Abstract
To study the role of Actinobacillus pleuropneumoniae-RTX-toxin III (ApxIII) in the pathogenesis of porcine pleuropneumonia, we cloned and characterized the gene encoding this toxin. For that purpose, we screened an expression library of genomic DNA of serotype 8 with an ApxIII-specific monoclonal antibody and isolated a 425-bp fragment of an immunoreactive clone. Using this fragment as a probe, we identified and cloned an overlapping chromosomal NsiI restriction fragment of 5.0 kbp. Escherichia coli cells that contained this fragment produced a protein similar to ApxIII. Like ApxIII, the protein had a molecular mass of approximately 120 kDa, was recognized by an ApxIII-specific antibody, killed porcine lung macrophages, and was not lytic for sheep erythrocytes. We concluded from these data that the 5.0-kbp NsiI fragment contained the ApxIII-coding gene. Nucleotide sequence analysis of the 5.0-kbp NsiI fragment revealed the presence of two genes, apxIIIC and apxIIIA. These genes coded for proteins ApxIIIC and ApxIIIA, respectively, which were 53 and 50% identical to the prototypic RTX proteins HlyC and HlyA of E. coli. We assumed that the apxIIIA gene coded for the structural RTX toxin and that the apxIIIC gene coded for its activator. In addition, we found that ApxIII could be secreted from E. coli by the heterologous RTX transporter proteins HlyB and HlyD. The deduced amino acid sequence of ApxIIIA was 50% identical to that of ApxIA and 41% identical to that of ApxIIA. We concluded that, beside ApxI and ApxII, ApxIII is the third RTX toxin produced by A. pleuropneumoniae.
Full text
PDF

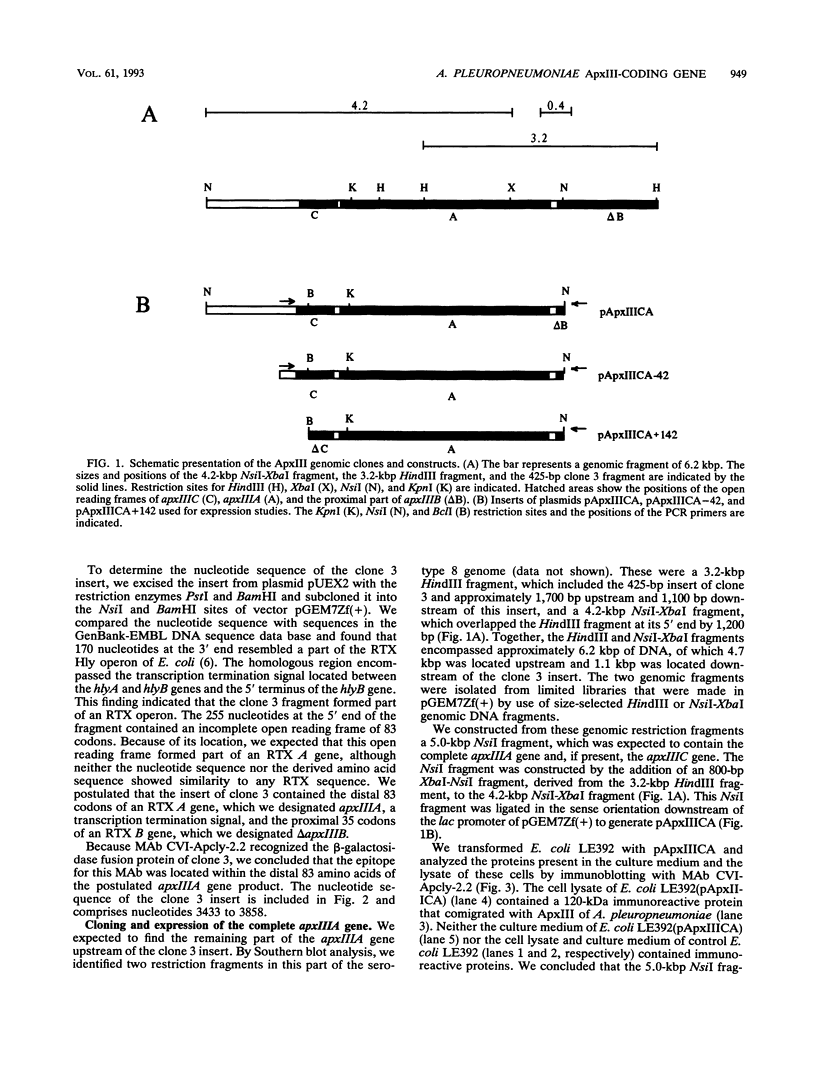


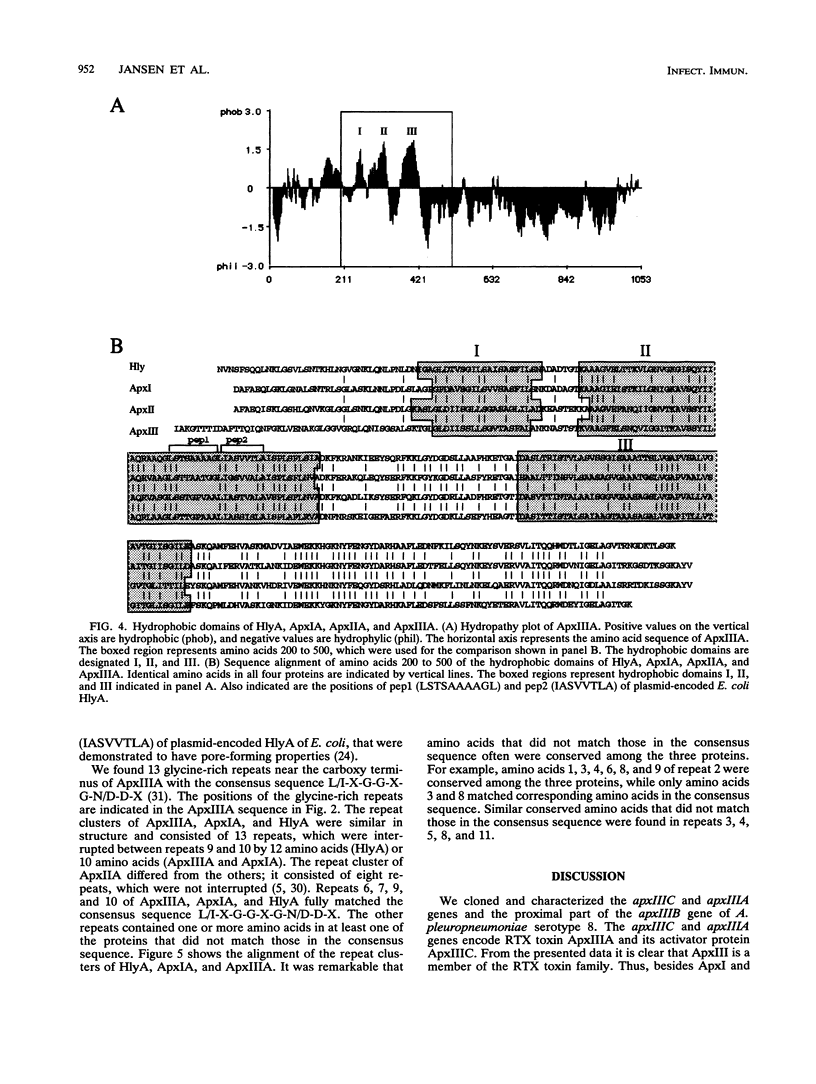


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertram T. A. Actinobacillus pleuropneumoniae: molecular aspects of virulence and pulmonary injury. Can J Vet Res. 1990 Apr;54 (Suppl):S53–S56. [PubMed] [Google Scholar]
- Boehm D. F., Welch R. A., Snyder I. S. Calcium is required for binding of Escherichia coli hemolysin (HlyA) to erythrocyte membranes. Infect Immun. 1990 Jun;58(6):1951–1958. doi: 10.1128/iai.58.6.1951-1958.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D. F., Welch R. A., Snyder I. S. Domains of Escherichia coli hemolysin (HlyA) involved in binding of calcium and erythrocyte membranes. Infect Immun. 1990 Jun;58(6):1959–1964. doi: 10.1128/iai.58.6.1959-1964.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan G. M., Stanley K. K. pUEX, a bacterial expression vector related to pEX with universal host specificity. Nucleic Acids Res. 1987 Dec 10;15(23):10056–10056. doi: 10.1093/nar/15.23.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Struck D. K. Cloning and characterization of a hemolysin gene from Actinobacillus (Haemophilus) pleuropneumoniae. DNA. 1989 Nov;8(9):635–647. doi: 10.1089/dna.1.1989.8.635. [DOI] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Struck D. K. The Actinobacillus pleuropneumoniae hemolysin determinant: unlinked appCA and appBD loci flanked by pseudogenes. J Bacteriol. 1991 Aug;173(16):5151–5158. doi: 10.1128/jb.173.16.5151-5158.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Welch R. A. Alterations of amino acid repeats in the Escherichia coli hemolysin affect cytolytic activity and secretion. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5269–5273. doi: 10.1073/pnas.85.14.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Meier R., Gygi D., Nicolet J. Nucleotide sequence of the hemolysin I gene from Actinobacillus pleuropneumoniae. Infect Immun. 1991 Sep;59(9):3026–3032. doi: 10.1128/iai.59.9.3026-3032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Nicolet J. Hemolysin patterns of Actinobacillus pleuropneumoniae. J Clin Microbiol. 1990 Feb;28(2):232–236. doi: 10.1128/jcm.28.2.232-236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., van den Bosch H., Segers R., Nicolet J. Identification of a second hemolysin (HlyII) in Actinobacillus pleuropneumoniae serotype 1 and expression of the gene in Escherichia coli. Infect Immun. 1992 Apr;60(4):1671–1676. doi: 10.1128/iai.60.4.1671-1676.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygi D., Nicolet J., Frey J., Cross M., Koronakis V., Hughes C. Isolation of the Actinobacillus pleuropneumoniae haemolysin gene and the activation and secretion of the prohaemolysin by the HlyC, HlyB and HlyD proteins of Escherichia coli. Mol Microbiol. 1990 Jan;4(1):123–128. doi: 10.1111/j.1365-2958.1990.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Issartel J. P., Koronakis V., Hughes C. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature. 1991 Jun 27;351(6329):759–761. doi: 10.1038/351759a0. [DOI] [PubMed] [Google Scholar]
- Iwase M., Lally E. T., Berthold P., Korchak H. M., Taichman N. S. Effects of cations and osmotic protectants on cytolytic activity of Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun. 1990 Jun;58(6):1782–1788. doi: 10.1128/iai.58.6.1782-1788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R., Briaire J., Kamp E. M., Smits M. A. Comparison of the cytolysin II genetic determinants of Actinobacillus pleuropneumoniae serotypes. Infect Immun. 1992 Feb;60(2):630–636. doi: 10.1128/iai.60.2.630-636.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp E. M., Popma J. K., Anakotta J., Smits M. A. Identification of hemolytic and cytotoxic proteins of Actinobacillus pleuropneumoniae by use of monoclonal antibodies. Infect Immun. 1991 Sep;59(9):3079–3085. doi: 10.1128/iai.59.9.3079-3085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp E. M., van Leengoed L. A. Serotype-related differences in production and type of heat-labile hemolysin and heat-labile cytotoxin of Actinobacillus (Haemophilus) pleuropneumoniae. J Clin Microbiol. 1989 Jun;27(6):1187–1191. doi: 10.1128/jcm.27.6.1187-1191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig E., Dailey T., Kolodrubetz D. Nucleotide sequence of the leukotoxin gene from Actinobacillus actinomycetemcomitans: homology to the alpha-hemolysin/leukotoxin gene family. Infect Immun. 1990 Apr;58(4):920–929. doi: 10.1128/iai.58.4.920-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo R. Y., Strathdee C. A., Shewen P. E. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica A1. Infect Immun. 1987 Sep;55(9):1987–1996. doi: 10.1128/iai.55.9.1987-1996.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A., Jarchau T., Benz R., Goebel W. The repeat domain of Escherichia coli haemolysin (HlyA) is responsible for its Ca2+-dependent binding to erythrocytes. Mol Gen Genet. 1988 Nov;214(3):553–561. doi: 10.1007/BF00330494. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Schmid A., Benz R., Goebel W. Mutations affecting pore formation by haemolysin from Escherichia coli. Mol Gen Genet. 1991 Apr;226(1-2):198–208. doi: 10.1007/BF00273604. [DOI] [PubMed] [Google Scholar]
- Oropeza-Wekerle R. L., Muller S., Briand J. P., Benz R., Schmid A., Goebel W. Haemolysin-derived synthetic peptides with pore-forming and haemolytic activity. Mol Microbiol. 1992 Jan;6(1):115–121. doi: 10.1111/j.1365-2958.1992.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Rosendal S., Devenish J., MacInnes J. I., Lumsden J. H., Watson S., Xun H. Evaluation of heat-sensitive, neutrophil-toxic, and hemolytic activity of Haemophilus (Actinobacillus) pleuropneumoniae. Am J Vet Res. 1988 Jul;49(7):1053–1058. [PubMed] [Google Scholar]
- Rycroft A. N., Williams D., Cullen J. M., Macdonald J. The cytotoxin of Actinobacillus pleuropneumoniae (pleurotoxin) is distinct from the haemolysin and is associated with a 120 kDa polypeptide. J Gen Microbiol. 1991 Mar;137(3):561–568. doi: 10.1099/00221287-137-3-561. [DOI] [PubMed] [Google Scholar]
- Rycroft A. N., Williams D., McCandlish I. A., Taylor D. J. Experimental reproduction of acute lesions of porcine pleuropneumonia with a haemolysin-deficient mutant of Actinobacillus pleuropneumoniae. Vet Rec. 1991 Nov 16;129(20):441–443. doi: 10.1136/vr.129.20.441. [DOI] [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Smits M. A., Briaire J., Jansen R., Smith H. E., Kamp E. M., Gielkens A. L. Cytolysins of Actinobacillus pleuropneumoniae serotype 9. Infect Immun. 1991 Dec;59(12):4497–4504. doi: 10.1128/iai.59.12.4497-4504.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Cloning, nucleotide sequence, and characterization of genes encoding the secretion function of the Pasteurella haemolytica leukotoxin determinant. J Bacteriol. 1989 Feb;171(2):916–928. doi: 10.1128/jb.171.2.916-928.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee C. A., Lo R. Y. Extensive homology between the leukotoxin of Pasteurella haemolytica A1 and the alpha-hemolysin of Escherichia coli. Infect Immun. 1987 Dec;55(12):3233–3236. doi: 10.1128/iai.55.12.3233-3236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabarovsky E. R., Allikmets R. L. An improved technique for the efficient construction of gene libraries by partial filling-in of cohesive ends. Gene. 1986;42(1):119–123. doi: 10.1016/0378-1119(86)90158-7. [DOI] [PubMed] [Google Scholar]
- van Leengoed L. A., Dickerson H. W. Influence of calcium on secretion and activity of the cytolysins of Actinobacillus pleuropneumoniae. Infect Immun. 1992 Feb;60(2):353–359. doi: 10.1128/iai.60.2.353-359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]