Summary
The circadian clock provides robust, ∼24 hr biological rhythms throughout the eukaryotes. The clock gene circuit in plants comprises interlocking transcriptional feedback loops, reviewed in [1], whereby the morning-expressed transcription factors CIRCADIAN CLOCK-ASSOCIATED 1 (CCA1) and LATE ELONGATED HYPOCOTYL (LHY) repress the expression of evening genes, notably TIMING OF CAB EXPRESSION 1 (TOC1). EARLY FLOWERING 3 (ELF3) has been implicated as a repressor of light signaling to the clock [2, 3] and, paradoxically, as an activator of the light-induced genes CCA1 and LHY [4, 5]. We use cca1-11 lhy-21 elf3-4 plants to separate the repressive function of ELF3 from its downstream targets CCA1 and LHY. We further demonstrate that ELF3 associates physically with the promoter of PSEUDO-RESPONSE REGULATOR 9 (PRR9), a repressor of CCA1 and LHY expression, in a time-dependent fashion. The repressive function of ELF3 is thus consistent with indirect activation of LHY and CCA1, in a double-negative connection via a direct ELF3 target, PRR9. This mechanism reconciles the functions of ELF3 in the clock network during the night and points to further effects of ELF3 during the day.
Highlights
► ELF3 is a regulator of TOC1, PRR9, GI, and PRR7 gene expression ► Repression by ELF3 is genetically separable from repression by LHY and CCA1 ► ELF3 physically associates with the promoter of PRR9 in a time-dependent manner
Results
Mutual Regulation of ELF3 and CCA1/LHY Expression
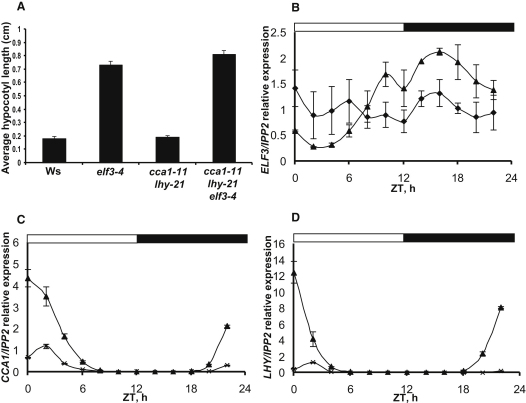
Hypocotyl growth is a circadian output and can be used as an indicator of clock function [6]. elf3-4 seedlings show abnormally elongated hypocotyls as the clock-controlled repression of hypocotyl growth is lost in these plants [7, 8]. To study the interaction between ELF3 and CCA1/LHY, we examined hypocotyl length in loss-of-function mutant backgrounds. Seedlings were grown under short day conditions (6:18 hr light:dark cycles) for 6 days, and hypocotyl length was assessed on day 7. The wild-type ecotype, Wassilewskija (Ws), and cca1-11 lhy-21 seedlings showed hypocotyls of similar length, whereas cca1-11 lhy-21 elf3-4 showed a phenotype very similar to elf3-4 seedlings (Figure 1A). This suggests that the elf3-4 mutant effect on aberrant growth of hypocotyls does not require the LHY and CCA1 transcription factors. Imaging of rhythms in delayed chlorophyll fluorescence (see Figure S1 available online) showed that, like elf3-4 mutants, cca1-11 lhy-21 elf3-4 plants were arrhythmic for this physiological marker in constant light.
Figure 1.
ELF3 Affects Clock Outputs and Clock Genes
Hypocotyl measurements of 7-day-old seedlings are shown as an average hypocotyl length (Wassilewskija [Ws] n = 12, elf3-4 n = 18, cca1-11 lhy-21 n = 19, and cca1-11 lhy-21 elf3-4 n = 23), with the error being represented as a standard error of the mean (SEM) (A). Data are representative of two biologically independent experiments. qPCR measurements are shown of RNA levels for ELF3 in Ws wild-type plants (filled triangles) and cca1-11 lhy-21 double mutants (filled diamonds) (B), CCA1 (C) and LHY (D) in Ws (filled triangles), and elf3-4 mutants (crosses). Data are all normalized against IPP2 expression [25]. Graphs are an average of two to three biologically independent experiments, with normalized data being used to generate SEM error bars. Seedlings were grown in 12:12 white light:dark cycles and sampled every 2 hr from Zeitgeber time (ZT) = 0. ZT = 0 is defined as the time of lights-on. See also Figures S1 and S2.
CCA1 and LHY RNA expression levels were shown to be very low in elf3 mutant seedlings, suggesting a mechanism for their arrhythmia [5]. We confirmed this through quantitative PCR (qPCR) analysis on 7-day-old seedlings under 12:12 white light:dark (LD) cycles (Figure 1) or transferred from 12:12 red LD to constant light (LL; Figure S2). The high amplitude of CCA1 and LHY expression rhythms in wild-type (100-fold to 1000-fold in LD, 10-fold in LL) collapsed in the elf3-4 plants, which became arrhythmic in LL. Transcript analysis under LD was more informative. The low-amplitude rhythm in both CCA1 and LHY transcripts (reaching at most 15% of wild-type peak level, Figures 1C and 1D; 40% of wild-type peak, Figures S2A and S2C) showed that the clock's morning functions were severely impaired in the elf3-4 mutant, though a rhythm could still be driven by the LD cycle. ELF3 RNA levels had a lower-amplitude rhythm in the wild-type (at most 10-fold in LD), whereas in cca1-11 lhy-21 mutants, ELF3 RNA showed little rhythmicity under LD and arrhythmia under LL (Figure 1B and Figure S2F). Circadian control of ELF3 expression [5] requires the morning loop components CCA1 and LHY. ELF3, in turn, regulates these clock genes and gates entrainment signals [2].
ELF3 Is a Key Repressor of Core Circadian Genes
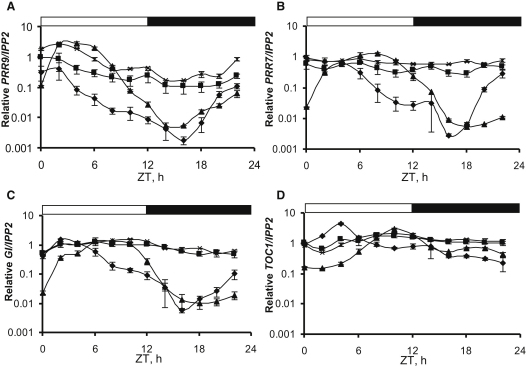
An evening loop, involving at least TOC1 and GIGANTEA (GI), is proposed to generate the short-period rhythms observed in lhy cca1 double mutants [9]. Through the comparison of clock gene expression in Ws, elf3-4, cca1-11 lhy-21, and cca1-11 lhy-21 elf3-4 plants, we aimed to test the role of ELF3 in the proposed evening loop. Plants were grown under 12:12 LD cycles for 6 days, sampled on day 7, and tested for expression of PRR9, PRR7, GI, and TOC1 (Figure 2; Figure S3). In cca1-11 lhy-21 plants, the evening genes (TOC1 and GI) showed an early-morning peak of high amplitude (Figures 2C and 2D). This is in agreement with previously published data [9–11] and also supports the hypothesis that CCA1 and LHY act to repress evening gene expression in the early morning. In the double mutant, PRR9 showed a lower amplitude rhythm, probably because of the loss of activation of expression by CCA1 and LHY (Figure 2A). The elf3-4 mutant showed a lower amplitude rhythm in gene expression for all measured genes, with notably higher levels (over 10-fold increase compared to wild-type) of PRR9, PRR7, and GI expression in the night, as reported in [12] for GI, as well as slightly higher nighttime expression of TOC1. The aberrant gene expression continued into the early morning, when CCA1 and LHY should be active in the wild-type (Figures 1C and 1D). Such results are consistent with a combination of indirect and direct mechanisms, whereby CCA1 and LHY repress evening gene expression in the morning (Zeitgeber time [ZT] 0–4, where ZT = 0 is defined as the time of lights-on) and ELF3 represses many genes at night (ZT 12–20), before CCA1 and LHY are expressed. From this it could be expected that the cca1-11 lhy-21 elf3-4 triple mutant would show high expression of certain clock genes throughout the LD cycle. This was not observed. Instead, in the triple mutant, all genes were expressed at intermediate levels, without strong responses to the ongoing LD. PRR9 and PRR7 expressions were higher than in cca1-11 lhy-21 but lower than in elf3-4. Evening genes (TOC1 and GI) lost the early peak observed in cca1-11 lhy-21, but then had the higher nighttime expression characteristic of elf3-4. This suggests that ELF3 influences the circadian network at more than one point and thus affects both morning and evening loops.
Figure 2.
ELF3 Regulates the Expression of Core Circadian Genes
qPCR measurements of RNA levels for PRR9 (A), PRR7 (B), GI (C), and TOC1 (D) normalized against IPP2 and between replicates in Ws (filled triangles), elf3-4 (crosses), cca1-11 lhy-21 (filled diamonds), and cca1-11 lhy-21 elf3-4 (filled squares). Graphs are an average of three biologically independent experiments, each containing triplicate samples. Normalized data were used to generate SEM error bars. Seedlings were grown and sampled as in Figure 1. See also Figure S3.
ELF3 Binds In Vivo to the Promoter of PRR9 in the Early Night
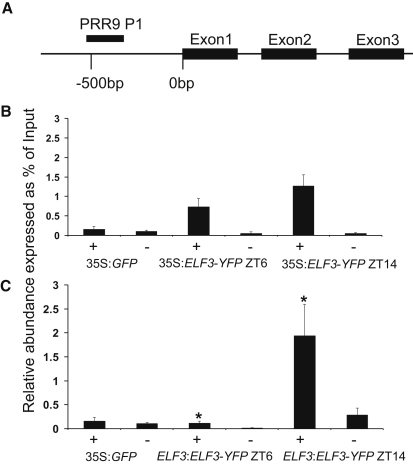
Because ELF3 shows some sequence homology with transcription factors [13], we investigated whether ELF3 physically associates with circadian-controlled promoters. Chromatin immunoprecipitation (ChIP) experiments were conducted using transgenic plants that expressed an ELF3::YFP fusion protein from either the native ELF3 promoter or the 35SCaMV promoter. We also used 35S::ELF4::YFP to investigate whether ELF3 and ELF4 act on the same promoters. EARLY FLOWERING 4 (ELF4) is a circadian-controlled gene that shows similar gene expression patterns and clock phenotypes to ELF3 [14]. ELF3 and ELF4 were both able to associate with the PRR9 promoter (Figures 3B and 3C; Figure S4). However, when ELF3 was expressed from its native promoter, it showed time-dependent affinity for the PRR9 promoter, being bound at ZT = 14 but not significantly (by Students t test) at ZT = 6 (Figure 3C). ELF3's apparently rhythmic association with the PRR9 promoter and the increased PRR9 expression observed in the elf3-4 mutant suggest that ELF3 acts as one of the repressors of PRR9 gene expression. Association of ELF3 with the PRR7 promoter was weak, because it was detected only in the 35S::ELF3::YFP plants (Figure S4). Association of ELF4 with PRR7 was comparable to results for PRR9 (Figure S4). Testing 1.3 kbp of sequences upstream of the ATG codon of CCA1 did not reveal any ELF3 or ELF4 association (data not shown), although this promoter fragment is sufficient for rhythmic transcription [15]. However, derepression of the PRR9 promoter is sufficient to explain low levels of CCA1 and LHY expression in the elf3-4 mutant (Figures 1C and 1D), because PRR9 is a known repressor of CCA1 and LHY [16]. The promoter regions required for rhythmic expression of PRR5, TOC1, and GI were also tested, and ELF3 and ELF4 were not found to associate with these (data not shown), suggesting that ELF3 is involved in the regulation of their expression indirectly.
Figure 3.
ELF3 Binds In Vivo to the Promoter of PRR9 in the Early Night, but Not during the Day
(A) Schematic of the PRR9 genomic region tested. The black bar indicates the specific region amplified from ChIP DNA by primer set P1.
(B and C) Chromatin of 3-week-old plants was immunoprecipitated using either no antibody (−) or anti-GFP antibody (+). Resultant DNA extracted from 35S::GFP (B and C), 35S::ELF3::YFP (B), and ELF3::ELF3::YFP (C) plants was analyzed by qPCR. Each signal is expressed as a percentage of the signal in nonimmunoprecipitated DNA (input) extracted from the same tissue sample. Data represent the mean of at least six samples taken from three independent ChIP experiments. Error bars represent the SEM. Student's t test showed that only ELF3::ELF3::YFP had significantly different chromatin association between ZT = 6 and ZT = 14, marked with ∗p < 0.05. See also Figure S4.
A Combination of Repressors Is Required for the Control of Circadian-Regulated Light Responses
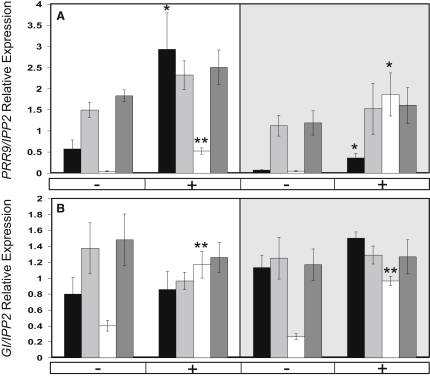
In order to investigate the regulation of light signaling via ELF3, a 20 min white-light pulse was applied to seedlings entrained in 12:12 white LD cycles and released into darkness. PRR9 and GI were specifically investigated because they have both been implicated in light signaling to the clock [16, 17], showed misregulation of gene expression in the elf3-4, cca1-11 lhy-21, and cca1-11 lhy-21 elf3-4 mutants, and represented the morning and evening loops of the circadian network. Wild-type plants showed strong light induction of PRR9 (Figure 4A). In cca1-11 lhy-21 double mutants, the expression levels of PRR9 were very low, and a clear acute response to light was observed, which was as large or larger than that in Ws during the predicted night, ZT = 38 (Figure 4A). In elf3-4 and cca1-11 lhy-21 elf3-4 seedlings, PRR9 had a higher level of basal expression in the night, consistent with Figure 2A and with ELF3's function as a repressor of gene expression in the dark. Little change in expression was observed following a light pulse at either predicted day ZT = 30 or night ZT = 38 (Figure 4A). Notably, the PRR9 expression level was not maximal compared to peak levels (Figure 2A), suggesting that another factor is involved in the gating of light responses in the dark. GI expression was not light responsive at these times in Ws and showed light induction in cca1-11 lhy-21, but not in elf3-4 or the triple mutant (Figure 4B). This again indicates that ELF3 affects clock gene expression in darkness, that ELF3 still controls clock genes in cca1-11 lhy-21 seedlings, and that some repressive functions remain in the triple mutant.
Figure 4.
ELF3 Is Required for the Control of Circadian-Regulated Light Responses in GI and PRR9
Acute light induction of PRR9 (A) and GI (B) gene expression was measured by qPCR in Ws (black bars), elf3-4 (light gray bars), cca1-11 lhy-21 (white bars), and cca1-11 lhy-21 elf3-4 (dark gray bars). Seedlings were grown for 5 days under white-light 12:12 LD cycles and released into continuous dark from ZT = 12 on day 5. On day 6, samples were either treated with (+) or without (−) a white-light pulse (20 min, 80 μmol m−2 s−1) 1 hr before sampling on the predicted day at ZT = 30 (white background) and on the predicted night at ZT = 38 (gray background). Error bars indicate the SEM from 4–6 samples. Student's t test was used to compare treated and untreated samples within a time point and genotype. For clarity, only treated samples that differ significantly from their control are marked with ∗p < 0.05 or ∗∗p < 0.005.
Discussion
This work tests the possibility that ELF3 acts as an activator of CCA1 through both the investigation of the transcriptional loops with which ELF3 is involved and the determination of whether ELF3 protein can associate with DNA. We show that ELF3 has repressive effects on several clock genes. The observed activation of CCA1 in elf3-4 mutants can be explained consistently with ELF3's repressive function by a double-negative effect via PRR9, the repressor of CCA1 and LHY [18]. ELF3 protein associates with the PRR9 promoter (Figure 3). In elf3-4, the levels of PRR9 are high, so the repression of CCA1 and LHY is greater. However, the high expression of evening genes GI and TOC1 in elf3-4 mutants cannot simply be explained by low levels of CCA1 and LHY, because this high baseline was not observed in cca1-11 lhy-21 mutants.
To investigate the role of ELF3 independently of the influence of CCA1 and LHY, we generated cca1-11 lhy-21 elf3-4 plants. These plants have a growth phenotype similar to the elf3-4 plants (Figure 1A). cca1-11 lhy-21 elf3-4 mutants show high basal levels of clock gene expression in the dark period of 12:12 LD cycles, as in elf3-4, but do not show the characteristic early peaks of PRR7, GI, and TOC1 expression observed in cca1-11 lhy-21 (Figure 2). This high level of gene expression in the dark is also observed in the acute light pulse response data set (Figure 4). Thus, through comparison of the cca1-11 lhy-21 and cca1-11 lhy-21 elf3-4 data, it seems that ELF3 allows rhythmicity in the cca1-11 lhy-21 double mutant. It also suggests that there may be another, normally redundant, factor, which is able to take the role of CCA1/LHY in the early morning (ZT 0–4) and repress the expression of circadian genes. This function is not observed in the cca1-11 lhy-21 double mutant because the component is still being repressed by ELF3.
Association with the PRR9 promoter provides a mechanism for ELF3's direct (PRR9) and indirect (CCA1/LHY) effects on the clock network. The fact that ELF3 affects the clock network beyond the times when ELF3 is detected at the PRR9 promoter is consistent with the known complexity of the clock circuit (Figure 2; Figure 3). Our current mathematical model of the Arabidopsis clock includes repression of PRR9 by an evening gene and assigns this role to TOC1 based on the known repression of PRR9 expression in TOC1-overexpressing plants [19]. It will now be important to understand the interaction of TOC1 and ELF3.
ELF3 is known to have a number of binding partners, including the red-light photoreceptor PHYB, the ubiquitin E3-ligase COP1, and clock-related proteins GI, SVP, and CCA1, suggesting that ELF3 may function in large signaling complexes. In this setting, ELF3 could participate in protein degradation [20] or transcriptional control through transcriptional complexes or histone and/or other chromatin modifications. Such an interpretation is supported by the mild phenotypic effect of the ELF3 overexpressor on the clock network [3] compared to the severe effect of the mutant; the ELF3 protein is required for correct clock function, but its level might not be so important.
This work identifies ELF3 as repressing gene expression of clock components, resulting in widespread effects on the clock gene network. Thus, ELF3 is essential for the normal operation of the circadian transcriptional feedback loops in light-grown plants, as reported in dark-grown seedlings [21]. The mechanism of ELF3 action presented here links ELF3 directly to the circadian network.
Experimental Procedures
Construction of Multiple Mutant Lines and Transgenic Plants
To create the cca1-11 lhy-21 elf3-4 triple mutant, we crossed the cca1-11 lhy-21 [22] double mutant to elf3-4 [6]. In the F2 progeny, individuals with long hypocotyls were selected and verified as homozygous elf3-4 mutants. These plants were then screened for cca1-11 and lhy-21 mutations. For details on the molecular markers used for genotyping, see Table S1.
The ELF3 promoter and the ELF3 and ELF4 coding sequences (CDS) were amplified by PCR from wild-type Ws genomic DNA by PCR primers with added restriction sites to facilitate cloning. The sequence of primers and the corresponding restriction sites are provided in Table S2. The amplified fragments were cloned in pBlueScript SK plasmids and verified by sequencing. The ELF3 promoter fragment contained 2695 nucleotides upstream of the start codon of the ELF3 gene and included the full 5′ untranslated region. The ELF3 and ELF4 CDS fragments included the full coding sequence but not the translational termination codons. The 35S:PHYA-YFP pPCVB812 binary vector has been described [23]. The PHYA cDNA in 35S:PHYA-YFP pPCVB812 was replaced with the ELF3 or ELF4 CDS fragments, resulting in 35S:ELF3-YFP pPCVB812 and 35S:ELF4-YFP pPCVB812. Then the 35S promoter in 35S:ELF3-YFP was replaced by the ELF3 promoter fragment, which yielded ELF3:ELF3-YFP pPCVB812. The binary vectors containing the gene constructs described above were transferred to Agrobacterium tumefaciens GV3101 cells. The constructs were transformed into wild-type Ws (ELF4 construct) and elf3-4 mutant plants (ELF3 constructs) by the floral-dip method [24]. Primary transformant plants were isolated based on resistance to Basta herbicide. Ten to 15 independent transgenic lines were produced for each combination of construct and host plant. Lines carrying a single copy of the transgene were selected based on the segregation of Basta resistance and were used for experiments.
Plant Materials and Growth Conditions
All plant lines are in the Ws ecotype. Surface sterilized seeds were stratified for 4 days in the dark at 4°C before being grown under cool-white fluorescent tubes (70–100 μmol m−2 s−1) in LD cycles at constant 22°C. All plants were grown on 1% agar Murashige-Skoog (MS) plates. Photoperiod light conditions were either short day (SD) 6:18 or standard 12:12, as shown in figures. ZT = 0 is defined as the last dark:light transition before measurements start.
Analysis of Gene Expression
For LD time courses, approximately 70 seedlings per sample were harvested for each genotype into 1 ml of RNAlater solution (Ambion). Samples were taken at 2 hr intervals starting at ZT = 0. Total RNA was extracted (QIAGEN RNeasy kit, 74106) according to manufacturer's instructions. cDNA was synthesized from 1 μg of total RNA, and random hexamer primers were supplied with the Fermentas cDNA synthesis kit. cDNA was diluted 1:5 in RNase-free dH2O, and qPCR plates (LightCycler 480 multiwell plate 384, Roche) were set up using a Tecan Freedom EVO robot controlled by EVOware standard software with Master Mix containing SYBR Green (Roche), gene-specific primers at 3 μM, and RNase-free dH2O. The qPCR was conducted in triplicate in a Roche LightCycler 480 controlled by LightCycler 480 SW1.5 software. Transcript levels were normalized to the control transcript IPP2 [25] and were normalized between replicates.
All presented measurements are an average of three independent experiments. Gene-specific primer pairs are listed in Table S2.
Measurement of Hypocotyl Length
Plants were grown under SD (6:18 LD) white-light (70–100 μmol m−2 s−1) photoperiod conditions on MS and 1% agar plates for 6 days, and hypocotyls with centimeter ruler were imaged using a digital camera. Measurement of hypocotyl length was performed by ImageJ (http://rsb.info.nih.gov/ij/), with hypocotyl length being defined as from V in hypocotyls-cotyledon formation to hypocotyls-root junction.
Chromatin Immunoprecipitation
ChIP was carried out as previously described [26], with the following modifications: seedlings were grown for 3 weeks in 12:12 LD cycles and harvested at either ZT = 6 or ZT = 14; crosslinking with 1% formaldehyde was carried out under a vacuum for a total of 30 min; and samples were resuspended in 4 ml of ChIP dilution buffer and split into four samples. Chromatin was immunoprecipitated using anti-GFP (Clontech). ChIP DNA was analyzed by qPCR on an LC480 (Roche) using SYBR Green Master Mix (Roche). Relative quantities were calculated as a percentage of the input DNA for each sample. Primer pairs for each region tested are listed in Table S2 and were designed to cover the promoter regions previously shown to be sufficient for normal expression in promoter:LUC reporters [14, 15].
Acknowledgments
We gratefully acknowledge expert technical assistance from Adrian Thomson and Sarah Hodge. L.E.D. was supported by a Biotechnology and Biological Sciences Research Council (BBSRC) graduate studentship. K.K. and A.P. were supported by BBSRC grant BB/E015263/1. L.K.-B. was supported by the Hungarian Scientific Research Fund (grant OTKA-73362) and by the János Bólyai Research Scholarship from the Hungarian Academy of Sciences. The Centre for Systems Biology at Edinburgh is a Centre for Integrative Systems Biology funded by BBSRC and Engineering and Physical Sciences Research Council, reference BB/D019621/1.
Published online: January 13, 2011
Footnotes
Supplemental Information includes four figures and two tables and can be found with this article online at doi:10.1016/j.cub.2010.12.013.
Supplemental Information
References
- 1.Harmer S.L. The Circadian system in higher plants. Annu. Rev. Plant Biol. 2009;60:357–377. doi: 10.1146/annurev.arplant.043008.092054. [DOI] [PubMed] [Google Scholar]
- 2.McWatters H.G., Bastow R.M., Hall A., Millar A.J. The ELF3 zeitnehmer regulates light signalling to the circadian clock. Nature. 2000;408:716–720. doi: 10.1038/35047079. [DOI] [PubMed] [Google Scholar]
- 3.Covington M.F., Panda S., Liu X.L., Strayer C.A., Wagner D.R., Kay S.A. ELF3 modulates resetting of the circadian clock in Arabidopsis. Plant Cell. 2001;13:1305–1315. doi: 10.1105/tpc.13.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaffer R., Ramsay N., Samach A., Corden S., Putterill J., Carre I.A., Coupland G. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell. 1998;93:1219–1229. doi: 10.1016/s0092-8674(00)81465-8. [DOI] [PubMed] [Google Scholar]
- 5.Kikis E.A., Khanna R., Quail P.H. ELF4 is a phytochrome-regulated component of a negative-feedback loop involving the central oscillator components CCA1 and LHY. Plant J. 2005;44:300–313. doi: 10.1111/j.1365-313X.2005.02531.x. [DOI] [PubMed] [Google Scholar]
- 6.Dowson-Day M.J., Millar A.J. Circadian dysfunction causes aberrant hypocotyls elongation patterns in Arabidopsis. Plant J. 1999;17:63–71. doi: 10.1046/j.1365-313x.1999.00353.x. [DOI] [PubMed] [Google Scholar]
- 7.Hicks K.A., Millar A.J., Carre I.A., Somers D.E., Straume M., Meeks-Wagner D.R., Kay S.A. Conditional circadian dysfunction of the Arabidopsis early-flowering 3 mutant. Science. 1996;274:790–792. doi: 10.1126/science.274.5288.790. [DOI] [PubMed] [Google Scholar]
- 8.Hicks K.A., Albertson T.A., Wagner D.R. EARLY FLOWERING 3 encodes a novel protein that regulates circadian clock function and flowering in Arabidopsis. Plant Cell. 2001;13:1281–1292. doi: 10.1105/tpc.13.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Locke J.C.W., Kozma-Bognar L., Gould P.D., Feher B., Kevei E., Nagy F., Turner M.S., Hall A., Millar A.J. Experimental validation of a predicted feedback loop in the multi-oscillator clock of Arabidopsis thaliana. Mol. Syst. Biol. 2006;2:59. doi: 10.1038/msb4100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizoguchi T., Wright L., Fujiwara S., Cremer F., Lee K., Onouchi H., Mouradov A., Fowler S., Kamada H., Putterill J., Coupland G. Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. Plant Cell. 2005;17:2255–2270. doi: 10.1105/tpc.105.033464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niwa Y., Ito S., Nakamichi N., Mizoguchi T., Niinuma K., Yamashino T., Mizuno T. Genetic linkages of the circadian clock-associated genes, TOC1, CCA1 and LHY, in the photoperiodic control of flowering time in Arabidopsis thaliana. Plant Cell Physiol. 2007;48:925–937. doi: 10.1093/pcp/pcm067. [DOI] [PubMed] [Google Scholar]
- 12.Fowler S., Lee K., Onouchi H., Samach A., Richardson K., Morris B., Coupland G., Putterill J. GIGANTEA: A circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J. 1999;18:4679–4688. doi: 10.1093/emboj/18.17.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X.L., Covington M.F., Fankhauser C., Chory J., Wagner D.R. ELF3 encodes a circadian clock-regulated nuclear protein that functions in an Arabidopsis PHYB signal transduction pathway. Plant Cell. 2001;13:1293–1304. doi: 10.1105/tpc.13.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McWatters H.G., Kolmos E., Hall A., Doyle M.R., Amasino R.M., Gyula P., Nagy F., Millar A.J., Davis S.J. ELF4 is required for oscillatory properties of the circadian clock. Plant Physiol. 2007;144:391–401. doi: 10.1104/pp.107.096206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle M.R., Davis S.J., Bastow R.M., McWatters H.G., Kozma-Bognar L., Nagy F., Millar A.J., Amasino R.M. The ELF4 gene controls circadian rhythms and flowering time in Arabidopsis thaliana. Nature. 2002;419:74–77. doi: 10.1038/nature00954. [DOI] [PubMed] [Google Scholar]
- 16.Farre E.M., Harmer S.L., Harmon F.G., Yanovsky M.J., Kay S.A. Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr. Biol. 2005;15:47–54. doi: 10.1016/j.cub.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Tryon E.L., Kreps J.A., Harmer S.L. GIGANTEA acts in blue light signaling and has biochemically separable roles in circadian clock and flowering time regulation. Plant Physiol. 2005;143:473–486. doi: 10.1104/pp.106.088757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamichi N., Kiba T., Henriques R., Mizuno T., Chua N.-H., Sakakibara H. PSEUDO-RESPONSE REGULATORS 9, 7, and 5 are transcriptional repressors in the Arabidopsis circadian clock. Plant Cell. 2010;22:594–605. doi: 10.1105/tpc.109.072892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pokhilko A., Hodge S.K., Stratford K., Knox K., Edwards K.D., Thomson A.W., Mizuno T., Millar A.J. Data assimilation constrains new connections and components in a complex, eukaryotic circadian clock model. Mol. Syst. Biol. 2010;6:416. doi: 10.1038/msb.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J.-W., Rubio V., Lee N.-Y., Bai S., Lee S.-Y., Kim S.-S., Liu L., Zhang Y., Irigoyen M.L., Sullivan J.A. COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Cell. 2008;32:617–630. doi: 10.1016/j.molcel.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thines B., Harmon F.G. Ambient temperature response establishes ELF3 as a required component of the core Arabidopsis circadian clock. Proc. Natl. Acad. Sci. USA. 2010;107:3257–3262. doi: 10.1073/pnas.0911006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall A., Bastow R.M., Davis S.J., Hanano S., McWatters H.G., Hibberd V., Doyle M.R., Sung S., Halliday K.J., Amasino R.M., Millar A.J. The TIME FOR COFFEE gene maintains the amplitude and timing of Arabidopsis circadian clocks. Plant Cell. 2003;15:2719–2729. doi: 10.1105/tpc.013730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauer D., Viczian A., Kircher S., Nobis T., Nitschke R., Kunkel T., Panigrahi K.C., Adam E., Fejes E., Schafer E., Nagy F. Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell. 2004;16:1433–1445. doi: 10.1105/tpc.021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clough S.J., Bent A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 25.Imaizumi T., Schulz T.F., Harmon F.G., Ho L.A., Kay S.A. FKFI F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science. 2005;309:293–297. doi: 10.1126/science.1110586. [DOI] [PubMed] [Google Scholar]
- 26.Pien S., Grossniklaus U. Chromatin immunoprecipitation protocol for histone modifications and protein-DNA binding analyses in Arabidopsis. Methods Mol. Biol. 2010;631:209–220. doi: 10.1007/978-1-60761-646-7_15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.