Research highlights
▶ Neuronal tracer was injected into the abducens nucleus of monkey. ▶ Tracer positive axons were observed in the lateral rectus muscles. ▶ Tracer positive axons were cholinergic and established motor terminals. ▶ Palisade endings in the lateral rectus contained tracer as well. ▶ This study shows that palisade endings originate from motor nuclei.
Keywords: Proprioception, Monkey, Extraocular muscle, Palisade endings, Oculomotor
Abstract
Palisade endings are found in the extraocular muscles (EOMs) of almost every mammalian species, including primates. These nerve specializations surrounding the muscle fiber insertion have been postulated to be the proprioceptors of the EOMs. However, it was recently demonstrated that palisade endings have a cholinergic nature, which reopened the question of whether palisade endings are motor or sensory structures. In this work, we examined whether the cell bodies of palisade endings lie in EOM motor nuclei by injecting an anterograde tracer, biotinylated dextran amine, into the abducens nucleus of a macaque monkey. Tracer visualization in the lateral rectus muscle was combined with choline acetyltransferase (ChAT) and α-bungarotoxin staining. Analysis of the samples was performed by conventional light microscopy and confocal laser scanning microscopy. About 30% of the nerve fibers innervating the muscle were tracer positive. These were ChAT positive as well. Tracer positive nerve fibers established motor contacts on singly and multiply innervated muscle fibers, which were confirmed by α-bungarotoxin staining. At the transition between muscle and distal tendon, we found palisade endings that contained tracer. Palisade endings exhibited the classic morphology: axons arising from the muscle extend onto the tendon, then turn back 180° and terminate in a cuff of terminals around an individual muscle fiber tip. This finding suggests that the cell bodies of palisade endings lie in the EOM motor nuclei, which complements prior studies demonstrating a cholinergic, and possibly motor, phenotype for palisade endings.
Proprioception contributes to the sense of self position and self movement, and is mediated by specialized receptors – the proprioceptors – in the skeletal muscle. The classical proprioceptors for mammalian skeletal muscles are muscle spindles and Golgi tendon organs. However, the source of proprioception for eye position is not fully understood, since these types of proprioceptors are only present in a few species [14]. Interestingly, in extraocular muscles (EOMs) of nearly all species, an encapsulated nervous end organ, which is unique to EOMs, is present – the palisade ending (= innervated myotendinous cylinder) [1–3,7,9,21]. This structure has been assumed to be the source of proprioception in EOMs [1,2,6,8,9,20,25,28], although other studies suggest a motor function [3,12,18,19,22], or a combined motor and sensory function [13].
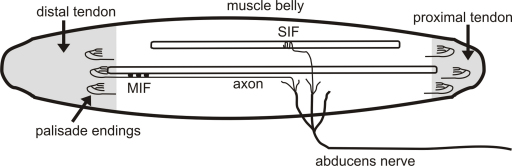
Palisade endings are located in the proximal and distal myotendinous junctions of the EOMs. The axons that form palisade endings run from the muscle belly to extend onto the tendinous insertion, where they turn back 180° to terminate in a complex cuff of preterminal and terminal axons around the tip of a muscle fiber [5,9]. Several studies indicate that palisade endings are associated with multiply innervated muscle fibers (MIFs, slow fibers) [1,21], which have several motor contacts along their length, as opposed to singly innervated muscle fibers (SIFs, fast fibers), which have only one motor contact [17]. The motoneurons innervating SIFs and MIFs have a characteristic localization in and around the EOM motor nuclei, respectively [6,26].
There is conflicting evidence on the location of the cell bodies that supply palisade endings, which impedes the clarification on whether palisade endings are sensory or motor structures. Presumably, if palisade endings are sensory, their cell bodies should be located in the trigeminal ganglion. If palisade endings are motor, their cell bodies should lie in the EOM motor nuclei. In sheep and pig, Manni et al. [15,16] stretched EOMs and recorded responses in the ipsilateral trigeminal ganglion, indicating that the cell bodies of EOM proprioceptors are located in this ganglion. Billig et al. [2] injected neuronal tracer into the trigeminal ganglia of cats and found three kinds of labeled nerve endings in the EOMs, one of which resembled palisade endings. Fackelmann et al. [10] found tracer positive cells in the ipsilateral trigeminal ganglion of monkeys after injection of neuronal tracer into the distal part of the EOMs. Alternatively, some have suggested that the mesencephalic trigeminal nucleus may contribute sensory input to the EOMs [27]. However, with the exception of the Billig study, none of these investigations provides direct evidence that palisade endings are proprioceptive, only that EOM proprioception is handled by trigeminal fibers. In fact, in an older study, Sas and Scháb [22] made lesions to the oculomotor nuclei in cats and found degeneration amongst palisade endings. Recently, it was demonstrated that palisade endings in EOMs of several species contain acetylcholine, the classical neurotransmitter for motor terminals [3,12,18,19]. Moreover, axons supplying palisade endings were found to establish motor contacts on the muscle fibers outside the palisade complex [3,12]. These novel findings reopened the debate over the function of palisade endings.
In the present study, we directly examined the source of palisade endings. Specifically, we injected a neuronal tracer into an EOM motor nucleus of a macaque monkey. Following an abducens nucleus injection, anterogradely labeled palisade endings were found in the lateral rectus muscle. These results complement our prior molecular findings in this primate species that indicated these EOM specific structures may have a motor function.
The animal procedures used in this study were approved by the University of Mississippi Medical Center IACUC, and conformed with the practices recommended by the Guide for Animal Care and Use. Male Macaca fascicularis monkeys (4–6 kg) were subdued with Ketamine HCl (35 mg/kg, IM) for transport, and were anesthetized with isoflurane to effect. Dexamethasone (1 mg/kg, IV) was given to suppress swelling and atropine (0.5 mg/kg, IM) was given to reduce secretions. Temperature, heart rate and blood gasses were monitored and maintained within normal ranges. A craniotomy was followed by the aspiration of the cortex overlying the pontomidbrain junction. Stereotaxic coordinates were adjusted with respect to the surface of the superior colliculus to reach the abducens nucleus in one monkey. The needle of a 5 μl Hamilton syringe held in a micromanipulator was inserted through a small opening in the tentorium, just caudal to the colliculi. The tracer, 0.2 μl of 10% biotinylated dextran amine (BDA), was injected into the left abducens nucleus. The wound site was closed and infused with sensorcaine (SQ). After cessation of the gas anesthesia, buprenex (0.01 mg/kg) was given as an analgesic. Following a 21 day survival, the animal was deeply anesthetized with sodium pentobarbital (50 mg/kg, IP), and perfused through the heart with a 4% paraformaldehyde fixative in 0.1 M, pH 7.2 phosphate buffer. The orbit was dissected, and the left and right (control) lateral rectus muscle including the distal tendon was removed. The muscles and brainstem were post-fixed in the same solution overnight, and then rinsed and stored at 4 °C in 0.1 M PBS, pH 7.4 until further processing.
In two monkeys, the same approach was used, but the needle was instead angled 20° in the sagittal plane and advanced through the superior colliculus to make a large injection (0.2–0.4 μl, 10% BDA) into the area containing the mesencephalic trigeminal nucleus.
The brainstem was frozen and sectioned in the frontal plane at 80 μm. The EOMs were cut into two pieces: the muscle belly and the distal muscle–tendon transition (i.e. the location of palisade endings). After freezing each muscle belly was cut longitudinally with a cryostat microtome (CM1950; Leica, Heidelberg, Germany) into 10 μm-thick sections. Sections were mounted onto gelatin-coated slides. The tissue containing the muscle–tendon transition was used as whole mount. Brainstem and EOM samples were reacted using conventional avidin–biotin procedures [27]. In both cases, the biotinylated tracer was tagged with horseradish peroxidase-conjugated streptavidin (Dako), and visualized with the chromagen, diaminobenzidine (DAB, Sigma–Aldrich) (without nickel ammonium sulfate and cobalt chloride in the case of the muscles). Alternatively, tracer was visualized by labeling with Alexa Fluor 488 conjugated avidin (Invitrogen). Fluorescence visualization of the tracer was combined with additional staining of cell structures with a variety of cell markers. Two different combinations of triple staining were performed: (1) anti-choline acetyltransferase (anti-ChAT), avidin, phalloidin and (2) α-bungarotoxin, avidin, phalloidin. Anti-ChAT was used to identify cholinergic neurons, α-bungarotoxin to label motor terminals, and phalloidin to stain muscle fibers. The incubation time for fluorescence staining of tracer with Alexa Fluor 488 conjugated avidin was 4 h. Staining with anti-ChAT, phalloidin and α-bungarotoxin were performed as described previously [12].
Analyses were performed by light microscopy (Zeiss Axioscope & Nikon Eclipse 600) and confocal laser scanning microscopy (Zeiss LSM 510). Digitized light microscopic images of palisade endings were recorded at different focal depths (1 μm) and 3D-reconstructed using the software Lucia G (Nikon GmbH, Düsseldorf). Fluorescence images were generated in 3 different fluorescence channels: excitation wavelength of 488, 568 and 633 nm, although in some cases, a transmission light image was recorded instead of the image with 633 nm excitation.
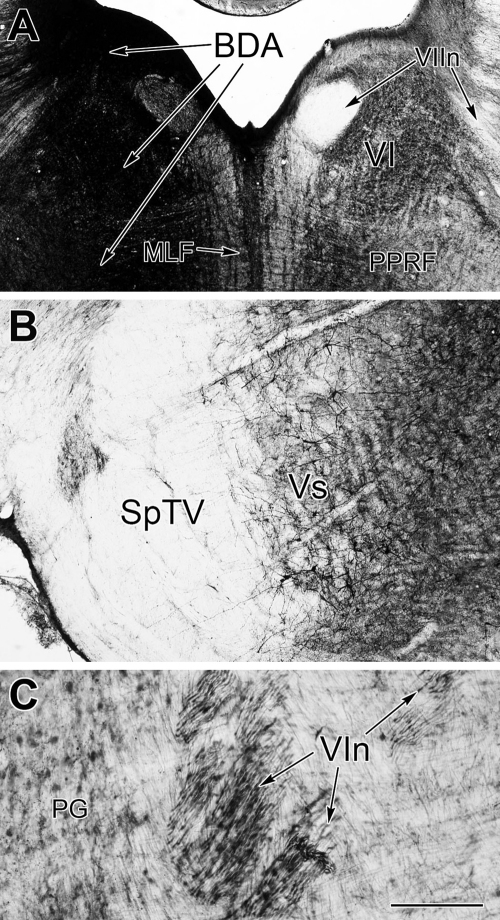
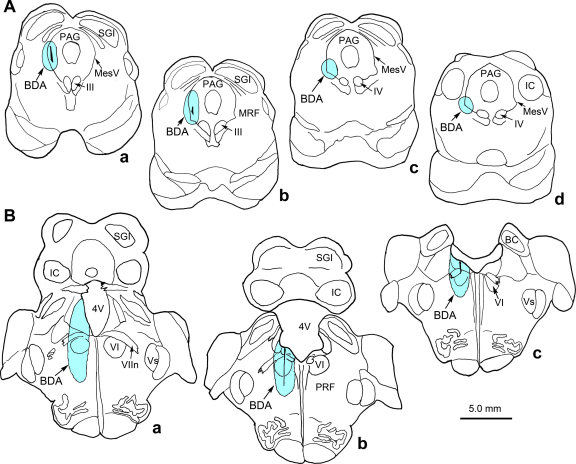
In the abducens case, the BDA injection site included all of the abducens nucleus, as well as overlying tissue and portions of the underlying paramedian pontine reticular formation (Fig. 1A, Supp. Fig. 1B). As such, it covered the area that is the source of innervation to both MIFs and SIFs in the abducens muscle [26]. The spread into regions adjacent to the abducens nucleus did not include other motoneurons, and there was no spread to the spinal trigeminal nucleus, although cells within this nucleus were clearly labeled (Fig. 1B). Black, BDA labeled axons were observed in the sixth nerve as it exited through the edge of the pontine gray (Fig. 1C), but were clearly absent from the trigeminal spinal tract (Fig. 1B).
Fig. 1.
Central BDA labeling. (A) The dark BDA injection site included the left abducens nucleus and extended into the subjacent PPRF. (B) The injection site did not spread into the spinal trigeminal nucleus, although labeled cells and terminals were present. Labeled fibers were not seen in the trigeminal spinal tract. (C) Many of the axons in the exiting sixth nerve fascicles were BDA labeled. BDA, biotinylated dextran amine; MLF, medial longitudinal fasciculus; PG, pontine gray; PPRF, paramedian pontine reticular formation, SpTV, trigeminal spinal tract, VI, abducens nucleus; VIn, abducens nerve, VIIn, facial nerve, Vs, spinal trigeminal nucleus. Scale for A = 1 mm, in B = 0.5 mm, C = 200 μm.
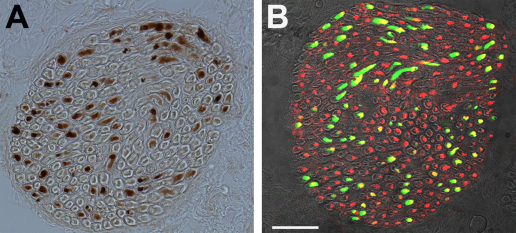
We tested whether the tracer was transported into the muscle belly of the lateral rectus muscle. With DAB labeling, we found tracer positive nerve fibers at the entry site of the nerve (Fig. 2A) and in the muscle belly. However, not all of the axons contained tracer: counts of tracer positive axons in 10 nerve branches on different sections revealed that on average 30% contained tracer, indicating that not all of the motoneurons had successfully taken up and transported BDA to the periphery.
Fig. 2.
Tracer visualization of the incoming nerve innervating the lateral rectus muscle. (A) Light microscopic image of tracer labeled axons. The tracer was visualized via the brown deposit of the DAB reaction product. Not all axons are tracer positive. (B) Fluorescence staining of tracer (green) and ChAT (red). The CLSM fluorescence image was overlaid with a transmission image. The red axons were ChAT positive, but did not contain tracer. Scale bar, 100 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
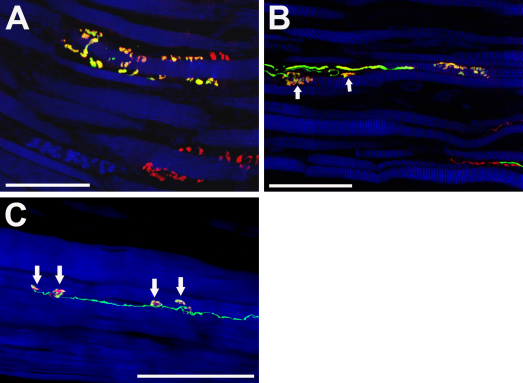
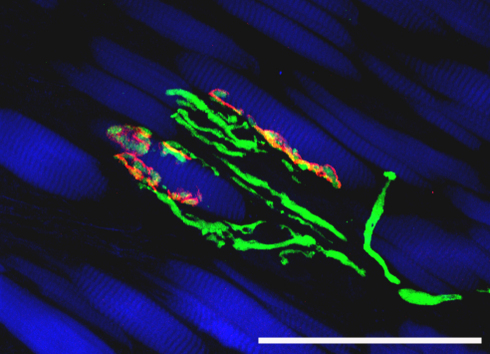
In the muscle belly of the left lateral rectus muscle of this monkey, we performed triple fluorescence labeling with avidin, anti-ChAT, and phalloidin. We found that all tracer positive nerve fibers exhibited ChAT immunoreactivity (Fig. 2B and Fig. 3). We further observed that tracer/ChAT positive axons established contacts on muscle fibers, whose morphology resembled motor terminals contacting SIFs (Fig. 3A) and MIFs (Fig. 3B). Triple labeling with avidin, α-bungarotoxin, and phalloidin confirmed that the tracer in these fibers was transported up to the motor terminals. In particular, we observed tracer/α-bungarotoxin positive motor endplates contacting SIFs (Supp. Fig. 2), and tracer/α-bungarotoxin positive en passant boutons contacting MIFs (Fig. 3C). On average 30% of the motor endplates/terminals were tracer positive, corresponding to the fraction of tracer labeled axons.
Fig. 3.
CLSM images showing triple fluorescence staining of muscle belly sections. (A, B) show labeling of tracer (green), ChAT (red) and muscle fiber (blue). (C) shows labeling of tracer (green), α-bungarotoxin (red) and muscle fiber (blue). (A) A tracer/ChAT positive nerve contact (yellow/green/red) on a muscle fiber resembling a motor endplate on a singly innervated muscle fiber. Another putative motor endplate (red) exhibits ChAT immunoreactivity, but contains no tracer. (B) A tracer/ChAT positive axon establishing two contacts (arrows) on the muscle fiber. (C) A tracer positive nerve fiber establishes several α-bungarotoxin positive motor contacts (arrows) which appear in yellow/green/red color. Scale bars, 100 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
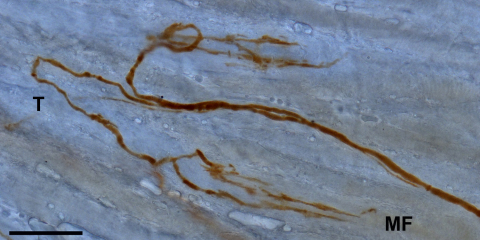
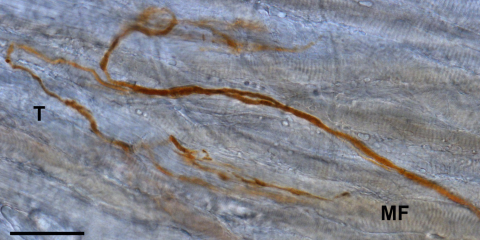
To determine, whether palisade endings also contain tracer, we performed DAB labeling in the wholemount of the distal myotendinous junction of the same muscle. We found 6 tracer labeled palisade endings, which displayed the classical morphology: the axon ran from the surface of the muscle belly, to extend onto the tendon, where it then turned back 180° and terminated in a cuff of nerve terminals around the tip of a muscle fiber (Fig. 4 and Supp. Fig. 3). A schematic drawing of the location and innervation of palisade endings in the lateral rectus muscle is shown in Supp. Fig. 4.
Fig. 4.
DAB staining of tracer in the muscle–tendon transition of a wholemount visualized by light microscopy. 3D-reconstruction from a stack of 10 sections showing two palisade endings labeled with tracer. The palisade endings show the classic shape: an axon originating from the muscle extends onto the tendon, where it turns back 180° and ends in arborizations at a muscle fiber tip. The junction of the muscle fibers with the tendon is more clearly discernable in single sections as shown in Supp. Fig. 2. Muscle fiber (MF), tendon (T). Scale bar, 100 μm.
In two monkeys, BDA was injected into the lateral periaqueductal gray so that the injection would include the mesencephalic trigeminal nucleus at its edge (Supp. Fig. 1A). No tracer was found in the EOM bellies. No labeling was observed in the palisade endings of the rectus muscle myotendinous junctions.
In this initial report, we described the use of BDA to determine whether the cells of origin of palisade endings found in EOMs are located within the EOM motor nuclei. After injecting this anterograde tracer into the abducens nucleus of a macaque monkey, we observed tracer labeled palisade endings in the distal tendon of the lateral rectus muscle indicating that these EOM specific structures originate from EOM motor nuclei. This conclusion must be tempered by the following points. First, as only one abducens-injected animal and one muscle have been investigated, more extensive experiments are required. Second, the possibility that labeling of mesencephalic trigeminal axons produced the palisade ending label must be entertained, as labeled fibers in the caudal portion of the nucleus made it impossible to ascertain whether cells in this nucleus were labeled either by the injection halo or via central branches of their axons. Arguing against this possibility is the fact that we did not see labeled palisade endings following control injections in the rostral part of the mesencephalic trigeminal nucleus. Finally, it should be noted that these findings do not rule out the possibility of other sources of input to the palisade endings.
In a prior study of macaque monkey, 50–73 palisade endings were counted in the medial rectus muscle, and 35–45 in the superior oblique muscle [3], while only 6 labeled palisades were observed in the present study. The fact that only 30% of the axons were labeled in nerve branches suggests this is due to insufficient transport of tracer. It seems unlikely that palisade endings with the same morphology would differ radically in their innervation patterns.
The function of palisade endings is a still an open question. Several studies support a sensory role of this EOM specific organ [1,2,6,8,9,20]. Specifically, fine structure studies demonstrated that all palisade endings, with the exception of rat's [9] and rabbit's [5], established contacts with the tendon [1,3,4,12,19,21]. In Golgi tendon organs, analogous nerve contacts are sensory [23]. Neuromuscular contacts in the palisade endings of some species (cat [1], sheep [4] and monkey [21]) lack a basal lamina in the synaptic cleft, a characteristic which is typical for sensory terminals in muscle spindles [11]. Clinically, patients have deficits in eye–hand coordination after repeated strabismus surgery. This has been interpreted as a proprioceptive loss resulting from damage to palisade endings during the surgical procedure [24,25]. After injection of tracer into the distal EOM of monkeys, Fackelmann et al. [10] found tracer positive cells in the trigeminal ganglion. Finally, Billig at al. [2] demonstrated that structures resembling palisade endings were labeled after tracer-injection into the sensory trigeminal ganglion of cats.
Alternatively, a motor role for palisade endings is suggested by other studies. Sas and Scháb [22] found that lesions to EOM motor nuclei led to a degeneration of palisade endings. Molecular studies in cat [12], sheep [18], dog [19] and monkey [3] showed that palisade endings are cholinergic. Additionally, the neuromuscular contacts present in the palisade endings of man [13], cat [12] and monkey [3] are α-bungarotoxin positive, a classic characteristic of motor terminals. In some cases, nerve fibers supplying palisade endings were observed to establish contacts on the fiber outside the palisade complex, indicating that palisade endings may be extensions of the axons supplying the rest of the muscle fiber [3]. Since these are MIFs, then presumably these fibers originate in the small motoneurons found in the periphery of each extraocular motor nucleus in contradistinction to large motoneurons in the core of the nucleus that supply SIFs [6,26]. Based on the location of the cells of origin and the neurochemical profile of the BDA labeled axons, our present findings are more compatible with a motor function of palisade endings. However, since the injection site includes both groups of motoneurons, we cannot distinguish whether palisades are innervated by small or large abducens motornuerons.
It is difficult to explain the conflicting findings regarding the function and origin of palisade endings. Species differences are unlikely since with the exception of rat and rabbit, palisade endings in all other species examined to date have a comparable morphology [3,12,18,19]. It is therefore unlikely that palisade endings with similar morphology would differ in their function. Lukas et al. [13] suggested that palisade endings could combine a motor and sensory function. However, if axons supplying palisade endings innervate the rest of the MIF [3], they would have to conduct information in two directions: from the central nervous system to the motor contacts on the MIF, and in the opposite direction, from palisade ending to the central nervous system. Since the idea that axons might be both efferent and afferent lacks physiological confirmation at present, the alternative possibility, that proprioception arises from non-palisade, sensory endings in the EOMs [2] must be considered.
In conclusion, this study provides evidence that the source of axons forming EOM palisade endings may be motoneurons in the EOM motor nuclei, and complements prior molecular findings [3,12,18,19], suggesting that palisade endings are motor rather than sensory features.
Acknowledgements
The authors thank Regina Mayer, Marietta Lipowec and Jinrong Wei for their valuable technical assistance. The study was supported by Grant P20881-B09 from the Fonds zur Foerderung der Wissenschaftlichen Forschung (FWF), an Austria-Spain concerted action AT2009-0039 to AMP and RB, and grant BFU2009-07121 from MEC-FEDER to AMP.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.neulet.2010.11.072.
Appendix A. Supplementary data
References
- 1.Alvarado Mallart R.M., Pincon Raymond M. The palisade endings of cat extraocular muscles: a light and electron microscope study. Tissue Cell. 1979;11:567–584. doi: 10.1016/0040-8166(79)90063-6. [DOI] [PubMed] [Google Scholar]
- 2.Billig I., Buisseret Delmas C., Buisseret P. Identification of nerve endings in cat extraocular muscles. Anat. Rec. 1997;248:566–575. doi: 10.1002/(SICI)1097-0185(199708)248:4<566::AID-AR8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 3.Blumer R., Konakci K.Z., Pomikal C., Wieczorek G., Lukas J.R., Streicher J. Palisade endings: cholinergic sensory organs or effector organs? Invest. Ophthalmol. Vis. Sci. 2009;50:1176–1186. doi: 10.1167/iovs.08-2748. [DOI] [PubMed] [Google Scholar]
- 4.Blumer R., Lukas J.R., Wasicky R., Mayr R. Presence and structure of innervated myotendinous cylinders in sheep extraocular muscle. Neurosci. Lett. 1998;248:49–52. doi: 10.1016/s0304-3940(98)00331-0. [DOI] [PubMed] [Google Scholar]
- 5.Blumer R., Wasicky R., Hotzenecker W., Lukas J.R. Presence and structure of innervated myotendinous cylinders in rabbit extraocular muscle. Exp. Eye Res. 2001;73:787–796. doi: 10.1006/exer.2001.1085. [DOI] [PubMed] [Google Scholar]
- 6.Buttner Ennever J.A., Horn A.K., Graf W., Ugolini G. Modern concepts of brainstem anatomy from extraocular motoneurons to proprioceptive pathways. Ann. N. Y. Acad. Sci. 2002;956:75–84. doi: 10.1111/j.1749-6632.2002.tb02810.x. [DOI] [PubMed] [Google Scholar]
- 7.Dogiel A. Die Endigungen der sensiblen Nerven in den Augenmuskeln und deren Sehnen beim Menschen und den Saeugetieren. Arch. Mikroskop. Anal. 1906;68:501–526. [Google Scholar]
- 8.Donaldson I.M. The functions of the proprioceptors of the eye muscles. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2000;355:1685–1754. doi: 10.1098/rstb.2000.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eberhorn A.C., Horn A.K., Eberhorn N., Fischer P., Boergen K.P., ButtnerEnnever J.A. Palisade endings in extraocular eye muscles revealed by SNAP-25 immunoreactivity. J. Anat. 2005;206:307–315. doi: 10.1111/j.1469-7580.2005.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fackelmann K., Nouriani A., Horn A.K., Buttner-Ennever J.A. Histochemical characterisation of trigeminal neurons that innervate monkey extraocular muscles. Prog. Brain Res. 2008;171:17–20. doi: 10.1016/S0079-6123(08)00603-1. [DOI] [PubMed] [Google Scholar]
- 11.Harker D.W. The structure and innervation of sheep superior rectus and levator palpebrae extraocular muscles. II. Muscle spindles. Invest. Ophthalmol. Vis. Sci. 1972;11:970–979. [PubMed] [Google Scholar]
- 12.Konakci K.Z., Streicher J., Hoetzenecker W., Blumer M.J., Lukas J.R., Blumer R. Molecular characteristics suggest an effector function of palisade endings in extraocular muscles. Invest. Ophthalmol. Vis. Sci. 2005;46:155–165. doi: 10.1167/iovs.04-1087. [DOI] [PubMed] [Google Scholar]
- 13.Lukas J.R., Blumer R., Denk M., Baumgartner I., Neuhuber W., Mayr R. Innervated myotendinous cylinders in human extraocular muscles. Invest. Ophthalmol. Vis. Sci. 2000;41:2422–2431. [PubMed] [Google Scholar]
- 14.Maier A., DeSantis M., Eldred E. The occurrence of muscle spindles in extraocular muscles of various vertebrates. J. Morphol. 1974;143:397–408. doi: 10.1002/jmor.1051430404. [DOI] [PubMed] [Google Scholar]
- 15.Manni E., Bortolami R., Desole C. Eye muscle proprioception and the semilunar ganglion. Exp. Neurol. 1966;16:226–236. doi: 10.1016/0014-4886(66)90101-4. [DOI] [PubMed] [Google Scholar]
- 16.Manni E., Bortolami R., Desole C. Peripheral pathway of eye muscle proprioception. Exp. Neurol. 1968;22:1–12. doi: 10.1016/0014-4886(68)90015-0. [DOI] [PubMed] [Google Scholar]
- 17.Morgan D.L., Proske U. Vertebrate slow muscle: its structure, pattern of innervation, and mechanical properties. Physiol. Rev. 1984;64:103–169. doi: 10.1152/physrev.1984.64.1.103. [DOI] [PubMed] [Google Scholar]
- 18.Rungaldier S., Heiligenbrunner S., Mayer R., Hanefl-Krivanek C., Lipowec M., Streicher J., Blumer R. Ultrastructural and molecular biologic comparison of classic proprioceptors and palisade endings in sheep extraocular muscles. Invest. Ophthalmol. Vis. Sci. 2009;50:5697–5706. doi: 10.1167/iovs.09-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rungaldier S., Pomikal C., Streicher J., Blumer R. Palisade endings are present in canine extraocular muscles and have a cholinergic phenotype. Neurosci. Lett. 2009;465:199–203. doi: 10.1016/j.neulet.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruskell G.L. Extraocular muscle proprioceptors and proprioception. Prog. Retin. Eye Res. 1999;18:269–291. doi: 10.1016/s1350-9462(98)00029-9. [DOI] [PubMed] [Google Scholar]
- 21.Ruskell G.L. The fine structure of innervated myotendinous cylinders in extraocular muscles of rhesus monkeys. J. Neurocytol. 1978;7:693–708. doi: 10.1007/BF01205145. [DOI] [PubMed] [Google Scholar]
- 22.Sas J., Scháb R. Die sogenannten “Palisaden-Endigungen” der Augenmuskeln. Acta Morph. Acad. Sci. Hung. 1952;2:259–266. [Google Scholar]
- 23.Schoultz T.W., Swett J.E. The fine structure of the Golgi tendon organ. J. Neurocytol. 1972;1:1–26. doi: 10.1007/BF01098642. [DOI] [PubMed] [Google Scholar]
- 24.Steinbach M.J., Kirshner E.L., Arstikaitis M.J. Recession vs marginal myotomy surgery for strabismus: effects on spatial localization. Invest. Ophthalmol. Vis. Sci. 1987;28:1870–1872. [PubMed] [Google Scholar]
- 25.Steinbach M.J., Smith D.R. Spatial localization after strabismus surgery: evidence for inflow. Science. 1981;213:1407–1409. doi: 10.1126/science.7268444. [DOI] [PubMed] [Google Scholar]
- 26.Ugolini G., Klam F., Doldan Dans M., Dubayle D., Brandi A.M., Buttner-Ennever J., Graf W. Horizontal eye movement networks in primates as revealed by retrograde transneuronal transfer of rabies virus: differences in monosynaptic input to “slow” and “fast” abducens motoneurons. J. Comp. Neurol. 2006;498:762–785. doi: 10.1002/cne.21092. [DOI] [PubMed] [Google Scholar]
- 27.Wang N., May P.J. Peripheral muscle targets and central projections of the mesencephalic trigeminal nucleus in macaque monkeys. Anat. Rec. 2008;291:974–987. doi: 10.1002/ar.20712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X., Zhang M., Cohen I.S., Goldberg M.E. The proprioceptive representation of eye position in monkey primary somatosensory cortex. Nat. Neurosci. 2007;10:640–646. doi: 10.1038/nn1878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.