4.355. [DOI] [PubMed] [Google Scholar]
- McKinney EF, Walton RT, Yudkin P, Fuller A, Haldar NA, Mant D, et al. Association between polymorphisms in dopamine metabolic enzymes and tobacco consumption in smokers. Pharmacogenetics. 2000;10:483–491. doi: 10.1097/00008571-200008000-00001. Retrieved from http://journals.lww.com/jpharmacogenetics/pages/default.aspx. [DOI] [PubMed] [Google Scholar]
- Munafo MR. Reliability and replicability of genetic association studies. Addiction. 2009;104:1439–1440. doi: 10.1111/j.1360-0443.2009.02662.x. doi:10.1111/j.1360-0443.2009.02662.x. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Clark T, Johnstone E, Murphy M, Walton R. The genetic basis for smoking behavior: A systematic review and meta-analysis. Nicotine & Tobacco Research. 2004;6:583–597. doi: 10.1080/14622200410001734030. doi:10.1080/14622200410001734030. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Flint J. Meta-analysis of genetic association studies. Trends in Genetics. 2004;20:439–444. doi: 10.1016/j.tig.2004.06.014. doi:10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Heron J, Araya R. Smoking patterns during pregnancy and postnatal period and depressive symptoms. Nicotine & Tobacco Research. 2008;10:1609–1620. doi: 10.1080/14622200802412895. doi:10.1080/14622200802412895. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Johnstone EC, Guo B, Murphy MF, Aveyard P. Association of COMT Val108/158Met genotype with smoking cessation. Pharmacogenetics and Genomics. 2008;18:121–128. doi: 10.1097/FPC.0b013e3282f44daa. doi:10.1097/FPC.0b013e3282f44daa. [DOI] [PubMed] [Google Scholar]
- Nedic G, Nikolac M, Borovecki F, Hajnsek S, Muck-Seler D, Pivac N. Association study of a functional catechol-o-methyltransferase polymorphism and smoking in healthy Caucasian subjects. Neuroscience Letters. 2010;473:216–219. doi: 10.1016/j.neulet.2010.02.050. doi:10.1016/j.neulet.2010.02.050. [DOI] [PubMed] [Google Scholar]
- Omidvar M, Stolk L, Uitterlinden AG, Hofman A, Van Duijn CM, Tiemeier H. The effect of catechol-O-methyltransferase Met/Val functional polymorphism on smoking cessation: Retrospective and prospective analyses in a cohort study. Pharmacogenetics and Genomics. 2009;19:45–51. doi: 10.1097/fpc.0b013e328317f3f8. doi:10.1097/FPC.0b013e328317f3f8. [DOI] [PubMed] [Google Scholar]
- Saccone SF, Pergadia ML, Loukola A, Broms U, Montgomery GW, Wang JC, et al. Genetic linkage to chromosome 22q12 for a heavy-smoking quantitative trait in two independent samples. American Journal of Human Genetics. 2007;80:856–866. doi: 10.1086/513703. doi:10.1086/513703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shield AJ, Thomae BA, Eckloff BW, Wieben ED, Weinshilboum RM. Human catechol O-methyltransferase genetic variation: Gene resequencing and functional characterization of variant allozymes. Molecular Psychiatry. 2004;9:151–160. doi: 10.1038/sj.mp.4001386. doi:10.1038/sj.mp.4001386. [DOI] [PubMed] [Google Scholar]
- Shiels MS, Huang HY, Hoffman SC, Shugart YY, Bolton JH, Platz EA, et al. A community-based study of cigarette smoking behavior in relation to variation in three genes involved in dopamine metabolism: Catechol-O-methyltransferase (COMT), dopamine beta-hydroxylase (DBH) and monoamine oxidase-A (MAO-A) Preventive Medicine. 2008;47:116–122. doi: 10.1016/j.ypmed.2008.03.013. doi:10.1016/j.ypmed.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szreter SRS. The genesis of the Registrar-General's social classification of occupations. British Journal of Sociology. 1984;35:522–546. doi:10.2307/590433. [Google Scholar]
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nature Genetics. 2010;42:448–453. doi: 10.1038/ng.573. doi:10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacco-and-Genetics-Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nature Genetics. 2010;42:441–447. doi: 10.1038/ng.571. doi:10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton TG, Rossing MA, Bowen DJ, Srinouanprachan S, Wicklund K, Farin FM. Genetic polymorphisms in dopamine-related genes and smoking cessation in women: A prospective cohort study. Behavioral and Brain Functions. 2007;3:22. doi: 10.1186/1744-9081-3-22. doi:10.1186/1744-9081-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg L, Moon G, Szatkowski L, Iggulden P. Smoking cessation in England: Intentionality, anticipated ease of quitting and advice provision. Social Science and Medicine. 2009;68:610–619. doi: 10.1016/j.socscimed.2008.11.032. doi:10.1016/j.socscimed.2008.11.032. [DOI] [PubMed] [Google Scholar]
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: Catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annual Review of Pharmacology and Toxicology. 1999;39:19–52. doi: 10.1146/annurev.pharmtox.39.1.19. doi:10.1146/annurev.pharmtox.39.1.19. [DOI] [PubMed] [Google Scholar]