Abstract
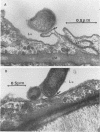
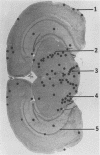
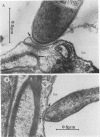
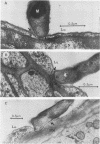
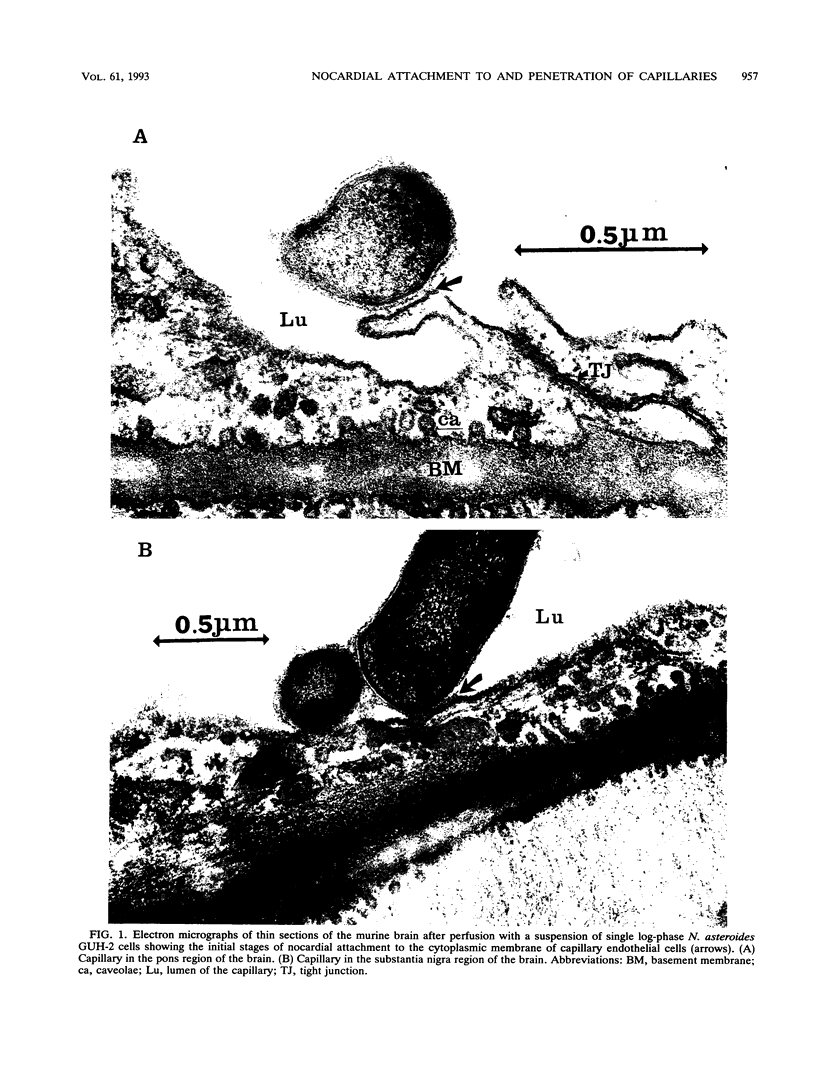
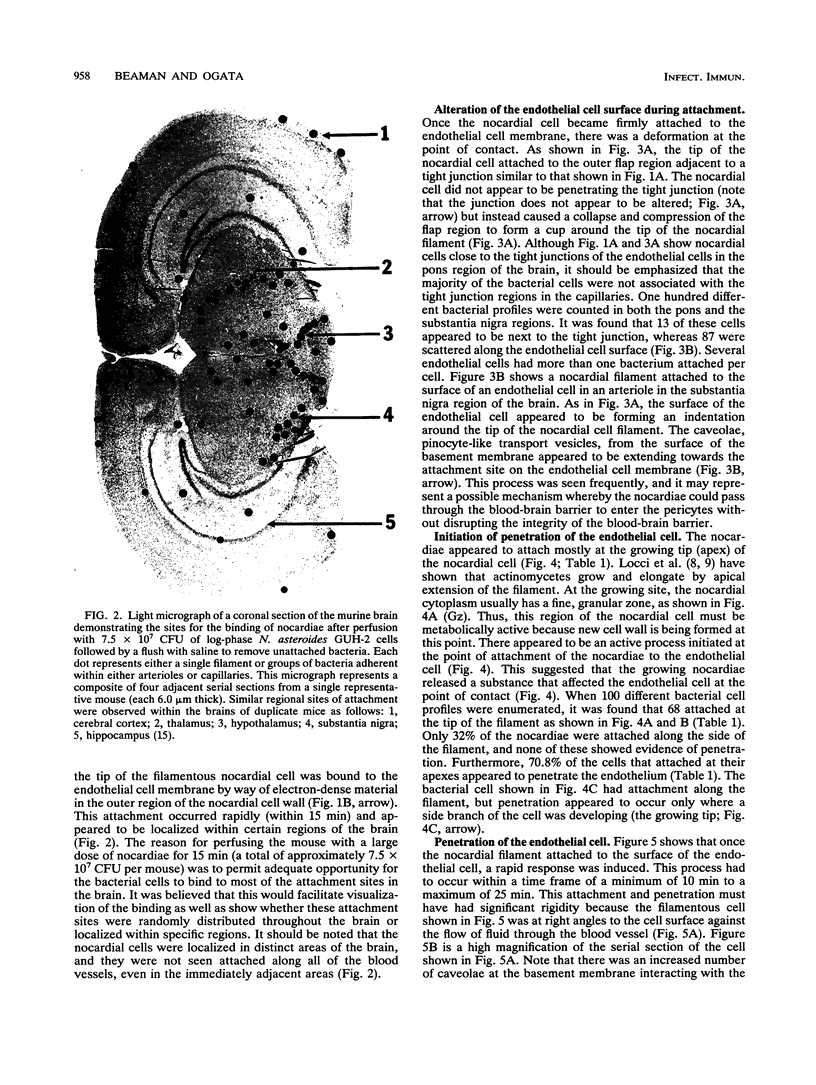
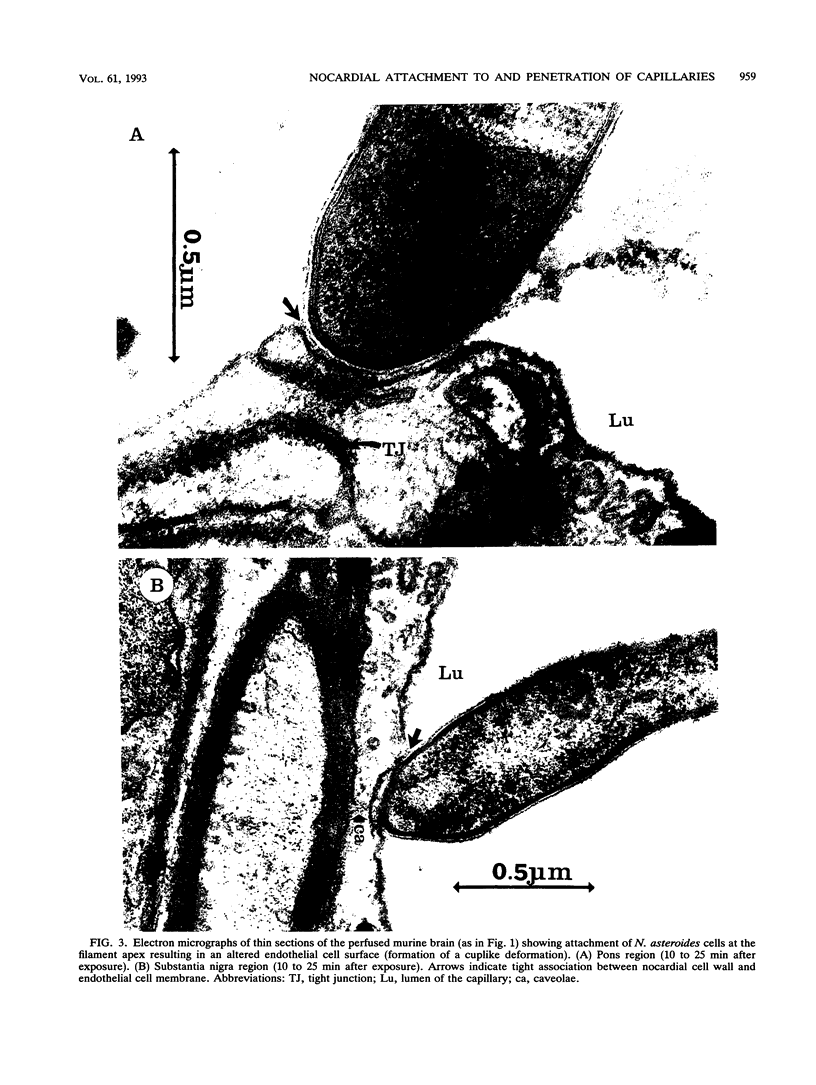
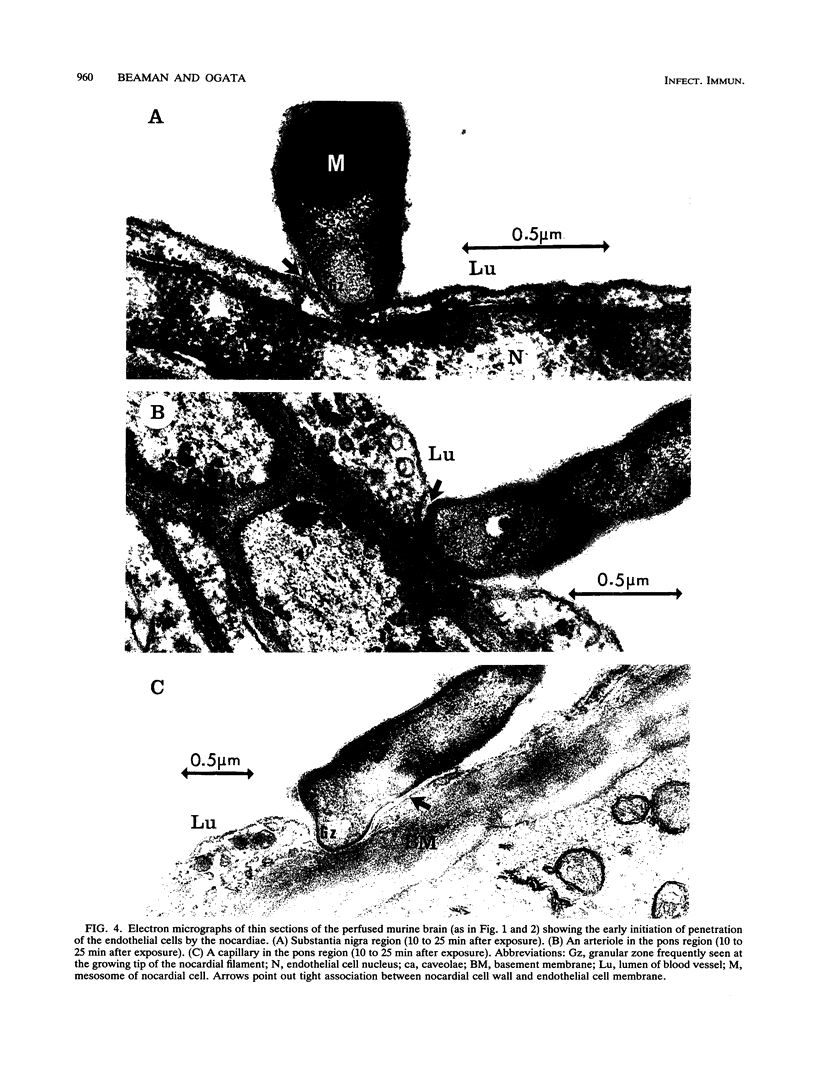
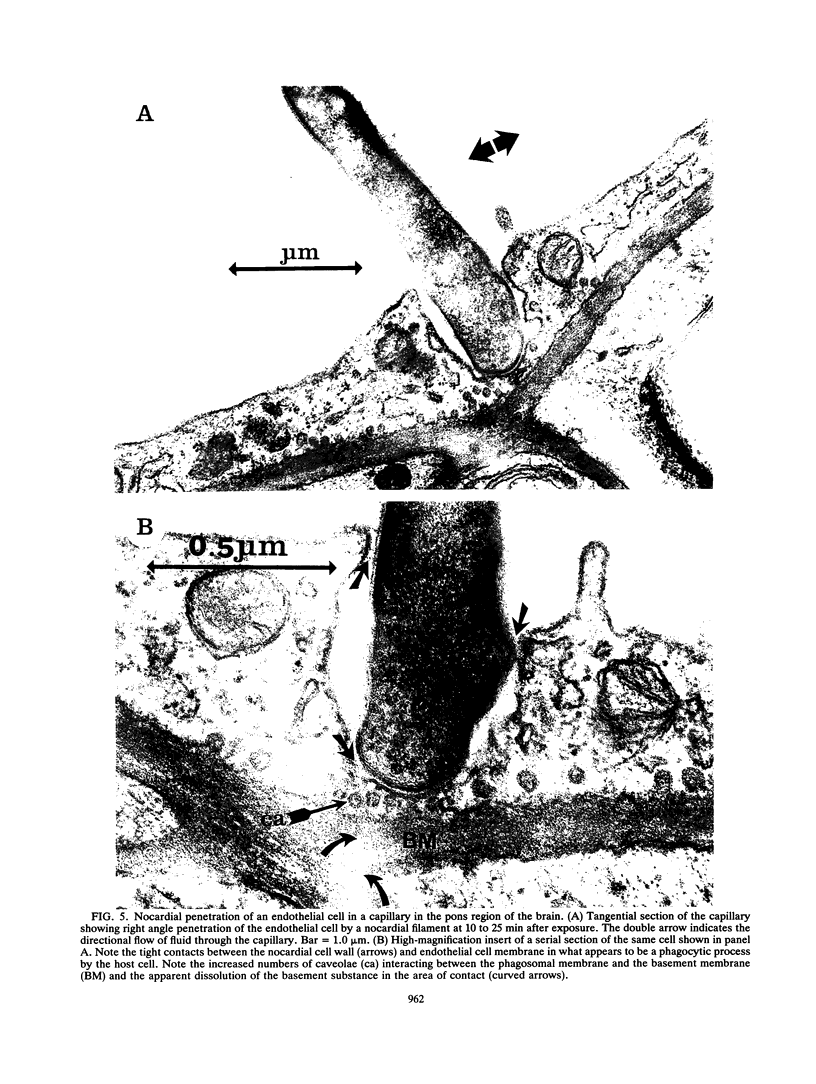
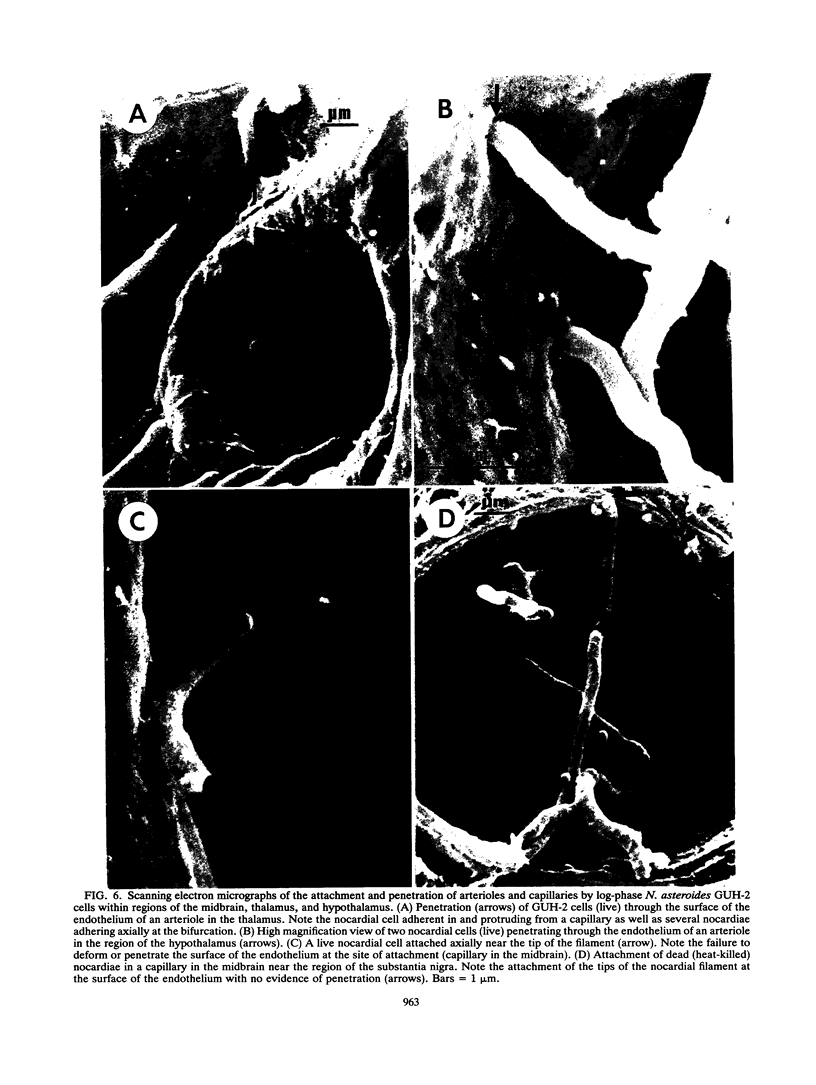
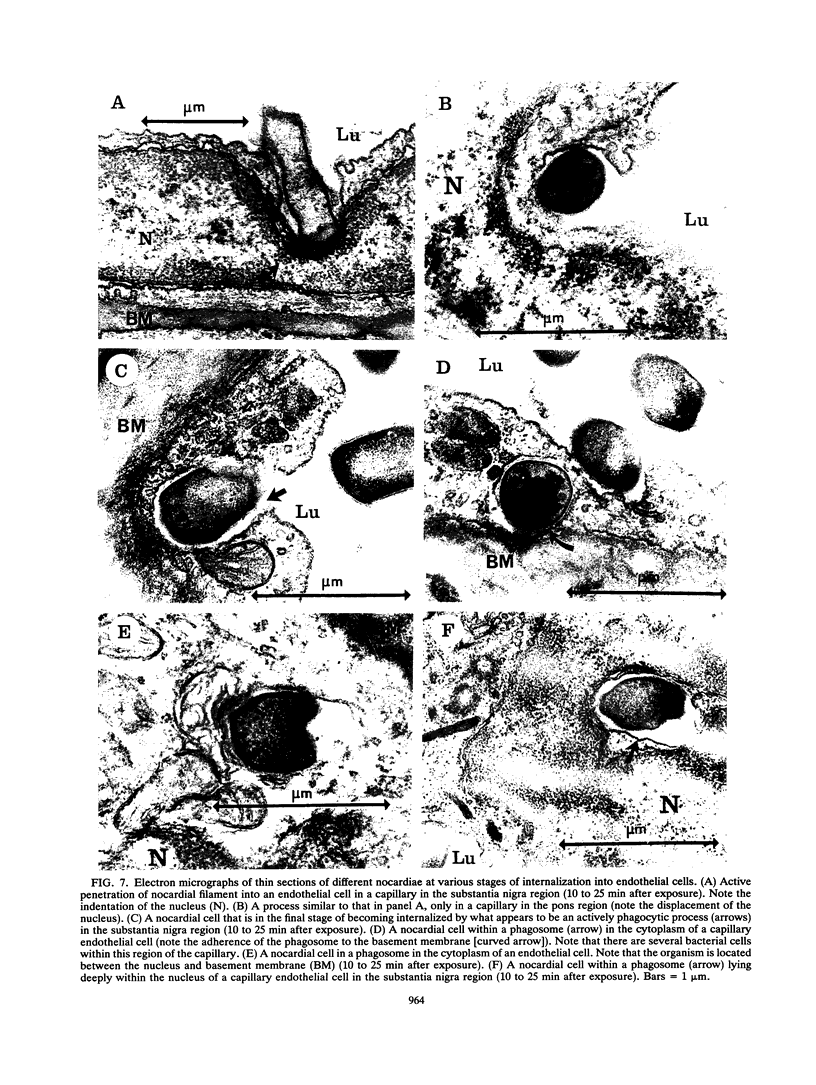
The attachment to and penetration of endothelial cells in the pons and midbrain (especially the substantia nigra) regions of the brains of BALB/c mice by log-phase Nocardia asteroides GUH-2 cells were determined by both scanning and transmission electron microscopic analysis. Within 15 min after exposure, the nocardiae attached to the surface of the endothelial cell membrane. This attachment occurred primarily at the growing tip of the nocardial filament, and the outermost layer of the nocardial cell wall had regions (electron-dense areas) that bound firmly to the cytoplasmic membrane of the host cell. There appeared to be specificity for this binding localized within the capillaries and arterioles because some regions had large numbers of bacteria bound, whereas adjacent areas had no bacterial cells. Nocardial filaments that attached by the apex induced a cuplike deformation of the endothelial cell membrane. This was followed by a rapid penetration of the endothelial cell so that within 25 min many of the bacteria were internalized within the host cell. These internalized bacteria remained within vesicles, and there was no ultrastructural evidence of damage to the nocardial cell during this process. Heat-killed GUH-2 cells still attached to endothelial surfaces (at a reduced frequency), but they did not penetrate into the endothelial cell. These data suggest that brain-invasive nocardiae possess both an adhesin for attachment to the membrane of endothelial cells and an invasion factor that promotes nocardial penetration of these cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman B. L., Gershwin M. E., Maslan S. Infectious agents in immunodeficient murine models: pathogenicity of Nocardia asteroides in congenitally athymic (nude) and hereditarily asplenic (Dh/+) mice. Infect Immun. 1978 May;20(2):381–387. doi: 10.1128/iai.20.2.381-387.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Gershwin M. E., Scates S. S., Ohsugi Y. Immunobiology of germfree mice infected with Nocardia asteroides. Infect Immun. 1980 Aug;29(2):733–743. doi: 10.1128/iai.29.2.733-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Maslan S. Virulence of Nocardia asteroides during its growth cycle. Infect Immun. 1978 Apr;20(1):290–295. doi: 10.1128/iai.20.1.290-295.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Moring S. E. Relationship among cell wall composition, stage of growth, and virulence of Nocardia asteroides GUH-2. Infect Immun. 1988 Mar;56(3):557–563. doi: 10.1128/iai.56.3.557-563.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L. Nocardia as a pathogen of the brain: mechanisms of interactions in the murine brain--a review. Gene. 1992 Jun 15;115(1-2):213–217. doi: 10.1016/0378-1119(92)90561-3. [DOI] [PubMed] [Google Scholar]
- Comstock L. E., Thomas D. D. Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect Immun. 1989 May;57(5):1626–1628. doi: 10.1128/iai.57.5.1626-1628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohbata S., Beaman B. L. L-dopa-responsive movement disorder caused by Nocardia asteroides localized in the brains of mice. Infect Immun. 1991 Jan;59(1):181–191. doi: 10.1128/iai.59.1.181-191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci R., Schaal K. P. Apical growth in facultative Anaerobic actinomycetes as determined by immunofluorescent labeling. Zentralbl Bakteriol A. 1980 Feb;246(1):112–118. [PubMed] [Google Scholar]
- Mounier J., Vasselon T., Hellio R., Lesourd M., Sansonetti P. J. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992 Jan;60(1):237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata S. A., Beaman B. L. Adherence of Nocardia asteroides within the murine brain. Infect Immun. 1992 May;60(5):1800–1805. doi: 10.1128/iai.60.5.1800-1805.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata S. A., Beaman B. L. Site-specific growth of Nocardia asteroides in the murine brain. Infect Immun. 1992 Aug;60(8):3262–3267. doi: 10.1128/iai.60.8.3262-3267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski A., Benach J. L. Lyme borreliosis: host responses to Borrelia burgdorferi. Microbiol Rev. 1991 Mar;55(1):21–34. doi: 10.1128/mr.55.1.21-34.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Sprinz H., LaBrec E. H., Formal S. B. Experimental bacillary dysentery. An electron microscopic study of the response of the intestinal mucosa to bacterial invasion. Am J Pathol. 1965 Dec;47(6):1011–1044. [PMC free article] [PubMed] [Google Scholar]
- Vasselon T., Mounier J., Hellio R., Sansonetti P. J. Movement along actin filaments of the perijunctional area and de novo polymerization of cellular actin are required for Shigella flexneri colonization of epithelial Caco-2 cell monolayers. Infect Immun. 1992 Mar;60(3):1031–1040. doi: 10.1128/iai.60.3.1031-1040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S. Rickettsial interactions with human endothelial cells in vitro: adherence and entry. Infect Immun. 1984 May;44(2):205–210. doi: 10.1128/iai.44.2.205-210.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]