Abstract
Introduction:
Nicotine replacement therapies (NRTs) have been demonstrated to be effective in clinical trials but may have lower efficacy when purchased over-the-counter (OTC). Premature discontinuation and insufficient dosing have been offered as possible explanations. The aims are to (a) investigate the prevalence of and reasons for premature discontinuation of stop-smoking medications (including prescription only) and (b) how these differ by type, duration of use, and source (prescription or OTC).
Methods:
The sample includes 1,219 smokers or recent quitters who had used medication in the last year (80.5% NRT, 19.5% prescription only). Data were from Waves 5 and 6 of the International Tobacco Control (ITC) Four-Country Survey.
Results:
Most of the sample (69.1%) discontinued medication use prematurely. This was more common among NRT users (71.4%) than in users of bupropion and varenicline (59.6%). OTC NRT users were particularly likely to discontinue (76.3%). Relapse back to smoking was the most common reason for discontinuation of medication reported by 41.6% of respondents. Side effects (18.3%) and believing that the medication was no longer needed (17.1%) were also commonly reported. Of those who completed treatment, 37.9% achieved 6-month continuous abstinence compared with 15.6% who discontinued prematurely. Notably, 65.6% who discontinued because they believed the medication had worked were abstinent.
Conclusions:
Premature discontinuation of stop-smoking medications is common but is not a plausible reason for poorer quit outcomes for most people. Encouraging persistence of medication use after relapse or in the face of minor side effects may help increase long-term cessation outcomes.
Introduction
Meta-analyses of clinical trials have concluded that all forms of nicotine replacement therapy (NRT) and the prescription-only medication bupropion increase the rate of smoking cessation over unaided quitting by 50%–70% (Eisenberg et al., 2008; Stead, Perera, Bullen, Mant, & Lancaster, 2008). The prescription-only medication varenicline may be even more effective, with increases of 200%–300% in quit rates reported (Cahill, Stead, & Lancaster, 2008). While NRT remains effective in the absence of formal behavioral support (West & Zhou, 2007), its effectiveness when purchased over-the-counter (OTC) without a prescription has been questioned (Pierce & Gilpin, 2002; Walsh, 2008). Few studies have explored either patterns of use or effectiveness of the prescription-only medications outside clinical settings.
Critics of OTC NRT argue that it is used differently in this context than when supplied on prescription; that essentially those who self-initiate use of NRT fail to use the product appropriately (Cummings & Hyland, 2005). Population-based studies have suggested that adherence to the recommended period of use of OTC NRT is generally poor (e.g., Paul, Walsh, & Girgis, 2003; Pierce & Gilpin, 2002; Thorndike, Biener, & Rigotti, 2002) and that it is typically used with very minimal additional support. Nonetheless, a meta-analysis of four studies found that quit rates using OTC NRT were similar to those of prescription NRT (Hughes, Shiffman, Callas, & Zhang, 2003). This analysis was criticized by Walsh (2008), who pointed to the difficulty of simulating typical OTC use in a randomized controlled trial, where the organization created by the research questioning may add to the base effect of the medication. Walsh concluded that the superiority of OTC NRT (with little structured support) over unaided cessation has not yet been demonstrated convincingly.
In this paper, we investigate why users of stop-smoking medication often fail to comply with the recommended treatment schedule in order to understand how adherence can be improved, particularly in the case of OTC NRT. Inadequate adherence is not necessarily evidence of inappropriate underuse if the reasons given for premature discontinuation are valid. Until recently, it has been recommended that NRT use ceases upon resumption of smoking (i.e., relapse). Therefore, it is valid to discontinue use following relapse, and discontinuation cannot have caused the relapse in such cases, although inappropriate levels of use could. It should be noted that use of NRT as a means of reducing cigarette consumption has been shown to be safe (Stead & Lancaster, 2007), and guidelines in this area are gradually being relaxed (Shahab et al., 2009).
To date, only one population-based study has investigated smokers’ reports of reasons for discontinuation of NRT in detail. Burns and Levinson (2008) found that while relapse was the most frequently cited reason for discontinuation, it was only endorsed by 34% of NRT users. Side effects were reported by only 17% of users as their main reason for discontinuation, followed by the perception that the NRT is not helping (14%), quitting successfully (10%), and cost (5%).
To our knowledge, no population-based studies have explored rates of adherence with bupropion or varenicline nor reasons for discontinuation of these medications. In part because they require a doctor consultation, these medications have really only been tested in the presence of structured support. For both, adverse events leading to discontinuation are commonly reported (Barrueco et al., 2005; Halperin et al., 2009), although side effects are typically mild (Cahill et al., 2008; Zwar & Richmond, 2003). In Australia and the United Kingdom, a second prescription is needed to receive the full course of treatment of both bupropion and varenicline, which may act as a barrier to adherence.
The likelihood of obtaining NRT via prescription differs in the four countries studied here (Hammond et al., 2008). NRT prescriptions are rare in Australia, Canada, and the United States but common in the United Kingdom where all stop-smoking medications are provided through the National Health Service prescription program. Those on income support are entitled to free NRT for 4–6 weeks obtained from an accredited smoking cessation provider. In the United States, some forms of NRT (nasal spray and inhaler) are available only on prescription, whereas the patch is available either OTC or on prescription. In Canada, NRT use is heavily subsidized in two provinces; however there is no national reimbursement scheme, and most smokers obtain it OTC.
The aims of this study were to (a) investigate the prevalence of premature discontinuation of use of stop-smoking medications, both NRT and prescription only; (b) examine reasons given for premature discontinuation; (c) investigate how these reasons are related to cessation outcome; and (d) explore differences, among NRT users, between those who obtained it OTC and via prescription on duration of use, dosage, reason for premature discontinuation, and cessation outcome. We explored whether discontinuation typically follows relapse (consistent with current smoking cessation guidelines) or whether users typically discontinue use prior to relapse. Based on the concerns expressed in the literature, we hypothesized that duration of use and cessation outcome would differ between those who obtain NRT OTC or via prescription, with those who obtained NRT via prescription more likely to use the product for the recommended time and more likely to quit successfully.
Methods
Sample
The sample was 1,219 adult (18+ years) smokers or recent ex-smokers who reported having made a quit attempt in the previous year and reported using medication to help them quit. Of these, 920 (75.5%) had relapsed and 299 were still quit. Data were from Waves 5 and 6 (conducted in October 2006–February 2007 and September 2007–February 2008, respectively) of the International Tobacco Control (ITC) Four-Country Survey, an annual cohort survey of smokers in Canada, United Kingdom, United States, and Australia conducted via computer-assisted telephone interview. Using a stratified random-digit dialing procedure, households are contacted and screened for adult smokers. Where more than one smoker is identified, the next birthday method is used to select the interviewee. At each wave, those lost to follow-up are replaced by a replenishment sample selected using the same procedure. The study recruits only current smokers who are retained in the cohort regardless of whether they subsequently quit. Detailed descriptions of the ITC study conceptual framework (Fong et al., 2006) and methods (Thompson et al., 2006) are available elsewhere.
Where a respondent used medication at both waves, we used data from Wave 6 (resulting in 833 cases from Wave 6 and 386 from Wave 5). Only those who had participated in at least one previous wave were eligible as including new recruits from the replenishment sample (all smokers) would have had the effect of excluding a proportion of those who quit successfully, potentially distorting the results relating to the proportion of medication users who completed treatment and the prevalence of each reason for discontinuation.
Measures
Use of Medication
Participants were asked which medication or combination of medications they had used in the last year. Responses for NRT were categorized into (a) patch and (b) oral forms, such as nicotine gum, lozenges, sublingual tablets, inhaler, or other NRT. Prescription-only medications were either bupropion (Zyban/Wellbutrin) or varenicline (Champix/Chantix). All questions on medication usage were asked separately for users of NRT and prescription-only medication. Because of the difficulty of identifying a single reason for discontinuation, users of both prescription-only medication and NRT could not be included.
Duration of medication use, for those who had used more than one type, was from the start of the first medication to the end of the last. Completion of a course of treatment was defined as use for 8 weeks, with those who terminated prior to this cutpoint considered to have stopped prematurely. This covers virtually all minimum use recommendations (e.g., Fiore et al., 2008; Zwar et al., 2007) and is a significant period of sustained use. We categorized duration of medication use into (a) less than 1 week, (b) 1–2 weeks, (c) 2–4 weeks, (d) 4–8 weeks, and (e) more than 8 weeks (completed treatment).
Among oral NRT users, medication dosage (only available at Wave 6) was classified into adequate use (10 or more pieces) compared with less.
Reason for Premature Discontinuation
Participants who had discontinued medication use prematurely were asked: “Why did you discontinue using the medication(s)?”, with reasons categorized for analysis as (a) relapsed (from the response “medication didn’t work/went back to smoking”), (b) the medication was no longer needed (from the response “quit/it worked”), (c) side effects or a legitimate medical reason, including pregnancy or advice from a health professional to discontinue, (d) low motivation to continue (a composite of citing the cost of the medication, running out, not liking the taste, or an excuse such as “sore jaw from chewing all day”), and (e) situational factors (a composite of citing a stressful or social situation). To determine in which category to place the 57 participants who reported multiple reasons for discontinuation, we applied a decision rule: Relapse (medication did not work) or quit (medication worked) took precedence over any other reason. This accounted for all cases with multiple reasons for discontinuation.
Other Sample Descriptors
We collected sociodemographic information at recruitment (age, gender, ethnic status, country, and education) and the Heaviness of Smoking Index (HSI), a measure of nicotine dependence (Heatherton, Kozlowski, Frecker, Rickert, & Robinson, 1989), using the data from the wave prior to the one with the medication use (or the last wave as a smoker). The HSI is scored from 0 to 6. We recoded it into three categories: low: 0–1, medium: 2–3, and high dependence: 4–6. Majority (as compared with minority) ethnic status was defined as “White” in Canada, the United States, and the United Kingdom and “speaks only English at home” in Australia. Education was categorized as low, medium, or high, with country-specific definitions, but in all cases, low education designated those who did not complete secondary school and high education those with a university degree.
Abstinence Outcome
The main outcome was 6-month continuous abstinence, accepted regardless of whether the person subsequently relapsed. For those quit for less than 6 months at the target wave, we used data from the next wave to resolve the outcome.
Outcome data were only available for 548 cases (45.0% of the sample). We were unable to determine outcome for those in the Wave 5 sample as the questions asked at this wave did not allow us to determine conclusively whether the reported medication usage took place on the most recent quit attempt (and was therefore relevant to the outcome). At Wave 6, 23% reported that the medication use did not take place on their most recent quit attempt, and a further 7% were excluded because they were still using medication and had been using for less than 8 weeks, and longer term use was unknown.
Data Analysis
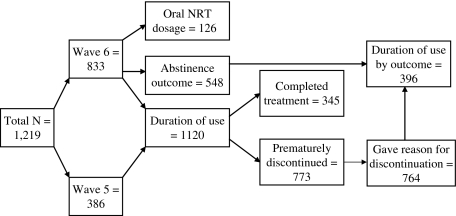
All analyses were performed using SPSS v.18. The Mann–Whitney U statistic was used for comparing groups on duration of medication use. Multiple logistic regression was used to examine predictors of completing a course of treatment and abstinence. Odds ratios (ORs) and 95% CIs were calculated for each predictor variable. A p value of less than .05 was considered statistically significant. Figure 1 shows the number of participants included in each analysis and the data wave from which they were sourced.
Figure 1.
Flow chart showing the number of participants included in each analysis and the data wave from which they were sourced.
Results
Characteristics of the sample are displayed in Table 1. The mean age was 45.5 years (SD = 13.0), and 60.5% of the sample were female. Participants smoked an average of 17.5 cigarettes/day (SD = 9.2) and had their first cigarette of the day within a median 30 min of waking with an interquartile range of 50 min.
Table 1.
Characteristics of the Sample by Type (NRT or prescription only) and Source of NRT (OTC or prescription)
| Characteristic | Overall NRT (n = 981) | OTC NRT (n = 670) | Prescription NRT (n = 311) | Prescription-only (n = 238) | Total (n = 1,219) |
| Gender (% female) | 60.8 | 58.8 | 65.0 | 59.2 | 60.5 |
| Age (years; %) | |||||
| 18–24 | 6.0 | 6.6 | 4.8 | 3.4 | 5.5 |
| 25–39 | 29.4 | 31.5 | 24.8 | 26.9 | 28.9 |
| 40–54 | 39.7 | 40.3 | 38.3 | 37.8 | 39.3 |
| 55+ | 25.0 | 21.6 | 32.2 | 31.9 | 26.3 |
| Country (%) | |||||
| Canada | 24.4 | 24.5 | 24.1 | 20.6 | 23.6 |
| United States | 15.7 | 16.4 | 14.1 | 51.7 | 22.7 |
| United Kingdom | 30.2 | 17.5 | 57.6 | 8.4 | 25.9 |
| Australia | 29.8 | 41.6 | 4.2 | 19.3 | 27.7 |
| Ethnic group (% majority) | 92.3 | 91.5 | 94.2 | 90.8 | 92.0 |
| Education (%) | |||||
| Low | 52.5 | 49.4 | 59.1 | 44.5 | 50.9 |
| Medium | 31.7 | 32.6 | 29.5 | 37.0 | 32.7 |
| High | 15.9 | 18.0 | 11.4 | 18.5 | 16.4 |
| Nicotine dependence (%) | |||||
| Low | 18.6 | 19.9 | 15.9 | 14.8 | 17.9 |
| Medium | 52.1 | 53.4 | 49.2 | 53.2 | 52.3 |
| High | 29.3 | 26.6 | 35.0 | 32.1 | 29.8 |
| No longer using medication (%) | 84.1 | 84.3 | 83.6 | 81.9 | 83.7 |
Note. NRT = nicotine replacement therapy; OTC = over-the-counter.
Type of Medication Used and Adherence to Dosage
Overall, 80.5% (n = 981) had used NRT (73.1% patch and 36.5% oral forms, mainly gum: 25.7%) and the remainder (19.5%, n = 238) used prescription-only medications.
Over two thirds of NRT users (68.3%) bought it OTC. This differed markedly by country. In total, 95.5% obtained NRT OTC in Australia compared with 71.4% in the United States, 68.6% in Canada, and only 39.5% in the United Kingdom, where the majority obtained it on prescription.
Of the 238 who used prescription-only medication, 50.8% used varenicline and 49.6% bupropion (one participant used both). All varenicline use was from the Wave 6 sample, and 79.2% of varenicline users were from the United States. Varenicline was not available in Australia at the time of the Wave 6 survey.
All patch users reported using the patch daily as recommended. Of the 126 participants from Wave 6 with oral NRT dosage data, only 15.9% complied with the recommended dosage.
Prevalence of Premature Discontinuation
Overall, 30.9% (n = 345) reported using medication for at least 8 weeks, with a further 63.4% (n = 773) having stopped prematurely. An additional 101 who had been using for less than 8 weeks were still using medication, and thus, duration of use could not be determined. Table 2 displays patterns of duration of use. Most striking is the difference in the proportion who stopped using within the first week (20.0% for NRT compared with 2.3% for prescription-only medication). Table 2 also shows that those who obtained NRT OTC and on prescription differed markedly; those who obtained OTC were more likely to discontinue in the first week of use (23.0% compared with 13.4% for prescription NRT) and less likely to use NRT for more than 1 month.
Table 2.
Duration of Medication Use by Type (NRT or prescription only) and Source of NRT (OTC or prescription)
| Duration of use | NRT (overall, n = 905), % | OTC NRT (n = 621), % | Prescription NRT (n = 284), % | Prescription only (n = 213), % |
| <1 week | 20.0 | 23.0 | 13.4 | 2.3 |
| 1–2 weeks | 12.5 | 13.5 | 10.2 | 7.0 |
| 2–4 weeks | 22.9 | 26.4 | 15.1 | 23.9 |
| 4–8 weeks | 16.0 | 13.4 | 21.8 | 26.3 |
| ≥8 weeks | 28.6 | 23.7 | 39.4 | 40.4 |
Note. NRT = nicotine replacement therapy; OTC = over-the-counter.
Completion of a full 8 weeks of medication use was significantly more likely among prescription medication users (either prescription-only or NRT) than among OTC NRT users, χ2(2) = 33.87, p < .001. However, it was not significantly related to nicotine dependence, χ2(2) = 3.04, p = .22.
A significant minority (5.5%, n = 62) reported use of medication for more than 6 months. This was more common among users of oral NRT only (10.2%) than in those who used both forms (7.3%) or the nicotine patch only (3.6%), χ2(2) = 13.85, p = .001. Prolonged use (>6 months) of prescription-only medication was also reported by 7.5% of bupropion users and 3.2% of those who used varenicline.
Excluding those who had used the patch and an oral form of NRT in combination, we compared patch users with users of oral NRT on duration of use. The proportion that completed a course of treatment did not differ significantly (26.7% patch compared with 30.5% oral, χ2(1) = 1.23, p = .27). Among those who discontinued prematurely (n = 574), the median duration of patch use was 14 days compared with 7 days for oral NRT (Mann–Whitney U: 24,637.5, p < .001). Among oral NRT users, completion of a course of treatment was unrelated to adherence to dosage (22.2% compared with 34.8% among those not compliant; χ2(1) = 1.08, p = .30).
There was also no difference in completion between varenicline users (43.7%) and bupropion users (36.7%), χ2(1) = 1.08, p = .30. Among those who discontinued prematurely (n = 127), the median duration of use was 21 days for both products (Mann–Whitney U: 1,952.5, p = .81).
We used logistic regression to examine predictors of completing a course of treatment. Bivariate associations with each predictor and the results of the logistic regression model are displayed in Table 3. Country, older age, high education, non-White ethnicity, type of medication (NRT or prescription only), and source of medication (OTC or prescription) were significant predictors. Each remained significant independent predictors in the logistic regression model, with the exception of type of medication (source of medication was the dominant predictor). A significant independent effect also emerged for high nicotine dependence.
Table 3.
Logistic Regression Analysis Predicting Completion of a Course of Medication
| Predictor | n | Bivariate associations | Overall model (n = 1,106) |
| Age | 1,118 | 1.02 (1.01–1.03) | 1.02 (1.00–1.03) |
| Gender | |||
| Male | 434 | 1.00 | 1.00 |
| Female | 684 | 1.24 (0.95–1.61) | 1.16 (0.88–1.53) |
| Country | |||
| Australia | 319 | 1.00 | 1.00 |
| Canada | 263 | 1.70 (1.17–2.48) | 1.41 (0.94–2.10) |
| United States | 250 | 2.19 (1.51–3.18) | 1.62 (1.07–2.46) |
| United Kingdom | 286 | 2.15 (1.50–3.09) | 1.57 (1.04–2.37) |
| Education | |||
| Low | 572 | 1.00 | 1.00 |
| Medium | 362 | 0.99 (0.74–1.32) | 0.94 (0.69–1.28) |
| High | 179 | 1.59 (1.12–2.26) | 1.81 (1.25–2.63) |
| Ethnic group | |||
| Majority | 1,028 | 1.00 | 1.00 |
| Identified minority | 87 | 0.52 (0.30–0.90) | 0.50 (0.28–0.89) |
| Nicotine dependence | |||
| Low | 200 | 1.00 | 1.00 |
| Medium | 580 | 0.84 (0.60–1.17) | 0.78 (0.55–1.12) |
| High | 334 | 0.72 (0.49–1.04) | 0.66 (0.44–0.98) |
| Type of medication | |||
| NRT | 905 | 1.00 | 1.00 |
| Prescription only | 213 | 1.69 (1.24–2.30) | 1.09 (0.72–1.65) |
| Source of medication | |||
| Over-the-counter | 621 | 1.00 | 1.00 |
| Prescription | 497 | 2.14 (1.65–2.76) | 1.82 (1.29–2.57) |
Reason for Premature Discontinuation
Of the 1,020 participants who had stopped using medication, 75.9% discontinued the medication prematurely (prior to 8 weeks of use). The most common reasons given for discontinuation were relapse/medication did not work (41.6%), side effects, or legitimate medical reasons (18.3%) and because the medication was no longer needed (17.1%). As shown in Table 4, almost half of NRT users reported that they discontinued because they had relapsed, but this was much less common among users of prescription-only medication. Premature discontinuation of prescription-only medication was more frequently reported because of side effects associated with the medications (reported by over a third).
Table 4.
Reason for Premature Discontinuation, Overall and by Type of Medication Used (NRT or prescription only)
| Reason for premature discontinuation | Overall (n = 764), % | NRT user (n = 637), % | Prescription-only user (n = 127), % | χ2(1) | p value |
| Relapse/medication didn’t work | 41.6 | 46.3 | 18.1 | 34.66 | <.001 |
| Side effects/legitimate medical reason | 18.3 | 14.8 | 36.2 | 32.59 | <.001 |
| No longer need | 17.1 | 16.3 | 21.3 | 1.81 | .18 |
| Low motivation (cost, ran out, taste, and other reason) | 12.4 | 12.9 | 10.2 | 0.68 | .41 |
| Situational (stressful or social) | 7.6 | 7.8 | 6.3 | 0.36 | .55 |
| Other (unclassified) | 2.9 | 1.9 | 7.9 | 13.59 | <.001 |
Note. NRT = nicotine replacement therapy.
Those high in nicotine dependence were more likely to have discontinued because they relapsed (49.6%) than those with medium (40.3%) or low dependence (30.7%), χ2(2) = 12.79, p = .002. Conversely, discontinuation because the medication was no longer needed was less likely in the highly dependent (12.1%) than in the medium- (18.7%) or low-dependence group (22.0%), χ2(2) = 7.16, p = .03. No other significant effects by level of dependence were found.
We explored whether the reasons given for premature discontinuation from NRT differed by form of use or source of product. Those who used patch and oral NRT in combination were excluded from the former analysis. Patch and oral NRT users did not differ in their reasons for discontinuation (all p values above .13).
There was some evidence of a trend for varenicline users to more frequently discontinue because the medication was no longer needed and because of relapse than bupropion users (both p values 0.11); however, power was low.
Relapse and side effects declined as reasons for discontinuation by duration of medication use (p = .001 and p = .027 respectively) while reporting that the medication was no longer needed became more common (p < .001).
Abstinence Outcome
Of the 548 cases for whom we were able to establish a smoking cessation outcome, 22.6% achieved 6-month continuous abstinence. More prescription-only medication users (30.6%) than NRT users (19.8%) achieved this, χ2(1) = 7.01, p = .008). There was no difference in outcome by whether oral NRT dosage was at recommended levels, χ2(1) = 0.06, p = .81.
In all analyses that follow, those who discontinued because they relapsed are excluded as none achieved abstinence (as is implicit). Table 5 shows the proportion of cases achieving 6-month continuous abstinence by reason for premature discontinuation and duration of medication use.
Table 5.
Proportion of Cases Achieving 6-Month Continuous Abstinence by Reason for Premature Discontinuation and Duration of Medication Use
| Duration of use | Side effects (n = 62), % | No longer need (n = 64), % | Low motivation (n = 45), % | Situational (n = 31), % | Total (n = 347), % |
| <1 week (n = 80) | 0 | 60.0 | 7.1 | 20.0 | 6.3 |
| 1–2 weeks (n = 54) | 25.0 | 37.5 | 25.0 | 0 | 11.1 |
| 2–4 weeks (n = 114) | 8.7 | 76.9 | 13.3 | 0 | 21.1 |
| 1–2 months (n = 99) | 7.1 | 64.0 | 16.7 | 0 | 19.2 |
| Total (n = 347) | 8.1 | 65.6 | 13.3 | 3.2 | 15.6 |
Likelihood of quit success was increased by duration of medication use. Of those who used medication for less than a week (n = 45), 11.1% achieved 6-month continuous abstinence compared with 21.4% of those who used for 1–2 weeks (n = 28), 33.3% of those who used between 2 and 4 weeks (n = 72), 29.0% of those who used between 4 and 8 weeks (n = 69), and 37.9% among those who completed a course of treatment (n = 182; χ2(4) = 13.81, p = .008).
Logistic regression was used to investigate whether duration of use and reason for premature discontinuation predicted continuous abstinence controlling for the demographic variables listed in Table 3 and nicotine dependence. Those who discontinued use prematurely were significantly less likely to achieve abstinence than those who completed the course of medication (OR = 0.16, 95% CI = 0.08–0.31), whereas those who stopped because they did not need it any more were significantly more likely to succeed (OR = 3.26, 95% CI = 1.75–6.07).
Comparison of OTC and Prescription NRT
No significant differences were found between those who obtained NRT OTC and on prescription in reasons for premature discontinuation (all p values > .11); in adherence to the recommended dosage, χ2(1) = 0.17, p = .68; or in outcome, 17.6% OTC abstinent compared with 23.8% prescription, χ2(1) = 2.20, p = .14.
Discussion
The results of this study confirm that only about a quarter of smokers using stop-smoking medications today are likely to complete the recommended 8 weeks of treatment. The main reasons for premature discontinuation of medication were relapse back to smoking, followed by reported side effects, and the perception that the medication had worked for them and was no longer needed.
In this study, almost half (46%) of NRT users who discontinued use prematurely did so because they had relapsed or they thought the medication had not worked. By contrast, only 18% of those who used prescription-only medication relapsed, then stopped taking their medication. Relapse also occurred earlier among those who used NRT. Some of the discontinuation may have been because of unreasonable expectations about the capacity of medication to eliminate cravings, but it is implausible in all cases that the premature discontinuation of medication early in the quitting process is the direct cause of relapse. We also observed that those higher in dependence were more likely to discontinue using medication because they had relapsed. For more dependent smokers, there may be a benefit of the prescription-only drugs in the early stages of quitting since adherence to the medication seems to be stronger.
A significant proportion of medication users discontinued prematurely because they believed they no longer needed to use medication (17% overall, with no difference by type of medication). This was particularly common between 1 and 2 months of use, accounting for 28% of discontinuation in this period. Of note, two thirds of those who believed the medication had worked were able to achieve 6-month continuous abstinence, which suggests that people are reasonably good at judging when it is appropriate to discontinue (although some of this might be after the event reinterpretation). It may be worth conducting a trial of participant choice of termination point, allowing both shorter and longer periods than currently recommended to see if this enhances outcomes in a cost-effective way.
Concerns have been raised about the incidence and severity of adverse events, particularly with regard to bupropion and varenicline (Barrueco et al., 2005; Halperin et al., 2009). Nonetheless, early discontinuation (within the first week) attributed to side effects or to a legitimate medical reason was uncommon, reported by only 4% of the sample overall. This is similar to rates observed by Ossip, Abrams, Mahoney, Sall, and Cummings (2009) in a sample of Quitline callers provided with free NRT. However, side effects were thereafter the most frequently cited reason for discontinuation, particularly among users of prescription-only medication. It is not clear whether side effects of prescription-only medication take time to appear or whether users are prepared to tolerate them for a while. We suspect the latter is more often the case, perhaps because users have been warned to expect them by the doctor and/or pharmacist. By contrast, side effects of NRT tended to precipitate discontinuation early on. Whether this is due to less discussion of what to expect and thus less preparedness to tolerate them is unclear, although we note that similar patterns were found regardless of whether the NRT was obtained OTC or by prescription.
The present study is consistent with numerous previous studies (e.g., Burns & Levinson, 2008; Hyland, Rezaishiraz, Giovino, Bauer, & Cummings, 2005) in finding that smokers typically do not use medication for the recommended duration. Including those still using medication who had used for over a month, over half (54%) of NRT users reported using NRT for less than 4 weeks (a similar proportion was reported by Hyland et al.), and discontinuation within a week was common. Duration of use did not differ between those who used the nicotine patch and those who used oral forms of NRT, while adherence to prescription-only medication was far higher than for NRT.
Premature discontinuation of stop-smoking medications may not be as large a consumer compliance problem as originally suggested in the literature (Pierce & Gilpin, 2002; Thorndike et al., 2002). Rather, we observed that most subjects discontinued medication use for what appear to be perfectly legitimate reasons. Clinical guidelines state that medication use should cease upon relapse (Zwar et al., 2004), while experiencing side effects is also a legitimate reason for discontinuation (although, depending on severity, should not necessarily lead to discontinuation or preclude switching to another form of medication). The present data indicate that premature discontinuation because of a belief that the medication has worked is also legitimate as it is associated with very high rates of continuous abstinence. Accepting this, we find that few cases of relapse can be attributed directly to premature discontinuation of medication. However, some of the reasons (motivational and situational) may reflect reduced motivation to persist and could be targeted. For example, those who report not liking the taste of nicotine gum could easily be advised to switch to another form of NRT or to accept that this is something they need to tolerate.
Overall, there was significantly more premature discontinuation among those who obtained NRT OTC (compared with on prescription) and more early relapse. The proportion of prescription NRT users who used the product for more than 8 weeks was comparable with that of users of prescription-only medication. Despite this, there was no difference between OTC and prescription NRT users in the likelihood of achieving 6-month continuous abstinence. This finding is consistent with the meta-analysis performed by Hughes et al. (2003) and a more recent study by West and Zhou (2007) and should serve to alleviate concerns that unsupervised NRT use is ineffective, although it should be noted that prescription NRT users were a more dependent group and thus might have been expected to have had inferior outcomes and in fact trended to superior ones.
Several limitations of the present study should be noted. We relied on retrospective reports, some several months after the event, which inevitably opens up the possibility that some results were due to post-hoc explanations (i.e., influenced by the outcome of the quit attempt). We also had limited information about the amount of support actually provided around quitting. There were insufficient cases reporting concurrent use of behavioral assistance to include this in the analysis. It would be interesting to do so in future as concurrent use of behavioral assistance can increase quit rates over medication alone by 50%–100% (Fiore et al., 2008).
Relapse is to be expected. Most former smokers progressed through a number of attempts before achieving long-term abstinence (U.S. Department of Health and Human Services, 1990). Current labeling of NRT products in which smokers are advised to stop using the product following relapse is inconsistent with this reality and with the evidence that concurrent use of NRT and cigarettes is safe (Fagerstrom & Hughes, 2002). Rather than recommend discontinuation of the product, it may be fruitful to advise smokers to continue using NRT after relapse, particularly if the relapse occurred within the first couple of weeks of cessation when withdrawal symptoms are most acute. Relapse is not necessarily evidence that the medication did not work as it may well have reduced the severity of nicotine withdrawal compared with an unassisted attempt. Rather, it may suggest that the medication was not used effectively. We found that few oral NRT users used to the recommended dosage, and only a minority of patch users combined the patch with an oral form, which has been shown to increase effectiveness (Stead et al., 2008). Education will be needed to reduce concern about side effects that may be a barrier to continued use of NRT (Bansal, Cummings, Hyland, & Giovino, 2004) and to use at the appropriate dosage.
In conclusion, most people who use medications do so appropriately, and inappropriate premature termination of use is not a major determinant of relapse. That said, the data suggest that some smokers may have unreasonable expectations of how effective stop-smoking medications are likely to be for them. Ensuring that smokers have a better appreciation of the limitations of these medications, and are more motivated to avoid potentially remediable reasons for premature discontinuation, may increase their likelihood of success. However, premature termination of use because of a belief that one has successfully quit seems to be a judgment many are able to accurately make. Encouraging smokers to engage more actively in the way they use medication may optimize their chances of cessation.
Funding
This work was supported by National Health and Medical Research Council of Australia (grant numbers 265903 and 450110), Cancer Research UK (grant number C312/A6465), U.S. National Cancer Institute (grant numbers RO1 CA100362, P50 CA111236, and P01 CA138389), and Canadian Institutes of Health Research (grant number 79551).
Declaration of Interests
James Balmford is currently employed part time, through the University of Freiburg, Germany, on a project funded by a Pfizer Global Health Partnership. This work is unrelated to this paper, which is part of his other employment at The Cancer Council Victoria. K. Michael Cummings is employed fulltime at Roswell Park Cancer Institute where he is involved in doing a stop-smoking study supported by Nabi Biopharmaceuticals; he also serves as a paid expert witness on behalf of plaintiffs in litigation against the tobacco industry.
Acknowledgments
The authors wish to thank Hua Yong for his assistance preparing the ITC dataset.
References
- Bansal MA, Cummings KM, Hyland A, Giovino GA. Stop-smoking medications: Who uses them, who misuses them, and who is misinformed about them? Nicotine & Tobacco Research. 2004;6:S303–S310. doi: 10.1080/14622200412331320707. doi:10.1080/14622200412331320707. [DOI] [PubMed] [Google Scholar]
- Barrueco M, Otero MJ, Palomo L, Jimenez-Ruiz C, Torrecilia M, Romero P, et al. Adverse effects of pharmacological therapy for nicotine addiction in smokers following a smoking cessation program. Nicotine & Tobacco Research. 2005;7:335–342. doi: 10.1080/14622200500124768. doi:10.1080/14622200500124768. [DOI] [PubMed] [Google Scholar]
- Burns EK, Levinson AH. Discontinuation of nicotine replacement therapy among smoking-cessation attempters. American Journal of Preventive Medicine. 2008;34:212–215. doi: 10.1016/j.amepre.2007.11.010. doi:10.1016/j.amepre.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews. 2008;3 doi:10.1002/14651858.CD006103.pub3. [Google Scholar]
- Cummings KM, Hyland A. Impact of nicotine replacement therapy on smoking behavior. Annual Review of Public Health. 2005;26:583–599. doi: 10.1146/annurev.publhealth.26.021304.144501. doi:10.1146/annurev.publhealth.26.021304.144501. [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Filion KB, Yavin D, Belisle P, Mottillo S, Joseph L, et al. Pharmacotherapies for smoking cessation: A meta-analysis of randomised controlled trials. Canadian Medical Association Journal. 2008;179:135–144. doi: 10.1503/cmaj.070256. doi:10.1503/cmaj.070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO, Hughes JR. Nicotine concentrations with concurrent use of cigarettes and nicotine replacement: A review. Nicotine & Tobacco Research. 2002;4:S73–S79. doi: 10.1080/1462220021000032753. doi:10.1080/1462220021000032753. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update—Clinical practice guideline. Rockville, MD: US Department of Health and Human Services; 2008. [Google Scholar]
- Fong GT, Cummings KM, Borland R, Hastings G, Hyland A, Giovino GA, et al. The conceptual framework of the International Tobacco Control (ITC) policy evaluation project. Tobacco Control. 2006;15:iii3–iii11. doi: 10.1136/tc.2005.015438. doi:10.1136/tc.2006.017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin AC, McAfee TA, Jack LM, Catz SL, McClure JB, Deprey TM, et al. Impact of symptoms experienced by varenicline users on tobacco treatment in a real world setting. Journal of Substance Abuse Treatment. 2009;36:428–434. doi: 10.1016/j.jsat.2008.09.001. doi:10.1016/j.jsat.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D, Reid JL, Driezen P, Cummings KM, Borland R, Fong GT, et al. Smokers’ use of nicotine replacement therapy for reasons other than stopping smoking: Findings from the ITC Four Country Survey. Addiction. 2008;103:1696–1703. doi: 10.1111/j.1360-0443.2008.02320.x. doi:10.1111/j.1360-0443.2008.02320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: Using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Addiction. 1989;84:791–800. doi: 10.1111/j.1360-0443.1989.tb03059.x. doi:10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Shiffman S, Callas P, Zhang J. A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tobacco Control. 2003;12:21–27. doi: 10.1136/tc.12.1.21. doi:10.1136/tc.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A, Rezaishiraz H, Giovino G, Bauer JE, Cummings KM. Over-the-counter availability of nicotine replacement therapy and smoking cessation. Nicotine & Tobacco Research. 2005;7:547–555. doi: 10.1080/14622200500185975. doi:10.1080/14622200500185975. [DOI] [PubMed] [Google Scholar]
- Ossip DJ, Abrams SM, Mahoney MC, Sall D, Cummings KM. Adverse effects with use of nicotine replacement therapy among quitline clients. Nicotine & Tobacco Research. 2009;11:408–417. doi: 10.1093/ntr/ntp005. doi:10.1093/ntr/ntp005. [DOI] [PubMed] [Google Scholar]
- Paul CL, Walsh RA, Girgis A. Nicotine replacement therapy products over the counter: Real-life use in the Australian community. Australian and New Zealand Journal of Public Health. 2003;27:491–495. doi: 10.1111/j.1467-842x.2003.tb00820.x. doi:10.1111/j.1467-842X.2003.tb00820.x. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Gilpin EA. Impact of over-the-counter sales on effectiveness of pharmaceutical aids for smoking cessation. Journal of the American Medical Association. 2002;288:1260–1264. doi: 10.1001/jama.288.10.1260. [DOI] [PubMed] [Google Scholar]
- Shahab L, Cummings KM, Hammond D, Borland R, West R, McNeill A. The impact of changing nicotine replacement therapy licencing laws in the United Kingdom: Findings from the International Tobacco Control Four Country Survey. Addiction. 2009;104:1420–1427. doi: 10.1111/j.1360-0443.2009.02641.x. doi:10.1111/j.1360-0443.2009.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead LF, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD005231.pub2. doi:10.1002/14651858.CD005231.pub2. [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2008;(1) doi: 10.1002/14651858.CD000146.pub3. doi:10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- Thompson ME, Fong GT, Hammond D, Boudreau C, Driezen P, Hyland A, et al. Methods of the International Tobacco Control (ITC) Four Country Survey. Tobacco Control. 2006;15:iii12–iii18. doi: 10.1136/tc.2005.013870. doi:10.1136/tc.2005.013870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike AN, Biener L, Rigotti NA. Effect on smoking cessation of switching nicotine replacement therapy to over the counter status. American Journal of Public Health. 2002;92:437–442. doi: 10.2105/ajph.92.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The health benefits of smoking cessation. A report of the Surgeon General. Atlanta, GA: Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion. Office on Smoking and Health; 1990. [Google Scholar]
- Walsh RA. Over-the-counter nicotine replacement therapy: A methodological review of the evidence supporting its effectiveness. Drug and Alcohol Review. 2008;27:529–547. doi: 10.1080/09595230802245527. doi:10.1080/09595230802245527. [DOI] [PubMed] [Google Scholar]
- West R, Zhou X. Is nicotine replacement therapy for smoking cessation effective in the “real world”? Findings from a prospective multinational cohort study. Thorax. 2007;62:930–931. doi: 10.1136/thx.2007.078758. doi:10.1136/thx.2007.081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwar N, Richmond R. Review of bupropion for smoking cessation. Drug and Alcohol Review. 2003;22:203–220. doi: 10.1080/09595230100100642. doi:10.1080/09595230100100642. [DOI] [PubMed] [Google Scholar]
- Zwar N, Richmond R, Borland R, Peters M, Stillman S, Litt J, et al. Smoking cessation pharmacotherapy: An update for health professionals. Melbourne, Australia: The Royal Australian College of General Practitioners; 2007. [Google Scholar]
- Zwar N, Richmond R, Borland R, Stillman S, Cunningham M, Litt J. Smoking cessation guidelines for Australian General Practice. Canberra, Australia: Commonwealth Department of Health and Ageing; 2004. [PubMed] [Google Scholar]