Abstract
Introduction:
The question of whether abstinence during the months following a planned quit attempt exacerbates or improves depressive symptoms is an important clinical issue. Extant research has primarily modeled between-person covariation between postquit abstinence and depressive symptom trajectories. However, this approach cannot account for potential third variables between participants that may affect both smoking and depression. Accordingly, the current study examined within-person covariation between time-varying abstinence and depressive symptom in a multilevel model (MLM), which allowed for transitions between smoking statuses within a participant.
Methods:
Participants were 236 heavy drinking smokers in a randomized clinical trial testing the efficacy of incorporating brief alcohol intervention into smoking cessation treatment. Depressive symptoms and biochemically verified abstinence were assessed 1 week prior to and 2, 8, 16, and 26 weeks after quit date.
Results:
MLMs indicated a slight increase in depressive symptoms over time in the sample as a whole. However, there was an inverse relation between time-varying abstinence (vs. smoking) and concurrent level of depressive symptoms, indicating that transitions from smoking to abstinence within individuals were associated with reductions in depressive symptoms.
Conclusions:
During the first 6 months following a planned quit attempt, being abstinent in a particular week appears to be associated with lower levels of concurrent depressive symptoms. These results are not concordant with the view that intentional smoking abstinence exacerbates depressive symptoms. Efforts to promote smoking cessation should highlight that individuals are likely to feel more rather than less psychologically healthy when they successfully quit smoking.
Introduction
The question of how successful and failed smoking cessation attempts affect depression remains open (Hughes, 2007a). Although one study of patients with a history of major depressive disorder (MDD) reported higher incidence of new major depressive episodes among those who successfully quit smoking (Glassman, Covey, Stetner, & Rivelli, 2001), others have found no association between abstinence and the odds of developing a major depressive episode (Kahler et al., 2002; Torres et al., 2010; Tsoh et al., 2000). Furthermore, Torres et al. (2010) found that failure to quit smoking was associated with greater depression incidence than abstinence. Less attention has focused on continuous measures of depressive symptoms, which can capture clinically important variation above and below the threshold of MDD and permit evaluation of both exacerbation and improvement in symptoms. Elevated depressive symptoms are associated with increased medical service utilization and social morbidity (Johnson, Weissman, & Klerman, 1992). Furthermore, even low levels of depressive symptoms predict increased risk of smoking relapse (Niaura et al., 2001). Therefore, depressive symptoms are an important outcome to monitor following smoking cessation treatment.
Kahler et al. (2002), in a sample of smokers with past MDD, found that those who successfully quit smoking for a full year after treatment showed significant reductions in depressive symptoms over that year, while depressive symptoms remained essentially unchanged in those who relapsed to smoking. These findings parallel studies that found self-reported stress (Chassin, Presson, Sherman, & Kim, 2002; Cohen & Lichtenstein, 1990; Parrott, 1995) and anxiety (Hughes, 1992; West & Hajek, 1997) decreased to below precessation levels in the weeks and months following successful smoking cessation. Only one study to date has examined changes in depressive symptoms as they relate to changes in smoking status over time (Munafò, Heron, & Araya, 2008). Trajectories associated with quitting smoking were associated with reductions in depressive symptoms. However, this study was limited to pregnant women, did not focus on individuals seeking cessation treatment, and did not quantify within-individual change in depressive symptoms associated with transitioning between smoking and abstinence.
Knowing whether smoking cessation is associated with reduction or exacerbation of depressive symptoms is crucial for clinical practice. If quitting smoking leads to worse psychological functioning even after withdrawal symptoms have ebbed, that must be addressed in treatment. On the other hand, if those who successfully quit experience less negative affect, stress, or depressive symptoms than those who return to smoking, that information could be used to encourage smokers to make and sustain quit attempts (Chassin et al., 2002; Parrott, 1999). A significant confound in studies addressing this question is that those smokers who experience the least affective disturbance upon quitting are those most likely to remain abstinent. Experimental studies that provide large incentives for abstinence can overcome this limitation by achieving very high abstinence rates (e.g., Gilbert et al., 1999). However, these studies may not parallel the real-world situation in which smokers quit for intrinsic reasons and may experience increased self-esteem and personal efficacy as a result (Cohen & Lichtenstein, 1990).
Although not a definitive test of causality, a promising method for understanding the cessation–depression link is to examine smoking abstinence as a time-varying correlate of depressive symptoms. The use of time-varying abstinence in multilevel models (MLMs) allows for transitions between smoking statuses within a participant. For example, we can observe a pattern in which a participant smokes prior to quit date, is abstinent at two and eight weeks and then returns to smoking at sixteen weeks. Using MLM, we can model whether changes in abstinence across these timepoints are associated with exacerbation or reduction in depressive symptoms. These participant-level effects allow us to account for the fact that those who have the worst smoking outcomes also may have the most vulnerability to depressive symptoms (Hughes, 2007b).
The purpose of the present study was to test, among smokers in cessation treatment, whether time-varying smoking abstinence covaried with depressive symptoms measured 1 week before making a quit attempt and 2, 8, 16, and 26 weeks after a quit attempt. Using MLM, we quantified the difference in depressive symptoms associated with being abstinent versus smoking at a given assessment. We also were able to test whether history of MDD, which has been associated with greater affective disturbance before and after quitting smoking (Berlin, Spreux-Varoquaux, Said, & Launay, 1997; Brown et al., 2007; Hall et al., 1996; Tsoh et al., 2000), significantly altered the effect of smoking abstinence on depressive symptoms.
Methods
Participants
Participants were 236 smokers seeking cessation treatment in a randomized clinical trial comparing standard smoking cessation treatment (ST) to smoking cessation treatment that incorporated brief alcohol intervention (ST-BI; Kahler et al., 2008). Participants had to be ≥18 years old, smoke ≥10 cigarettes/day, use no other tobacco/nicotine products, and drink heavily: for men >14 drinks per week or ≥5 drinks per occasion at least once a month and for women >7 drinks per week or ≥4 drinks per occasion at least once a month (National Institute on Alcohol Abuse and Alcoholism, 1995). Participants were excluded for current substance abuse or dependence (excluding nicotine dependence and alcohol abuse); current affective disorder, psychosis or suicidality; medical contraindications for nicotine patch; or current pregnancy. The sample was 45% female, 90.7% non-Hispanic White, and 33% married. Mean age was 41.5 (SD = 12.0) years, and mean education was 14.0 (SD = 2.6) years. Participants smoked 21.3 (SD = 9.4) cigarettes/day, and mean Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) score was 5.0 (SD = 2.2). A history of MDD, assessed by the Structured Clinical Interview for DSM-IV (Spitzer, Williams, Gibbon, & First, 1990), was reported by 79 (33.5%) participants; 24 of these reported recurrent MDD.
Procedure
Treatment consisted of four-weekly individual counseling sessions with quit date occurring at Session 2. ST-BI incorporated focused discussion of participant’s alcohol use but otherwise was similar to ST. All participants received nicotine patch (21 mg for four weeks, 14 mg for the next two weeks, and 7 mg during the last two weeks). Follow-ups were conducted 8, 16, and 26 weeks after quit date.
Measures
Seven-day point-prevalence abstinence was assessed at 2, 8, 16, and 26 weeks after quit date. Abstinence was verified by a combination of alveolar carbon monoxide ≤10 ppm and saliva cotinine ≤15 ng/ml (SRNT Subcommittee on Biochemical Verification, 2002) or by confirmation from a significant other. Missing self-report or confirmation data were coded as smoking (5.9%, 6.8%, 9.7%, and 5.9% at 2, 8, 16, and 26 weeks, respectively) when classifying smoking outcome patterns. However, no such assumptions were needed for MLM analyses given that all participants completing self-report measures at a given follow-up also provided complete smoking data.
Depressive symptoms during the past week were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D; Radloff, 1977), a 20-item self-report scale with scores ranging from 0 to 60. CES-D completion rates were 99.6% at Session 1 and 83.5%, 87.3%, 86.4%, and 87.3% at 2, 8, 16, and 26 weeks, respectively.
Data Analysis Plan
We first plotted depressive symptoms across follow-ups by common patterns of abstinence. We then used MLM (Singer & Willett, 2003) to test smoking abstinence’s association with depressive symptoms at concurrent assessments. In Level 1, we modeled within-participant change in depressive symptoms using mean level, rate of change (the linear effect of weeks since Session 1, coded 0, 3, 9, 17, or 27), and time-varying abstinence (abstinent = 1; smoking = 0) parameters. The Level 2 model tested whether parameters were significantly different from zero across participants. We covaried the effect of treatment condition, MDD history, gender, and FTND on mean levels of depressive symptoms, given that these variables could be related both to depressive symptoms and to smoking outcomes.
Results
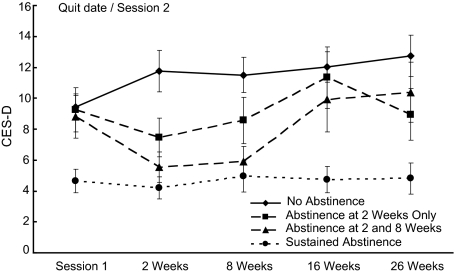
Given that all participants were smoking at Session 1 and that there were four postquit assessments of point-prevalence abstinence, participants could show 1 of 16 possible patterns of abstinence. However, all but 29 (12.3%) participants showed one of the following patterns: (a) continuously smoking (n = 99, 41.9%); (b) abstinent for just the two-week assessment (n = 44, 18.6%); (c) abstinent for just the two- and eight-week assessments (n = 33, 14.0%); or (d) abstinent at all assessments after Session 1 (n = 31, 13.1%). CES-D scores in these 207 participants are depicted in Figure 1. For Patterns 2 and 3, the lowest CES-D scores in absolute terms were at the assessments in which they were abstinent. MDD history was not significantly related to abstinence pattern.
Figure 1.
Depressive symptoms by patterns of smoking abstinence (n = 207). Session 1 represents one week before quit date. Weeks refer to the number of weeks since quit date. Abstinence is biochemically confirmed seven-day point-prevalence smoking abstinence. Errors bars shown are the standard errors of the means for that abstinence pattern at that assessment. Twenty-nine participants with patterns of smoking outcomes other than these four most common patterns are not depicted but are included in the multilevel model analyses. Note. CES-D = Center for Epidemiologic Studies Depression Scale.
Results of the MLM analyses (N = 235 due to one participant missing data on MDD history) are presented in Table 1. Overall, CES-D scores showed significant modest increases over time. Across participants, abstinence at a given assessment, compared with smoking, was associated with a reduction of 2.74 points (SE = 0.51, p < .0001) on the CES-D. Time-varying abstinence accounted for approximately 2.4% of within-participant variation. Follow-up analyses revealed no significant effect of MDD history on the effect of time-varying smoking abstinence (B = −0.51, SE = 1.10, p = .64), a result that was not changed by distinguishing recurrent versus single episode MDD in a subsequent model.
Table 1.
Results of Multilevel Model Predicting CES-D Scores Prior to and After Quit Date (N = 235)
| Fixed effects | B (SE) |
| Mean level (intercept) | |
| Intercept | 5.71 (1.27)*** |
| ST-BI (vs. ST) | 0.49 (0.83) |
| MDD history (vs. none) | 2.09 (0.89)* |
| Male gender (vs. female) | −1.03 (0.85) |
| Baseline FTND score | 0.56 (0.19)** |
| Rate of change (slope) | |
| Intercept | 0.062 (0.02)** |
| Time-varying abstinence | |
| Intercept | −2.74 (0.51)*** |
| Variance components | |
| Level 1 | |
| Within-person residuals | 33.86 (2.10)*** |
| Level 2 | |
| In mean level | 37.55 (5.56)*** |
| Level 2 | |
| In rate of change | 0.029 (0.011)** |
| Level 2 | |
| In time-varying abstinence | 4.77 (5.03) |
Note. Analyses were conducted with full maximum likelihood estimation using PROC MIXED in SAS. Time-varying abstinence refers to seven-day point-prevalence smoking abstinence versus nonabstinence at the assessment concurrent with the assessment of depressive symptoms (CES-D). Analyses included all participants with any data on the CES-D; one participant was not included due to missing data on MDD history. CES-D = Center for Epidemiologic Studies Depression Scale; FTND = Fagerström Test for Nicotine Dependence; MDD = major depressive disorder; ST = standard treatment; ST-BI = standard treatment plus brief alcohol intervention.
*p < .05, **p < .01, ***p < .0001.
Some participants smoked at all assessments, thereby restricting within-participant variability in smoking status. Thus, we re-ran the analyses including only participants who had confirmed abstinence at at least one timepoint (n = 136). In this subsample, the time-varying effect of smoking abstinence accounted for approximately 12.5% of within-person variance and was significant in the Level 2 model, B = −2.10, SE = 0.55, p = .0002.
Discussion
To our knowledge, this is the first examination of within-person covariation between smoking abstinence and depressive symptoms following a cessation attempt. On average, depressive symptoms increased slightly following a quit smoking attempt. However, there was significant variability in depressive symptoms based on abstinence patterns. Those abstinent at all timepoints had particularly low levels of depressive symptoms at baseline, which remained low throughout the follow-up. Smokers who transitioned from smoking to abstinence and back to smoking showed the lowest levels of depressive symptoms when abstinent, while those who were never abstinent showed gradual increases in depressive symptoms over time.
MLM analyses indicated that given an individual’s trajectory of depressive symptoms, being abstinent in a particular week was associated with significantly lower depressive symptoms. This effect did differ according to whether participants had a history of MDD. Accordingly, our results are inconsistent with the notion that abstaining from smoking after a planned quit attempt exacerbates depressive symptoms. Instead, it was only participants who never abstained whose depressive symptoms appeared consistently higher after quit date compared with before.
This study was limited to heavy drinkers seeking cessation treatment, and the sample was predominantly non-Hispanic White, calling for caution in generalizing results. Furthermore, analyses explored concurrent associations between abstinence and depressive symptoms, which leaves open the possibility that changes in depression precipitate transitions between smoking and abstinence rather than the other way around. However, the fact that depressive symptoms decreased in those who initially attained abstinence does suggest that initial success is associated with alleviation in symptoms; whether this reflects psychological mechanisms (e.g., improved self-esteem) or biological mechanisms (e.g., improved physical function or elimination of repeated nicotine withdrawal) cannot be discerned from these data.
Although a causal role cannot be established when participants are not randomized to abstinence, these results, taken together with prior research (Chassin et al., 2002; Cohen & Lichtenstein, 1990; Hughes, 1992; Munafò et al., 2008; Parrott, 1995; West & Hajek, 1997), suggest being successful in quitting is often associated with near-term improvement in psychological functioning. Thus, efforts to promote smoking cessation should highlight that smokers who attempt to quit are likely to feel more rather than less psychologically healthy when they meet their goal of smoking abstinence. Efforts to highlight and enhance reductions in depressive symptoms after quitting may reinforce maintenance of smoking abstinence and increase long-term smoking cessation. Efforts to reduce depressive symptoms prior to quitting also may be important, given that continuous abstinence is related to low pretreatment depressive symptoms.
Funding
This work was supported by the National Institute on Drug Abuse, grants R01 DA15534 to CK, K08 DA029094 to NS, and K08 DA025041 to AL.
Declaration of Interests
None declared.
References
- Berlin I, Spreux-Varoquaux O, Said S, Launay JM. Effects of past history of major depression on smoking characteristics, monoamine oxidase-A and -B activities and withdrawal symptoms in dependent smokers. Drug and Alcohol Dependence. 1997;45:31–37. doi: 10.1016/s0376-8716(97)01338-0. doi:10.1016/S0376-8716(97)01338-0. [DOI] [PubMed] [Google Scholar]
- Brown RA, Niaura R, Lloyd-Richardson EE, Strong DR, Kahler CW, Abrantes AM, et al. Bupropion and cognitive-behavioral treatment for depression in smoking cessation. Nicotine & Tobacco Research. 2007;9:721–730. doi: 10.1080/14622200701416955. doi:10.1080/14622200701416955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Sherman SJ, Kim K. Long-term psychological sequelae of smoking cessation and relapse. Health Psychology. 2002;21:438–443. doi: 10.1037//0278-6133.21.5.438. doi:10.1037/0278-6133.21.5.438. [DOI] [PubMed] [Google Scholar]
- Cohen S, Lichtenstein E. Perceived stress, quitting smoking, and smoking relapse. Health Psychology. 1990;9:466–478. doi: 10.1037//0278-6133.9.4.466. doi:10.1037/0278-6133.9.4.466. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Dibb WD, Plath LC, Hiyane S, et al. EEG, physiology, and task-related mood fail to resolve across 31 days of smoking abstinence: Relations to depressive traits, nicotine exposure, and dependence. Experimental and Clinical Psychopharmacology. 1999;7:427–443. doi: 10.1037//1064-1297.7.4.427. doi:10.1037/1064-1297.7.4.427. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Covey LS, Stetner F, Rivelli S. Smoking cessation and the course of major depression: A follow-up study. Lancet. 2001;357:1929–1932. doi: 10.1016/S0140-6736(00)05064-9. doi:10.1016/S0140-6736(00)05064-9. [DOI] [PubMed] [Google Scholar]
- Hall SM, Muñoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, et al. Mood management and nicotine gum in smoking treatment: A therapeutic contact and placebo-controlled study. Journal of Consulting and Clinical Psychology. 1996;64:1003–1009. doi: 10.1037//0022-006x.64.5.1003. doi:10.1037/0022-006X.64.5.1003. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. Retrieved from http://web.ebscohost.com/ehost/pdfviewer/pdfviewer?vid=8&hid=105&sid=8c44ca53-4abc-4f87-866a-c2ca6f0f5191%40sessionmgr111. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Tobacco withdrawal in self-quitters. Journal of Consulting and Clinical Psychology. 1992;60:689–697. doi: 10.1037//0022-006x.60.5.689. doi:10.1037/0022-006X.60.5.689. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Depression during tobacco abstinence. Nicotine & Tobacco Research. 2007a;9:443–446. doi: 10.1080/14622200701243185. doi:10.1080/14622200701243185. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine & Tobacco Research. 2007b;9:315–327. doi: 10.1080/14622200701188919. doi:10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Johnson J, Weissman MM, Klerman GL. Service utilization and social morbidity associated with depressive symptoms in the community. Journal of the American Medical Association. 1992;267:1478–1483. Retrieved from http://jama.ama-assn.org/cgi/reprint/267/11/1478. [PubMed] [Google Scholar]
- Kahler CW, Brown RA, Ramsey SE, Niaura R, Abrams DB, Goldstein MG, et al. Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. Journal of Abnormal Psychology. 2002;111:670–675. doi: 10.1037//0021-843x.111.4.670. doi:10.1037/0021-843X.111.4.670. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Metrik J, LaChance HR, Ramsey SE, Abrams DB, Monti PM, et al. Addressing heavy drinking in smoking cessation treatment: A randomized clinical trial. Journal of Consulting and Clinical Psychology. 2008;76:852–862. doi: 10.1037/a0012717. doi:10.1037/a0012717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Heron J, Araya R. Smoking patterns during pregnancy and postnatal period and depressive symptoms. Nicotine & Tobacco Research. 2008;10:1609–1620. doi: 10.1080/14622200802412895. doi:10.1080/14622200802412895. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. The physicians’ guide to helping patients with alcohol problems (Vol. NIH Publication No. 95-3769) Rockville, MD: National Institutes of Health; 1995. [Google Scholar]
- Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychology of Addictive Behaviors. 2001;15:13–17. doi: 10.1037/0893-164x.15.1.13. doi:10.1037/0893-164X.15.1.13. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Stress modulation over the day in cigarette smokers. Addiction. 1995;90:233–244. doi: 10.1046/j.1360-0443.1995.9022339.x. Retrieved from http://web.ebscohost.com/ehost/detail?vid=7&hid=105&sid=8c44ca53-4abc-4f87-866a-c2ca6f0f5191%40sessionmgr111&bdata=JnNpdGU9ZWhvc3QtbGl2ZQ%3d%3d#db=sih&AN=9503151139. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Does cigarette smoking cause stress? American Psychologist. 1999;54:817–820. doi: 10.1037//0003-066x.54.10.817. doi:10.1037/0003-066X.54.10.817. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. (Summer) doi:10.1177/014662167700100306. [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. Structured clinical interview for DSM-III-R—Non-patient edition (SCID-NP, Version 1.0) Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. doi:10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Torres LD, Barrera AZ, Delucchi K, Penilla C, Perez-Stable EJ, Munoz RF. Quitting smoking does not increase the risk of major depressive episodes among users of Internet smoking cessation interventions. Psychological Medicine. 2010;40:441–449. doi: 10.1017/S0033291709990560. doi:10.1017/S0033291709990560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoh JY, Humfleet GL, Munoz RF, Reus VI, Hartz DT, Hall SM. Development of major depression after treatment for smoking cessation. American Journal of Psychiatry. 2000;157:368–374. doi: 10.1176/appi.ajp.157.3.368. Retrieved from http://ajp.psychiatryonline.org/cgi/content/full/157/3/368. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P. What happens to anxiety levels on giving up smoking? American Journal of Psychiatry. 1997;154:1589–1592. doi: 10.1176/ajp.154.11.1589. Retrieved from http://ajp.psychiatryonline.org/cgi/content/full/154/11/1589. [DOI] [PubMed] [Google Scholar]