Abstract
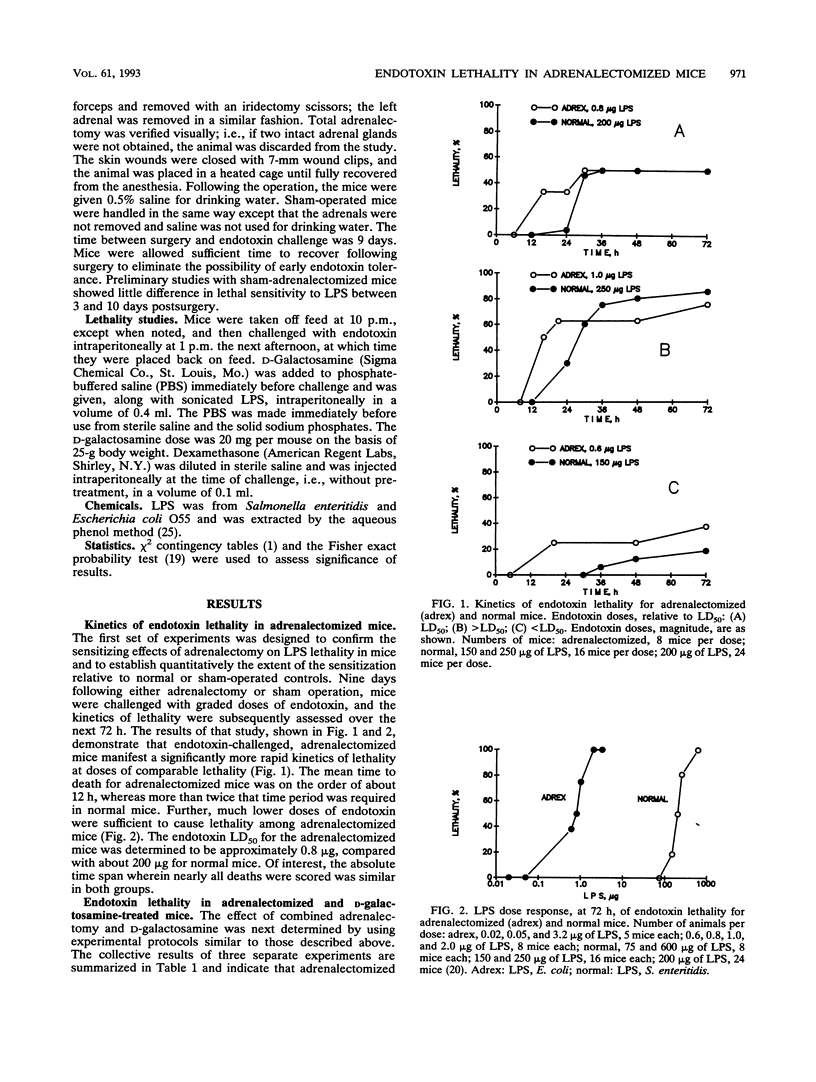
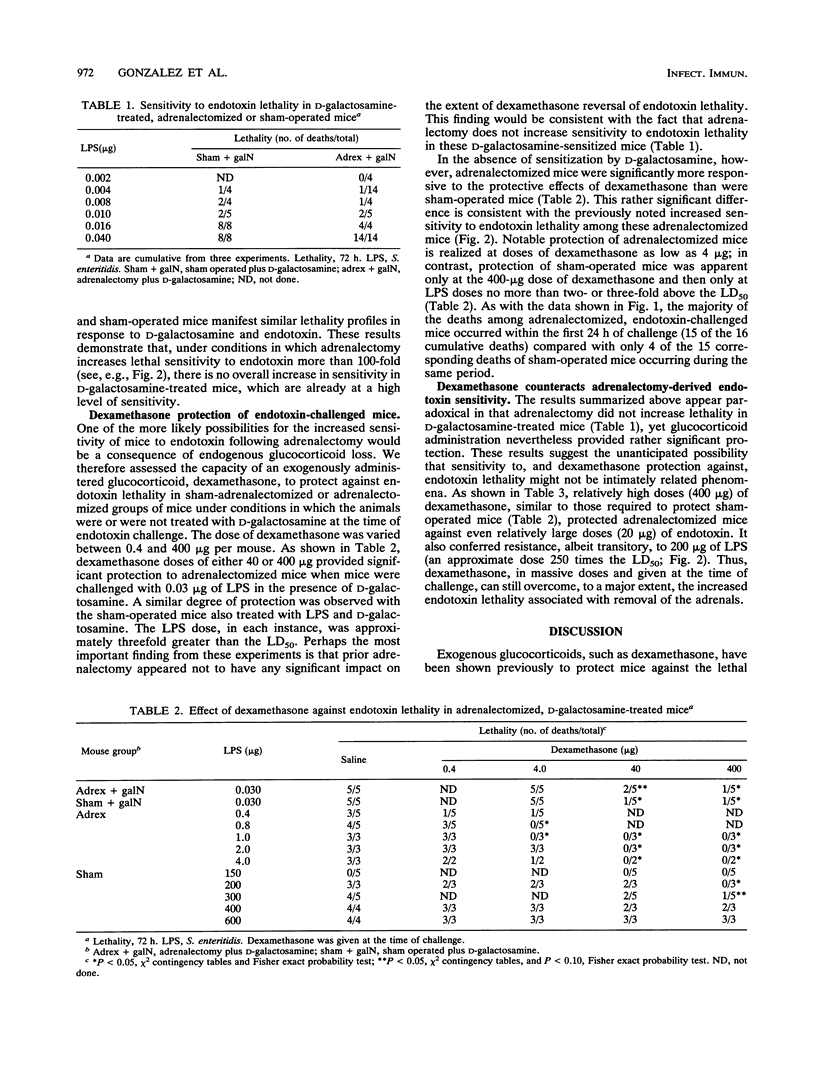
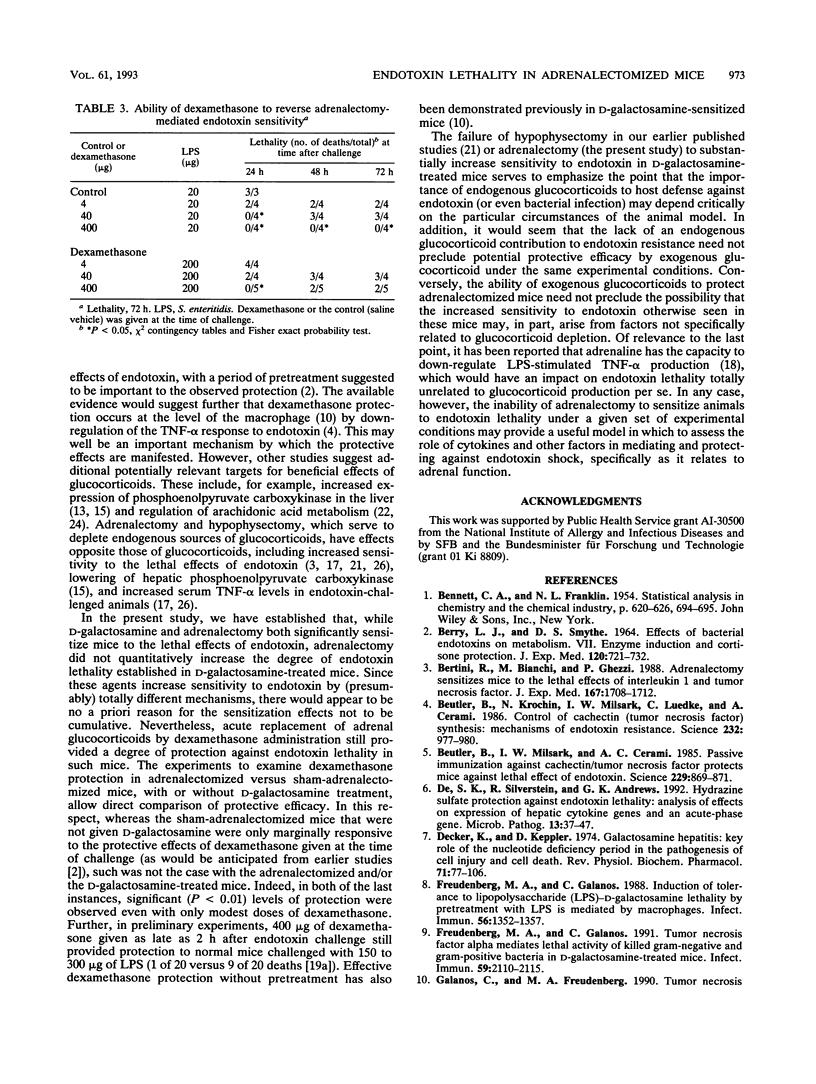
Endotoxin sensitivity and dexamethasone protection have been assessed in mice that were adrenalectomized and also treated with D-galactosamine at the time of endotoxin challenge. Our data establish that adrenalectomy did not detectably alter the magnitude of the increased sensitivity induced by D-galactosamine alone. Furthermore, protection provided by acute exogenous glucocorticoid treatment was still demonstrable in these mice and was not influenced by chronic experimentally induced glucocorticoid deficiency. Our data confirm that the adrenalectomized mouse model of endotoxin lethality is characterized by increased sensitivity to endotoxin and establish that the magnitude of this sensitizing effect is more than 100-fold. We also show for the first time that adrenalectomy causes an appreciable kinetic shift in the endotoxic crisis and that dexamethasone, given at the time of endotoxin challenge, will significantly reverse the increased sensitivity to lethality. Our results indicate that the protective effects of corticosteroids may involve important chronic as well as acute responses. In particular, we conclude that endogenous glucocorticoid need not always increase host resistance to endotoxin, nor does such a circumstance eliminate the possibility for exogenous glucocorticoid-mediated protective effects.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERRY L. J., SMYTHE D. S. EFFECTS OF BACTERIAL ENDOTOXINS ON METABOLISM. VII. ENZYME INDUCTION AND CORTISONE PROTECTION. J Exp Med. 1964 Nov 1;120:721–732. doi: 10.1084/jem.120.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini R., Bianchi M., Ghezzi P. Adrenalectomy sensitizes mice to the lethal effects of interleukin 1 and tumor necrosis factor. J Exp Med. 1988 May 1;167(5):1708–1712. doi: 10.1084/jem.167.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- De S. K., Silverstein R., Andrews G. K. Hydrazine sulfate protection against endotoxin lethality: analysis of effects on expression of hepatic cytokine genes and an acute-phase gene. Microb Pathog. 1992 Jul;13(1):37–47. doi: 10.1016/0882-4010(92)90030-r. [DOI] [PubMed] [Google Scholar]
- Decker K., Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;(71):77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pretreatment with LPS is mediated by macrophages. Infect Immun. 1988 May;56(5):1352–1357. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in D-galactosamine-treated mice. Infect Immun. 1991 Jun;59(6):2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann V., Freudenberg M. A., Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987 Mar 1;165(3):657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum R. E., Seale T. W., Stith R. D. Influence of endotoxin treatment on dexamethasone induction of hepatic phosphoenolpyruvate carboxykinase. Infect Immun. 1983 Jan;39(1):213–219. doi: 10.1128/iai.39.1.213-219.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Silverstein R., Bright S. W., Chen T. Y., Flebbe L. M., Lei M. G. Monoclonal antibody to mouse lipopolysaccharide receptor protects mice against the lethal effects of endotoxin. J Infect Dis. 1990 Nov;162(5):1063–1068. doi: 10.1093/infdis/162.5.1063. [DOI] [PubMed] [Google Scholar]
- Parant M., Le Contel C., Parant F., Chedid L. Influence of endogenous glucocorticoid on endotoxin-induced production of circulating TNF-alpha. Lymphokine Cytokine Res. 1991 Aug;10(4):265–271. [PubMed] [Google Scholar]
- Severn A., Rapson N. T., Hunter C. A., Liew F. Y. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J Immunol. 1992 Jun 1;148(11):3441–3445. [PubMed] [Google Scholar]
- Silverstein R., Christoffersen C. A., Morrison D. C. Modulation of endotoxin lethality in mice by hydrazine sulfate. Infect Immun. 1989 Jul;57(7):2072–2078. doi: 10.1128/iai.57.7.2072-2078.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein R., Turley B. R., Christoffersen C. A., Johnson D. C., Morrison D. C. Hydrazine sulfate protects D-galactosamine-sensitized mice against endotoxin and tumor necrosis factor/cachectin lethality: evidence of a role for the pituitary. J Exp Med. 1991 Feb 1;173(2):357–365. doi: 10.1084/jem.173.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiegs G., Wolter M., Wendel A. Tumor necrosis factor is a terminal mediator in galactosamine/endotoxin-induced hepatitis in mice. Biochem Pharmacol. 1989 Feb 15;38(4):627–631. doi: 10.1016/0006-2952(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Wendel A., Tiegs G. Leukotriene D4 mediates galactosamine/endotoxin-induced hepatitis in mice. Biochem Pharmacol. 1987 Jun 15;36(12):1867–1867. doi: 10.1016/0006-2952(87)90482-5. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. H., Shellhaas J., Butler L. D. Differential regulation of lipopolysaccharide-induced interleukin 1 and tumor necrosis factor synthesis: effects of endogenous and exogenous glucocorticoids and the role of the pituitary-adrenal axis. Eur J Immunol. 1989 Feb;19(2):301–305. doi: 10.1002/eji.1830190213. [DOI] [PubMed] [Google Scholar]


