Abstract
Stimulation of the cystic fibrosis transmembrane conductance regulator (CFTR) by protease-activated receptors (PARs) at the basolateral membranes and by adenosine receptors (ADO-Rs) at the apical membrane maintain airway surface liquid (ASL) volume, which is required to ensure hydrated and clearable mucus. Both pathways involve the release of prostaglandin E2 (PGE2) and the stimulation of their basolateral receptors (EP-Rs). We sought to determine whether gap junctions contribute to the coordination of these pathways for modulating CFTR activity and mucus hydration. We used RT-PCR and Western blotting to determine connexin (Cx), CD73, and EP-R expression in a Calu-3 airway epithelial cell line grown on Transwell (Corning Costar, Cambridge, MA) inserts. We used dye coupling to evaluate gap junctional intercellular communication (GJIC). We used Ussing chamber studies and X-Z confocal microscopy to monitor Cl− secretion and ASL volume regulation. We found that connexin 43 (Cx43)–mediated GJIC was increased either by endogenous ADO after the hydrolysis of purine nucleotides by CD73 or by the direct activation of ADO-Rs. Inhibition of phospholipase A2 and cyclooxygenase prevented ADO-dependent increases in GJIC, suggesting the involvement of PGE2. PGE2 was found to increase GJIC markedly by stimulating EP4-Rs. The modulation of ADO signaling also affected the PAR-dependent activation of CFTR. The reduction of GJIC by CD73 or Cx43 inhibition prevented PAR-evoked CFTR currents in Ussing chambers. The inhibition of GJIC resulted in a failure of PGE2 to increase ASL volume in Calu-3 cells and in primary cultures of well-differentiated human airway epithelial cells. Thus, gap junctions coordinate a signaling network comprising CFTR, ADO-Rs, PARs, and EP-Rs, and are required for ASL volume homeostasis.
Keywords: CFTR, connexin, nucleotides, airway surface liquid, PGE2
CLINICAL RELEVANCE.
We show that gap junction channels coordinate a signaling network, activated by adenosine, proteases, and prostaglandin E2, to activate the cystic fibrosis transmembrane conductance regulator in human airway epithelial cells. This coordination is required for airway surface liquid homeostasis, and thus the maintenance of a proper epithelial host defense. Altered regulation of gap junctions may represent an additional layer of dysfunction in airway diseases.
The airway epithelium plays a crucial role in protecting pulmonary tissue from foreign substances by orchestrating mechanical, innate, and acquired host defense systems. Airborne particles, including pathogens, are absorbed by the layer of mucus lining the airways, where they are inactivated by the innate mucosal defense system and removed via mucociliary and cough clearance (1). The mucus lining the upper airways is mostly produced by submucosal glands, whereas the overall airway surface liquid (ASL) volume is determined by active transepithelial salt transport involving the amiloride-sensitive epithelial Na+ channel (ENaC) and the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel (2, 3). In cystic fibrosis (CF), the impairment of CFTR channels leads to decreased ASL volume and a dehydrated airway surface, which favor infection by opportunistic pathogens, resulting in a progressively destructive chronic inflammation of the lung (4).
Efficient mucociliary clearance is achieved by the continuous regulation of airway surface hydration by multiple effectors of CFTR activity. In this context, G-protein–coupled receptors (GPCRs), including adenosine (ADO) receptors (ADO-Rs) and protease-activated receptors (PARs), are well-known activators of CFTR. ADO-Rs and PARs each comprise a family of four subtypes (A1-R, A2A-R, A2B-R, and A3-R, and PAR-1, PAR-2, PAR-3, and PAR-4, respectively). ADO is a product of ATP hydrolysis by ecto-ATPDase (CD39) and ecto-5′-nucleotidase (CD73), two ectoenzymes bound to the membranes of epithelial cells (5, 6). A2B-Rs are mostly expressed at the surface of airway epithelial cells, and their stimulation activates CFTR via intracellular 3′-5′-cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA), whereas their inhibition results in ASL height collapse (7–9). The activation of PAR involves the enzymatic cleavage of the NH2-terminus by proteases such as thrombin, trypsin, or elastase. After PAR stimulation, G proteins stimulate phospholipase C-β to produce inositol 1,4,5-trisphosphate, which in turn increases intracellular Ca2+ concentrations and PKC activity. The activation of PAR in the basal membrane by trypsin or with PAR-2–selective peptides was shown to regulate ion transport function in airway epithelial cells (10, 11). In the Calu-3 airway epithelial cell line, the PAR-dependent activation of CFTR was abolished with cyclooxygenase (COX) inhibitors and prostaglandin E2 (PGE2) receptor antagonists (12). Thus, PAR stimulation of Cl− secretion occurs in part through an indirect mechanism involving the synthesis and release of PGE2. Consistent with this idea, the ADO-dependent activation of Cl− secretion in airway epithelial cells was reported to be sensitive to phospholipase A2 (PLA2) and COX inhibitors, again implicating arachidonic acid and eicosanoid metabolism in the regulation of CFTR activity (7, 8). These observations indicate that the rate of ADO formation by CD39/CD73 and the presence of proteases are modulators of ASL volume and inflammation via PGE2 signaling. PGE2 is a major COX product in a number of physiologic settings, and acts on four subtypes of GPCRs (EP1-R, EP2-R, EP3-R, and EP4-R) (13). According to increasing evidence, PGE2 exerts both proinflammatory and anti-inflammatory effects, depending on receptor subtype, cell population, and context of activation. Interestingly, ADO production, PLA2 activity, the production of arachidonic acid, and the release of eicosanoid are thought to be elevated in chronic airway diseases, including CF (14–19).
The coordination of signaling generated in airway cells by the activation of GPCR is critical for an appropriate regulation of ASL height, efficient mucociliary clearance, and inflammatory response. In this context, gap junctions may contribute to the coordination of intercellular signaling, because they provide direct aqueous pores between neighboring cells, thus allowing the intercellular spread of ions, nucleotides, and second messengers (20). Gap junctions are present in airway epithelial cells, and the regulation of their channel activity is defective in CF (21, 22). Thus, this study set out to determine whether gap junctions mediate the GPCR-induced activation of CFTR, and if their blockade could inhibit ASL volume homeostasis.
MATERIALS AND METHODS
Cell Cultures
The human airway epithelial Calu-3 cell line was purchased from the American Type Culture Collection (Rockville, MD). A Sleeping Beauty transposon vector pT2/si-PuroV2 was used for the stable expression in Calu-3 cells of short hairpin RNAs (shRNAs) directed against CFTR. Calu-3 cells that knock out CFTR (SH3) or express an altered shRNA CFTR sequence containing five nucleotide replacements (Alter cells) were generated, as previously described (12). Calu-3, SH3, and Alter cells were maintained in DMEM/F12 (3:1 vol/vol), supplemented with 10% FCS, 30 U/ml penicillin, and 30 mg/ml streptomycin. SH3 and Alter cells were continuously selected in the presence of 4 μg/ml puromycin, as reported previously (12). For all experiments, 100,000 cells were seeded onto 0.33-cm2 porous (0.4 μm) Transwell polyester membranes (Transwell 3470; Corning Costar, Cambridge, MA) and cultured for up to 15 days. In some experiments, the apical medium was removed after 5–6 days, and cells were kept at the air–liquid interface for up to 10 more days.
Primary cultures of well-differentiated human airway epithelial cells (HAECs) were purchased from Epithelix Sàrl (Geneva, Switzerland). HAEC cultures were grown on Transwell inserts and maintained at the air–liquid interface, as previously described (23).
RNA Isolation and RT-PCR
Cellular RNA was extracted from cell lines with NucleoSpin RNA II (Macheley-Nagel, Oensingen, Switzerland). After reverse transcription, 40 cycles of PCR were performed (at 94°C for 30 seconds, 62°C for 45 seconds, and 72°C for 90 seconds), using the following primer pairs: EP1 (forward, TTGTCGGTATCATGGTGGTG; reverse, ATGTACACCCAAGGGTCCAG), EP2 (forward, GCAGTCTCCCTGCTCTTCTG; reverse, CACCGAGACAATGAGAAGCA), EP4 (forward, GACCTGTTGGGCACTTTGTT; reverse, TGGACGCATAGACTGCAAAG), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (forward, ACTCCACTCACGGCAAATTC; reverse, TCTCCATGGTGGTGAAGACA).
Western Blotting
Western blotting was performed as previously described (24), using antibodies against connexin 43 (Cx43; B.D. Biosciences, Basel, Switzerland), CD73 (Lab Force AG, Nunningen, Switzerland), and CFTR (clone M24.1; R&D Systems GmbH, Wiesbaden, Germany). An anti–β-actin (clone AC-15; Sigma-Aldrich Chemie GmbH, Buchs, Switzerland) was used as control for protein loading. Culture inserts were rinsed with PBS and scraped into an ice-cold solubilization buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, 1 mM PMSF, and a mixture of protease inhibitors. After 30 minutes of incubation, samples were centrifuged at 4°C for 10 minutes at 10,000 × g. Supernatants were recovered, and total amounts of protein were determined by a bicinchoninic acid quantification assay (Pierce, Rockford, IL). An equal amount of protein was electrophoresed in 12% SDS-PAGE and electrotransferred onto Immobilon-P polyvinylidene difluoride membranes (Millipore AG, Volketswill, Switzerland). Membranes were then blocked overnight at 4°C in a 5% defatted milk saturation buffer containing 20 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.1% Tween-20, and 0.2% sodium azide. Next, proteins were immunoblotted for 1 hour with appropriate antibodies. This step was followed by a 1-hour incubation with goat anti-mouse or anti-rabbit IgG secondary antibodies conjugated to peroxidase (Jackson Laboratories, West Grove, PA). Immunoreactivity was detected via the Super Signal West Pico Kit (Pierce).
Ussing Chambers
The bioelectric properties of Calu-3 cell cultures were studied by mounting the filters in Ussing chambers. The apical and basal chambers were filled with a Krebs-Ringer-bicarbonate (KRB) buffer containing (in mM) 134 NaCl, 4 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2.5 CaCl2, 5 NaHCO3, 1 glucose, and 10 Hepes (pH 7.4), maintained at 37°C in an O2/CO2 incubator. The transepithelial potential difference was voltage-clamped at zero, and the resulting short-circuit current (Isc) was recorded continuously through Calomel electrodes (Radiometer Analytical SAS, Villeurbanne, France) and 3 M KCl agar bridges. Voltage-clamp experiments were performed using a VCC MC6 amplifier (Physiological Instruments, San Diego, CA), and data were sampled using the interface DI-720 (DataQ Instruments, Akron, OH) and recorded/displayed using Acquire and Analyze version 2.3 software (Physiological Instruments). PAR-induced Isc was evoked by a basolateral application of 10 μM of trypsin (Sigma). No difference was detected between the trypsin-induced Isc recorded in polarized cultures at the air–liquid or liquid–liquid interface for 10–15 days.
Determination of ASL Volume
The measurement of ASL volume was performed according to Rollins and colleagues (8). ASL was stained by adding to the apical surface of Calu-3 cell cultures 2 mg/ml Texas Red–Dextran (10 kD, Invitrogen, Basel, Switzerland) in 15 μL of PBS for 24 hours. During the experiment, 100 μL of the Novec fluid HFE-7200 (3M Novec, Rüschlikon, Switzerland) were added to the preparation to prevent the evaporation of ASL. Transwell cultures were placed on the stage of an inverted Zeiss LSM510 laser scanning confocal microscope (Carl Zeiss, Inc., Oberkochen, Germany), and were imaged using a ×63 oil immersion lens. Because of irregularities in Calu-3 cell height and cellular debris, ASL volume instead of height was calculated from three-dimensional reconstructed images using Imaris 3.2 (Bitplane AG, Zurich, Switzerland). Five random images were acquired from each culture before and after exposure of the basolateral membrane to KRB alone or KRB supplemented with 10 nM PGE2 or 10 nM PGE2 + 100 μM of the gap junction blocker 18α-glycyrrhethinic acid for 20 minutes. The time for capture of the five images was 4–5 minutes. To quantify changes in the volume of ASL (ΔASL volume), the five images before and after the 20-minute treatment were averaged, and the difference was calculated. A similar protocol was used for HAEC cultures, except that they were stimulated with 10 μM of PGE2 for 15 minutes. Four microliters of Texas Red–Dextran-containing and amiloride-containing PBS were added to the apical surface 1 hour before initiating image acquisition, using a ×40 oil immersion lens. In independent experiments, the effect of amiloride (100 μM) on ASL absorption was evaluated by adding to the apical surface the Na+-channel blocker in 15 μl of Texas Red–Dextran-containing PBS. Images were then acquired at 0, 30, and 45 minutes.
Dye Coupling
Gap junctional intercellular communication (GJIC) was determined by an intracellular microinjection of Lucifer Yellow in Calu-3 cells cultured on Transwell inserts and incubated in KRB. Lucifer Yellow was prepared in 150 mM LiCl and 10 mM Hepes (pH 7.2). The tracer was injected, using a thin-tipped glass microelectrode, into one cell for 3 minutes, and the number of labeled cells was counted at the end of the injection period. Fluorescent cells were viewed on an inverted TMD300 microscope (Nikon AG, Kürsnacht, Switzerland) equipped with a high-sensitivity black and white CCD Visicam camera (Visitron Systems GmbH, Puchheim, Germany). Images were captured using Metafluor version 4.01 software (Universal Imaging Corp., Downington, PA), and processed using Adobe Photoshop 5.5 (Adobe Systems, Mountain View, CA).
Experimental Conditions
The expression and activity of CD73 were increased by incubating Calu-3 cells with 100 nM of the methotrexate analogue aminopterine (AMT) for 16 hours in culture medium to enhance the release of ADO (25). The activity of CD73 was blocked by exposing cells treated or not treated with AMT to the CD73 inhibitor α,β-methyleneadenosine 5′-diphosphate (AMP-CP, 100 μM) for 3 hours. The ADO receptor agonist 5′-(N-ethylcarboxamido)adenosine (NECA, 10 μM) was applied to the apical membrane for 3 hours. The PLA2 inhibitor methyl arachidonyl fluorophosphonate (MAFP, 10 μM) or the COX inhibitor indomethacin (Indo, 10 μM) was applied 45 minutes before exposing cells to NECA or trypsin. EP-R inhibitors for EP1 (SC19220), EP1 plus EP2 (AH6809), or EP4 (GW627368X) were used at 10–20 μM and were applied 45 minutes before exposing cells to trypsin or PGE2. For dye coupling, PGE2 (10 nM) was applied to the basolateral membrane for 3 hours. GJIC was inhibited by treating Calu-3 cells twice, every 24 hours, with 0.25 μg/μl of the mimetic Cx43 peptide 43Gap26 (VCYDKSFPISHVR). A mimetic peptide specific for Cx26, 26Gap26 (VCYDHYFPISHIR), was used as control. The rapid and complete inhibition of GJIC was achieved with either 18α-glycyrrhethinic acid (50–100 μM) or heptanol (1 mM). The cAMP cocktail containing a mixture of forskolin (10 μM), (4-chlorophenylthio)-cAMP (1 mM), and 3-isobutyl-1-methylxanthine (50 μM) was applied for 3 hours. Drugs were dissolved in water, ethanol, urea, or DMSO, and controls were performed using identical dilutions of the solvents. All reagents were from Sigma-Aldrich Chemie, except for PGE2 (Biomol International, Plymouth Meeting, PA), GW627368X and MAFP (Cayman Chemical, Ann Harbor, MI), and 43Gap26 and 26Gap26 (Alpha Diagnostic, San Antonio, TX).
Statistical Analysis
Experiments were compared using unpaired t tests, one-way ANOVA, and nonparametric Mann–Whitney U test, where appropriate. Values are expressed as mean ± SEM. P < 0.05 was considered significant.
RESULTS
CD73/ADO Modulates PAR-Evoked CFTR Current in Polarized Calu-3 Cells
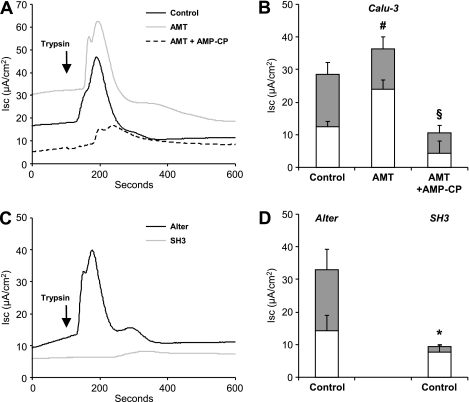
The expression of CFTR increased during the process of polarization and differentiation in Calu-3 cells grown on Transwell inserts (Figure 1A, top). Under these conditions, the basal activation of PARs with trypsin treatment led to a characteristic rapid but transient increase in Isc, as evaluated in Ussing chambers (Figure 2A). To confirm that CFTR contributes to the PAR-induced Isc, we used Calu-3 cells stably transfected with a plasmid containing shRNA designed to silence CFTR selectively (SH3 cells). SH3 cells failed to express CFTR (Figure 1B) and to generate Isc in response to PAR activation (Figures 2C and 2D), compared with control Calu-3 cells (Alter cells) expressing a nonsilencing shRNA sequence containing five nucleotide replacements (12). The Calu-3 cell lines did not exhibit amiloride-sensitive currents (data not shown).
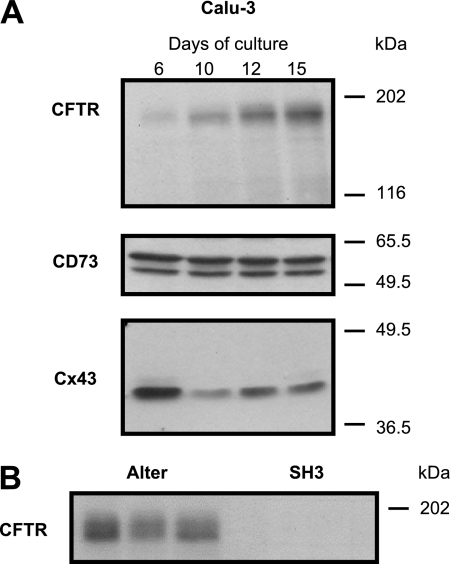
Figure 1.
Polarized Calu-3 cells express cystic fibrosis transmembrane conductance regulator (CFTR), CD73, and connexin 43 (Cx43). (A) The expression of CFTR, CD73, and Cx43 was determined by Western blotting on total protein extracted from Calu-3 cells grown on Transwell inserts for 6, 10, 12, and 15 days. CFTR expression increased with time of culture, whereas the expression of CD73 did not change. Cx43 expression was highest in less well-differentiated cells on Day 6, but was still expressed on Day 15. Two bands were detected for CD73, presumably representing different glycosylation levels of the protein. Blots are representative of at least five experiments. (B) Western blot shows lack of CFTR in three samples of Calu-3 cells transfected with a short hairpin RNA (shRNA) construct designed to silence CFTR selectively (SH3). In contrast, Calu-3 cells transfected with a control shRNA (Alter) expressed normal levels of CFTR.
Figure 2.
CD73/adenosine (ADO) modulates protease-activated receptor (PAR)-evoked CFTR current in polarized Calu-3 cells. (A) Representative short-circuit currents (Isc) in Calu-3 cells mounted in Ussing chambers after basolateral PAR activation with trypsin (arrow) in control condition and after pretreatment with aminopterine (AMT) or AMT + α,β-methyleneadenosine 5′-diphosphate (AMP-CP). (B) Quantitative analyzes of basal (open columns) and PAR-evoked (gray columns) Isc. Overnight exposure of Calu-3 cells to AMT increased the basal but not stimulated current compared with control conditions. This increase was likely attributable to the continuous production of ADO by CD73. In the presence of the CD73 inhibitor AMP-CP for 3 hours, both the basal and the PAR-evoked Isc was markedly reduced. #P < 0.05 compared with basal Isc in Control. §P < 0.05 compared with PAR-evoked Isc in Control. n = 4–18 filters per condition. (C) Representative short-circuit recordings of SH3 cells and Alter–Calu-3 cells exposed to trypsin. No PAR-evoked Isc was observed in SH3 cells, as expected after silencing CFTR with a shRNA strategy. (D) Quantitative analyzes of basal (open columns) and PAR-evoked (gray columns) Isc in SH3 and Alter cells. *P < 0.05 compared with PAR-evoked Isc in Alter cells. n = 7 filters per condition.
As shown in Figure 1A (middle), Calu-3 cells express CD73, a rate-limiting ectoenzyme at the surface of airway epithelial cells hydrolyzing adenine nucleotides into ADO, which in turn generates intracellular cAMP after the stimulation of apical A2B receptors. The methotrexate analogue AMT is known to promote the release of adenine nucleotide from cells, and thus to stimulate the production of ADO via CD73 activity (25). The treatment of Calu-3 cells with AMT increased the basal Isc, but did not affect PAR-induced CFTR currents in Ussing chambers (Figures 2A and 2B). In contrast, both the basal and the PAR-induced Isc was decreased in the presence of AMP-CP, an inhibitor of CD73. These results suggest that ADO signaling, regulated by CD73 activity, sets up the responsiveness of Calu-3 cells to additional PAR stimulation.
CD73/ADO Modulates GJIC in Calu-3 Polarized Cells
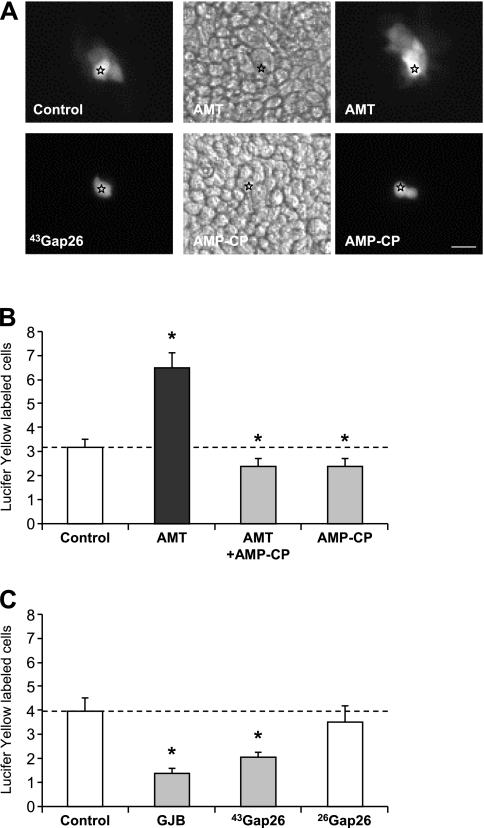
ADO signaling may interfere with PAR signaling in many ways. To investigate the possible contributions of direct intercellular communication in signaling integration by Calu-3 cells, we first evaluated the functional expression of gap junctions. The gap junction protein Cx43 was detected in well-polarized Calu-3 cells (by RT-PCR, immunofluorescence [data not shown], and Western blotting) (Figure 1A, bottom). Gap junctional intercellular communication (GJIC) was evaluated in Calu-3 cells grown on Transwell inserts by dye coupling, using Lucifer Yellow. AMT increased the extent of Lucifer Yellow diffusion, an effect that could be reversed by the CD73 inhibitor AMP-CP (Figures 3A and 3B). Blocking CD73 activity was sufficient to reduce GJIC significantly (Figure 3B). The specificity of dye transfer experiments was evidenced by the inhibitory effects of the gap junction blocker (GJB) heptanol and of a specific mimetic peptide blocking Cx43-mediated GJIC (43Gap26). A mimetic peptide of Cx26 (26Gap26), which is not expressed in Calu-3 cells (Figure 3C), had no effect on GJIC. Thus, CD73 activity modulates the extent of Cx43-mediated GJIC in polarized Calu-3 cells.
Figure 3.
CD73 activity modulates gap junctional intercellular communication (GJIC) in polarized Calu-3 cells. (A) Fluorescent views of Lucifer Yellow spreading after microinjection of Calu-3 cells treated or not treated with AMT, AMP-CP, or connexin 43-mediated GJIC (43Gap26). Bright-field images for AMT and AMP-CP are also shown. Stars indicate injected cells. Bar, 20 μm. (B) Quantitative analyzes of dye injections. AMT increased dye coupling between Calu-3 cells (n = 20 dye injections). This increase was prevented by the inhibition of CD73 activity with AMP-CP (n = 20). AMP-CP alone (n = 25) or in combination with AMT reduced GJIC. *P < 0.05 compared with Control (n = 34). (C) The gap junction blocker heptanol (GJB; n = 17) and 43Gap26 (n = 19), but not the mimetic peptide 26Gap26 (n = 12) from an irrelevant connexin (Cx26) in Calu-3 cells, decreased dye coupling. *P < 0.01 compared with Control (n = 10). Dashed line in B and C indicates control level of dye coupling.
PGE2-Dependent Regulation of GJIC in Polarized Calu-3 Cells
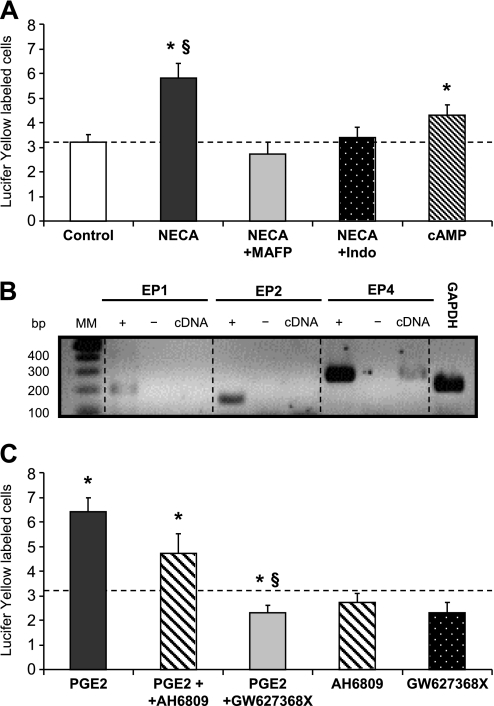
To investigate further the signaling pathway involved in GJIC regulation, dye coupling was evaluated after apical stimulation of ADO-R with the analogue NECA. As shown in Figure 4A, NECA increased GJIC, an effect that was prevented by PLA2 and COX inhibitors (MAFP and indomethacin, respectively). PGE2 is a major COX product, and is involved in CFTR activation in response to A2B receptor activation. Using RT-PCR, we detected mRNA for the PGE2 receptor EP4, but not EP1-R and EP2-R (Figure 4B). Although the apical stimulation of Calu-3 cells had no effect on GJIC (3.7 ± 0.7 fluorescent cells, n = 10), the number of Lucifer Yellow–labeled cells increased when PGE2 was applied basolaterally (Figure 4C). This increase was prevented by the specific EP4 receptor inhibitor (GW627368X), but not by an inhibitor (AH6809) of EP1-Rs and EP2-Rs (Figure 4C). The treatment of Calu-3 cells with a cAMP cocktail enhanced GJIC, but to a lower extent than that achieved with NECA or PGE2, suggesting that additional mechanisms beyond PKA activation regulate Cx43 channel activity in polarized Calu-3 cells. Altogether, these results indicate that the apical CD73/ADO pathway regulates GJIC by the release of PGE2 and subsequent activation of basolateral EP4-Rs.
Figure 4.
PGE2 signaling modulates GJIC between polarized Calu-3 cells. (A) Extent of dye coupling in Calu-3 cells exposed to ADO receptor agonist 5′-(N-ethylcarboxamido)adenosine (NECA) and inhibitors of phospholipase A2 (PLA2) (MAFP) or cyclooxygenase (COX) (Indo). NECA (n = 26 dye injections) increased dye coupling between Calu-3 cells, an effect that was prevented by inhibition of PLA2 (n = 11) or COX (n = 23). Treatment of Calu-3 cells with a cAMP cocktail (n = 25) increased dye coupling but to a lower extent than NECA. Dashed line indicates the control (n = 34) level of dye coupling. *P < 0.001 compared with Control. §P < 0.001 compared with cAMP. (B) mRNA was isolated from Calu-3 cells grown in Transwell filters for 15 days and subjected to RT-PCR, using specific primer pairs for EP1-receptors, EP2-receptors, EP4-receptors, or GAPDH. Only an amplification product for EP4 was detected (cDNA). + and − indicate positive (genomic DNA) and negative (no RT) controls, respectively. MM, molecular markers. (C) Extent of dye coupling in Calu-3 cells exposed to PGE2 and EP2 (AH6809) or EP4 (GW627368X) receptor antagonists. PGE2 increased dye coupling between Calu-3 cells (n = 30) compared with the control level of dye coupling indicated by dashed line (value from A). AH6809 (n = 16) and GW627368X (n = 12) alone did not affect GJIC. Although AH6809 did not change (P > 0.05) the extent of dye coupling evoked by PGE2 (PGE2 + AH6809, n = 15 compared with PGE2 alone, n = 16), GJIC was markedly reduced in the presence of GW627368X (PGE2 + GW627368X, n = 16 compared with PGE2 alone, n = 17). Because the effect of PGE2 on GJIC was not different in the three groups of experiments, PGE2 values were pooled (n = 63) for display. Pooling the PGE2 values did not affect the outcome of the statistics performed for each of three groups of experiments. *P < 0.05 compared with Control. §P < 0.001 compared with PGE2.
GJIC-Dependent Regulation of PAR-Evoked CFTR Current in Polarized Calu-3 Cells
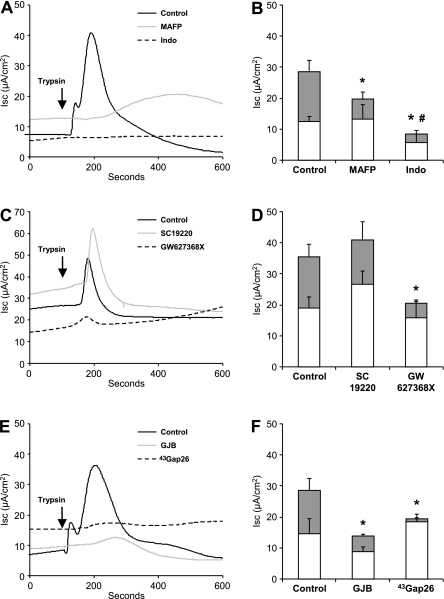
PGE2 was shown to mediate PAR-induced CFTR activation in Calu-3 cells (12). In Ussing chambers, PAR-evoked CFTR currents were indeed prevented by MAFP and indomethacin (Figures 5A and 5B). Finally, the inhibition of EP4 receptors was sufficient to abolish the PAR-evoked CFTR current, compared with the EP1 blocker SC19220 used as a control (Figures 5C and 5D). Because the release of PGE2 appears to be a means to modulate GJIC within Calu-3 cells, we tested the effect of Cx43 channel inhibition on the PAR-evoked CFTR current. As shown in Figures 5E and 5F, both GJB and 43Gap26 blocked the PAR-evoked Isc increase, indicating that the activation of CFTR current required functional GJIC.
Figure 5.
PGE2-dependent and GJIC-dependent regulation of PAR-evoked CFTR current in polarized Calu-3 cells. Representative short-circuit recordings (A, C, and E) and quantitative analyzes (B, D, and F) of basal (open columns) and trypsin-evoked (gray columns) Isc of Calu-3 cells. (A and B) PAR-evoked Isc was markedly reduced by inhibition of PLA2 with MAFP and of COX with Indo, which also decreased the basal current. (C and D) PAR-evoked currents were also inhibited by the EP4 receptor antagonist GW627368X, but not by SC19220, an EP1 receptor antagonist. (E and F) Inhibition of GJIC with GJB and 43Gap26 fully prevented PAR-evoked Isc. *P < 0.05 compared with PAR-evoked Isc in Control. #P < 0.05 compared with basal Isc in Control. n = 4–18 filters per condition.
GJIC-Dependent Regulation of ASL Volume
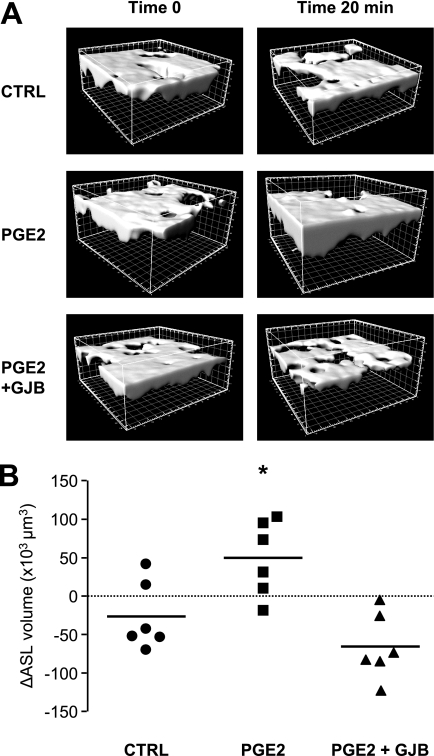
The activation of CFTR is needed to move Cl− out of cells and trigger the diffusion of water to the apical surface of the airway epithelium. Airway surface liquid in polarized Calu-3 cells was stained with Texas Red–Dextran, and its volume was monitored by confocal microscopy and three-dimensional reconstruction before and 20 minutes after the basal application of PGE2 (Figure 6A). The volume of ASL slightly decreased with time under control conditions, likely mediated by passive Na+ flux through tight junctions or because of cell leakage. However, the process was fully reversed and the volume of ASL increased markedly in response to PGE2 (Figure 6B). This increase was dependent on CFTR activity, because PGE2 failed to enhance the volume of ASL in SH3 cells (ΔASL volume = −2,083 ± 10,874 μm3, n = 4). To determine if GJIC underlay the increase in ASL volume elicited by PGE2, we repeated the experiment in the presence of a GJB. As shown in Figures 6A and 6B, the PGE2-induced increase of ASL volume was abolished in the presence of the GJB 18α-glycyrrhethinic acid.
Figure 6.
GJIC-dependent regulation of ASL volume in Calu-3 cells. Texas Red–Dextran was added to the apical surface of polarized Calu-3 cells 24 hours before the experiments. (A) Representative three-dimensional reconstruction views from Z-scanning confocal imaging of Texas Red–Dextran-labeled ASL. Reconstructions were performed at time 0 and after 20 minutes of exposure of the basolateral cell membrane to control medium (CTRL) or medium supplemented with PGE2 or with PGE2 + GJB. Scale, 10 × 10 μm per square. (B) Quantification of ASL volume changes (ΔASL) using Imaris software 20 minutes after addition of control medium (CTRL) or medium supplemented with PGE2 or PGE2 + GJB. Calu-3 cells showed a weak absorption of ASL in control medium. PGE2 increased ASL volume, an effect that was blocked in the presence of the gap junction blocker. Bars indicate mean values. *P < 0.05 compared with CTRL. n = 6 filters per condition.
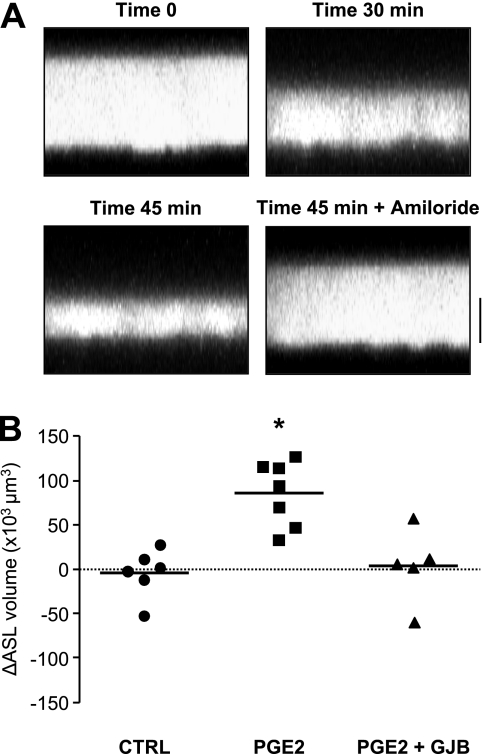
To confirm the importance of GJIC on ASL volume regulation in a cell system closer to human airway physiology, we performed additional experiments on primary cultures of well-polarized HAECs at the air–liquid interface. In contrast to Calu-3 cells, HAEC cultures showed the properties of an absorbing epithelium. A consistent decrease in ASL volume was detected with time (30 and 45 minutes) after the addition to the apical surface of PBS labeled with Texas Red–Dextran (Figure 7A). This decrease was blocked in the presence of amiloride, indicating that absorption was dependent on active Na+ transport via ENaC channels. Under these conditions, no change in ASL volume was detected in the absence of PGE2 (Figure 7B). In contrast, PGE2 induced an increase of ASL volume, which was prevented in the presence of 18α-glycyrrhethinic acid (Figure 7B). These results indicate that gap junctions contribute to ASL homeostasis in human airway epithelial cells.
Figure 7.
GJIC-dependent regulation of ASL volume in primary HAEC cultures. (A) Z-scanning confocal images of Texas Red–Dextran-labeled ASL. Images were acquired at 0, 30, and 45 minutes after addition of Texas Red–Dextran or 45 minutes after addition of Texas Red–Dextran + amiloride. ASL absorption was prevented in the presence of the epithelial Na+ channel blocker. Bar. 20 μm. (B) Quantification of ASL volume changes (ΔASL), using Imaris software 15 minutes after addition of control medium (CTRL) or medium supplemented with PGE2 or PGE2 + GJB. PGE2 increased ASL volume in primary HAECs, an effect that was blocked in the presence of the gap junction blocker. Bars indicate mean values. *P < 0.01 compared with CTRL. n = 5–7 filters per condition.
DISCUSSION
Mucociliary clearance functions as a multisignaling process in which the release, metabolism, and conversion of purinergic agonists on the epithelial surface, as well as the apical and basolateral actions of proteases, play a crucial role (3, 6). We show that gap junction channels between Calu-3 cells mediate the PGE2-dependent activation of CFTR by A2B-R and PAR. We also show that gap junctional intercellular communication is required for the PGE2-dependent regulation of airway surface liquid homeostasis.
The airway epithelial Calu-3 cell line, which is derived from a lung adenocarcinoma, exhibits the properties of a mixed population of serous cells and mucin-secreting goblet-like cells (6). This cell line can be cultured as polarized monolayers on permeable supports, and was shown to reproduce many aspects of the airway transepithelial transport of ions and protein secretion. The regulation of CFTR activity by extracellular nucleotides (in particular ATP and ADO) was extensively described in this cell model. Szkotak and colleagues suggested that nucleoside transporters, together with adenosine kinase and CD73, play a crucial role in the regulation of CFTR through an ADO-dependent pathway in these cells (26). The A2B-Rs were identified as major transducers of nucleoside-regulated ion and water transport in airway epithelia, and blockade of the receptors impaired CFTR-dependent Cl− secretion (5, 6, 9). Our finding that the modulation of CD73 activity in Calu-3 cells results in variations in basal Isc, likely by regulating the intensity of A2B-R stimulation by ADO, is thus consistent with these observations. Interestingly, the activation of CFTR in response to PAR stimulation was impaired with CD73 inhibition. These results suggest that an A2B-dependent signaling is required to set up the responsiveness of Calu-3 cells to additional PAR stimulation.
Although A2B-R triggers the intracellular production of cAMP and the activation of PKA, evidence indicates that additional signaling pathways are involved for maximal CFTR activation. The production of PGE2 via PLA2 and COX activation and its paracrine stimulation of EP-Rs were proposed as a mechanism to amplify not only the engagement of A2B-Rs, but also of PARs (7, 8, 11, 12, 27, 28). Controversy continues regarding which PGE2 receptor subtype is involved in this signaling pathway. Palmer and colleagues showed in Calu-3 cells that PGE2 activated CFTR via EP2-Rs and EP4-Rs (12). Joy and Cowley, in contrast, reported that Calu-3 cells express all four known EP-R subtypes, but point to EP4-Rs in the regulation of CFTR-dependent iodide efflux (28). Via RT-PCR, we only detected EP4-Rs in our Calu-3 cells, and EP4 inhibition was sufficient to prevent CFTR activation, confirming the results of Clayton and colleagues (29).
We found that the modulation of CD73 activity, and thus ADO production, affects the extent of Cx43-mediated GJIC in polarized Calu-3 cells. Connexins form gap junction channels with distinct unitary conductance, molecular permeability, and gating. The opening and closing of gap junction channels respond to changes in cytoplasmic ion composition, posttranslational modifications such as phosphorylation (both referred to as chemical gating), and changes in the difference of membrane potential between cells (voltage gating). The cytoplasmic tail of Cx43 plays a critical role in these regulations, and truncation of the Cx43 C-terminus creates gap junction channels unable to close in response to chemical and voltage gating (30, 31). Cx43 was shown to be a target of A2B-Rs in pituitary folliculostellate cells (32). In contrast to that study, however, we did not observe significant changes in Cx43 expression in response to AMT, NECA, or PGE2 in Calu-3 cells (data not shown). Thus, enhanced GJIC in response to A2B-R/EP4 activation is likely attributable to an increase of Cx43 channel open probability or conductance. We also found that the exposure of Calu-3 cells to a cAMP cocktail only modestly increased GJIC, compared with A2B-R stimulation. In addition, blocking PGE2 production with MAFP or indomethacin fully prevented GJIC in the presence of the ADO analogue NECA. Altogether, these results indicate that A2B-R signaling regulates GJIC by the release of PGE2 and the subsequent activation of basolateral EP4 receptors. The results also point to the signaling pathways triggered by EP4-R activation in the regulation of Cx43 gap junctions and CFTR channels.
An interesting observation in our study involves the parallel regulation of CFTR and GJIC in response to ADO. Indeed, the inhibition of A2B-R signaling pathways, including the blockade of CD73, PLA2, COX, and EP4-R, all led to decreased GJIC and impaired CFTR activation. This led us to hypothesize that CFTR activity and GJIC may be interrelated. In support of this idea, we found that the inhibition of GJIC by well-known GJBs, including the 43Gap26 mimetic peptide for Cx43, fully prevented the activation of CFTR current. 43Gap26 is an analogue to part of the amino-acid sequence of the first extracellular loop of Cx43, and is known to inhibit Cx43-mediated cell-to-cell communication by preventing docking gap junction channels in airway cells (24, 33, 34). Finally, the inhibition of GJIC resulted in ASL volume collapse in the presence of PGE2, not only in Calu-3 cells but also in primary cultures of well-polarized HAECs grown at the air–liquid interface. GJIC appears to be a key mediator of ASL homeostasis. We propose that the endogenous activity of CD73 is important in setting the level of GJIC between airway epithelial cells, which in turn will modulate the threshold for CFTR activation. The mechanism by which GJIC modulates this threshold is at present unknown. Functional gap junctions may contribute to the spread of ions and second messenger or cofactor exchange between cells to activate CFTR fully and ensure efficient Cl− secretion. Whether GJIC contributes to the intrinsic regulation of ENaC channels, therefore modulating Na+ absorption, remains to be evaluated. In a heterogeneous cell system, gap junctions may coordinate a signaling network, comprising CFTR, A2B-R, PAR, and EP-R, which is required for ASL volume homeostasis.
Our results demonstrate tight regulatory links between ADO, PAR, PGE2, and CFTR. The evidence points toward the mediation of these processes by gap junctions. CFTR was shown to influence connexin function in several cell models. The co-expression of two protein-forming channels in paired Xenopus oocytes was shown to affect the gating of gap junction channels (35, 36). In addition, the inhibition of CFTR by ATP depletion abolished GJIC, whereas the activation of CFTR by cAMP opened gap junction channels within minutes (37, 38). Therefore, a reciprocal regulation of the activity of the two channels occurs, and interestingly, the gating of gap junctions is impaired in CF epithelial cells (21). Regarding the importance of gap junctions in integrating multiple signaling pathways for the regulation of ASL volume, and thus the maintenance of a proper epithelial host defense, the altered regulation of GJIC may represent an additional layer of dysfunction in airway diseases.
Acknowledgments
We thank Brenda Kwak for her careful reading of the manuscript, Serge Arnaudeau and Sergei Startchik from the BioImaging Core Facility, and Jennifer Brancato for early Ussing experiments.
This work was supported by the Swiss National Science Foundation (grant 310000–119739), Vaincre la Mucoviscidose, the Schweizerische Gesellschaft für Cystische Fibrose, and the National Institutes of Health (grant DK07401). K.E.L.S. was supported by the Schmidheiny Foundation and the Novartis Consumer Health Foundation. D.L. was supported by the Italian Cystic Fibrosis Research Foundation (grant FFC#19/2009), adopted by the FFC delegation “La Bottega delle Donne.”
Originally Published in Press as DOI: 10.1165/rcmb.2009-0361OC on February 18, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Whitsett JA. Intrinsic and innate defenses in the lung: intersection of pathways regulating lung morphogenesis, host defense, and repair. J Clin Invest 2002;109:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wine JJ, Joo NS. Submucosal glands and airway defense. Proc Am Thorac Soc 2004;11:47–53. [DOI] [PubMed] [Google Scholar]
- 3.Chambers LA, Rollins BR, Tarran R. Liquid movement across the surface epithelium of large airways. Respir Physiol Neurobiol 2007;159:256–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med 2007;58:157–170. [DOI] [PubMed] [Google Scholar]
- 5.Picher M, Burch LH, Hirsh AJ, Spychala J, Boucher RC. Ecto 5′-nucleotidase and nonspecific alkaline phosphatase: two AMP-hydrolyzing ectoenzymes with distinct roles in human airways. J Biol Chem 2003;278:13468–13479. [DOI] [PubMed] [Google Scholar]
- 6.Davis CW, Lazarowski E. Coupling of airway ciliary activity and mucin secretion to mechanical stresses by purinergic signaling. Respir Physiol Neurobiol 2008;163:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobb BR, Ruiz F, King CM, Fortenberry J, Greer H, Kovacs T, Sorscher EJ, Clancy JPA. (2)Adenosine receptors regulate CFTR through PKA and PLA(2). Am J Physiol Lung Cell Mol Physiol 2002;282:L12–L25. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Wang W, Parker W, Clancy JP. Adenosine regulation of cystic fibrosis transmembrane conductance regulator through prostenoids in airway epithelia. Am J Respir Cell Mol Biol 2006;34:600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rollins BM, Burn M, Coakley RD, Chambers LA, Hirsh AJ, Clunes MT, Lethem MI, Donaldson SH, Tarran R. A2B adenosine receptors regulate the mucus clearance component of the lung's innate defense system. Am J Respir Cell Mol Biol 2008;39:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danahay H, Withey L, Poll CT, van de Graaf SF, Bridges RJ. Protease-activated receptor-2–mediated inhibition of ion transport in human bronchial epithelial cells. Am J Physiol Cell Physiol 2001;280:C1455–C1464. [DOI] [PubMed] [Google Scholar]
- 11.Kunzelmann K, Sun J, Markovich D, König J, Mürle B, Mall M, Schreiber R. Control of ion transport in mammalian airways by protease activated receptors type 2 (PAR-2). FASEB J 2005;19:969–970. [DOI] [PubMed] [Google Scholar]
- 12.Palmer ML, Lee SY, Maniak PJ, Carlson D, Fahrenkrug SC, O'Grady SM. Protease-activated receptor regulation of Cl− secretion in Calu-3 cells requires prostaglandin release and CFTR activation. Am J Physiol Cell Physiol 2006;290:C1189–C1198. [DOI] [PubMed] [Google Scholar]
- 13.Hata AN, Breyer RM. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther 2004;103:147–166. [DOI] [PubMed] [Google Scholar]
- 14.Konstan MW, Walenga RW, Hilliard KA, Hilliard JB. Leukotriene B4 markedly elevated in the epithelial lining fluid of patients with cystic fibrosis. Am Rev Respir Dis 1993;148:896–901. [DOI] [PubMed] [Google Scholar]
- 15.Miele L, Cordella-Miele E, Xing M, Frizzell R, Mukherjee AB. Cystic fibrosis gene mutation (DeltaF508) is associated with an intrinsic abnormality in Ca2+-induced arachidonic acid release by epithelial cells. DNA Cell Biol 1997;16:749–759. [DOI] [PubMed] [Google Scholar]
- 16.Freedman SD, Katz MH, Parker EM, Laposata M, Urman MY, Alvarez JG. A membrane lipid imbalance plays a role in the phenotypic expression of cystic fibrosis in CFTR(−/−) mice. Proc Natl Acad Sci USA 1999;96:13995–14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medjane S, Raymond B, Wu Y, Touqui L. Impact of CFTR DeltaF508 mutation on prostaglandin E2 production and type IIA phospholipase A2 expression by pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol 2005;289:L816–L824. [DOI] [PubMed] [Google Scholar]
- 18.Holgate ST. The Quintiles Prize Lecture 2004: the identification of the adenosine A2B receptor as a novel therapeutic target in asthma. Br J Pharmacol 2005;145:1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varani K, Caramori G, Vincenzi F, Adcock I, Casolari P, Leung E, Maclennan S, Gessi S, Morello S, Barnes PJ, et al. Alteration of adenosine receptors in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;173:398–406. [DOI] [PubMed] [Google Scholar]
- 20.Harris A, Locke D. Connexins: a guide. New York: Humana Press; 2009.
- 21.Chanson M, Kotsias BA, Peracchia C, O'Grady SM. Interactions of connexins with other membrane channels and transporters. Prog Biophys Mol Biol 2007;94:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson LN, Koval M. Cross-talk between pulmonary injury, oxidant stress, and gap junctional communication. Antioxid Redox Signal 2009;11:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiszniewski L, Jornot L, Dudez T, Pagano A, Rochat T, Lacroix JS, Suter S, Chanson M. Long-term cultures of polarized airway epithelial cells from patients with cystic fibrosis. Am J Respir Cell Mol Biol 2006;34:39–48. [DOI] [PubMed] [Google Scholar]
- 24.Sarieddine MZ, Scheckenbach KEL, Foglia B, Maass K, Garcia I, Kwak BR, Chanson M. Connexin43 modulates neutrophil recruitment to the lung. J Cell Mol Med 2009;13:4560–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morabito L, Montesinos MC, Schreibmann DM, Balter L, Thomson LF, Resta R, Carlin G, Huie MA, Cronstein BN. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase–mediated conversion of adenine nucleotides. J Clin Invest 1998;101:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szkotak AJ, Ng AM, Man SF, Baldwin SA, Cass CE, Young JD, Duszyk M. Coupling of CFTR-mediated anion secretion to nucleoside transporters and adenosine homeostasis in Calu-3 cells. J Membr Biol 2003;192:169–179. [DOI] [PubMed] [Google Scholar]
- 27.Perng DW, Wu YC, Tsai MC, Lin CP, Hsu WH, Perng RP, Lee YC. Neutrophil elastase stimulates human airway epithelial cells to produce PGE2 through activation of p44/42 MAPK and upregulation of cyclooxygenase-2. Am J Physiol Lung Cell Mol Physiol 2003;285:L925–L930. [DOI] [PubMed] [Google Scholar]
- 28.Joy AP, Cowley EA. 8-iso-PGE2 stimulates anion efflux from airway epithelial cells via the EP4 prostanoid receptor. Am J Respir Cell Mol Biol 2008;38:143–152. [DOI] [PubMed] [Google Scholar]
- 29.Clayton A, Holland E, Pang L, Knox A. Interleukin-1β differentially regulates β2 adrenoreceptor and prostaglandin E2–mediated cAMP accumulation and chloride efflux from Calu-3 bronchial epithelial cells: role of receptor changes, adenyl cyclase, cyclooxygenase 2 and protein kinase A. J Biol Chem 2005;280:23451–23463. [DOI] [PubMed] [Google Scholar]
- 30.Thomas MA, Huang S, Cokoja A, Riccio O, Staub O, Suter S, Chanson M. Interaction of connexins with protein partners in the control of channel turnover and gating. Biol Cell 2002;94:445–456. [DOI] [PubMed] [Google Scholar]
- 31.Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J 2009;419:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis BM, Pexa A, Francic K, Verma V, McNicol AM, Scanlon M, Deussen A, Evans WH, Rees DA, Ham J. Adenosine stimulates connexin43 expression and gap junctional communication in pituitary folliculostellate cells. FASEB J 2006;20:2585–2587. [DOI] [PubMed] [Google Scholar]
- 33.Boitano S, Evans WH. Connexin mimetic peptides reversibly inhibit Ca2+-signaling through gap junctions in airway cells. Am J Physiol Lung Cell Mol Physiol 2000;279:623–630. [DOI] [PubMed] [Google Scholar]
- 34.Parthasarathi K, Ichimura H, Monma E, Lindert J, Quadri S, Issekutz A, Bhattacharya J. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J Clin Invest 2006;116:2193–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotsias BA, Peracchia C. Functional interaction between CFTR and Cx45 gap junction channels expressed in oocytes. J Membr Biol 2005;203:143–150. [DOI] [PubMed] [Google Scholar]
- 36.Kotsias BA, Salim M, Peracchia LL, Peracchia C. Interplay between cystic fibrosis transmembrane regulator and gap junction channels made of connexins 45, 40, 32 and 50 expressed in oocytes. J Membr Biol 2006;214:1–8. [DOI] [PubMed] [Google Scholar]
- 37.Brézillon S, Zahm JM, Pierrot D, Gaillard D, Hinnrasky J, Millart H, Klossek JM, Tümmler B, Puchelle E. ATP depletion induces a loss of respiratory epithelium functional integrity and down-regulates CFTR (cystic fibrosis transmembrane conductance regulator) expression. J Biol Chem 1997;272:27830–27838. [DOI] [PubMed] [Google Scholar]
- 38.Chanson M, Scerri I, Suter S. Defective regulation of gap junctional coupling in cystic fibrosis pancreatic duct cells. J Clin Invest 1999;103:1677–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]