Abstract
A 54-year-old woman presented to hospital after deliberate acute ingestion of paracetamol 20 g. Despite early administration of a standardised acetylcysteine regimen, the patient developed acute liver impairment and acute renal impairment. Prolonged acetylcysteine administration and supportive measures allowed restoration of normal liver and renal function. Early presentation to hospital and prolonged duration of follow-up gave an unusual opportunity to examine the onset and duration of paracetamol-induced hepatic and renal impairment.
BACKGROUND
Paracetamol remains the commonest means of deliberate self-poisoning in the Western world.1 Acetylcysteine substantially reduces the risk of acute hepatotoxicity if administered early, particularly if within 8 h of paracetamol ingestion.2 The decision to administer acetylcysteine is based on the quantity ingested, serum paracetamol concentration and interval after ingestion, and the presence of risk factors for hepatotoxicity including chronic excess ethanol consumption, malnutrition and the use of enzyme-inducing drugs.3 In the UK, acetylcysteine is administered as a standardised sequential intravenous infusion of 150 mg/kg over 15 min, 50 mg/kg over 4 h and 100 mg/kg over 16 h, based on patient weight up to a maximum of 110 kg. If, at the end of the infusion, prothrombin time and liver biochemistry are satisfactory, then the patient may be considered for discharge.2,4
Nephrotoxicity is a recognised complication of acute paracetamol overdose, and occurs in around 0.5% of patients that present to hospital.5 Onset is delayed in comparison to hepatotoxicity and, for example, peak creatinine concentrations occur at 5–6 days, whereas peak alanine transaminase activity is observed at 2–3 days.5 The true rate of occurrence of nephrotoxicity may be significantly higher than existing data suggest. Rising serum creatinine concentrations might not be detectable until more than 2 days after overdose, whereas few patients are observed for such a prolonged period of observation. Surprisingly few data are available concerning the onset and duration of hepatotoxicity and nephrotoxicity after acute paracetamol overdose.6 This case allowed us to observe the timecourse of the biochemical changes associated with acute toxicity from shortly after overdose until 20 days later.
CASE PRESENTATION
A 54-year-old woman absconded from a local psychiatric unit where she had been receiving treatment for depression. Shortly after, she presented to the Emergency Department claiming to have bought and ingested 40 paracetamol tablets (20 g). There were empty drug blister packs that corresponded with the stated dose. She denied co-ingestion of other drugs or ethanol, and was asymptomatic. Medical history included type 2 diabetes and depression, and her regular medications were simvastatin 20 mg every night, metformin 500 mg twice daily, trazodone 150 mg every night, venlafaxine modified release 225 mg daily and lorazepam 1 mg twice daily.
The patient was tearful but cooperative with assessment. Temperature was 36.3°C, heart rate 78 beats/min, respiratory rate 14 breaths/min, blood pressure 138/72 mm Hg and physical examination was normal. Peripheral oxygen saturation was 97%, resting 12-lead electrocardiogram was normal, near-patient testing showed capillary glucose 8.5 mmol/litre and breath alcohol meter did not detect ethanol. At 4 h after ingestion, serum paracetamol concentration was 222 mg/litre, creatinine 86 μmol/litre, alanine transaminase activity 19 U/litre and the international normalised ratio (INR) was 1.0. Salicylates were not detected, and a urinary toxicology screen was negative for amphetamines, benzodiazepines, cannabinoids, cocaine metabolites, methadone and opioids.
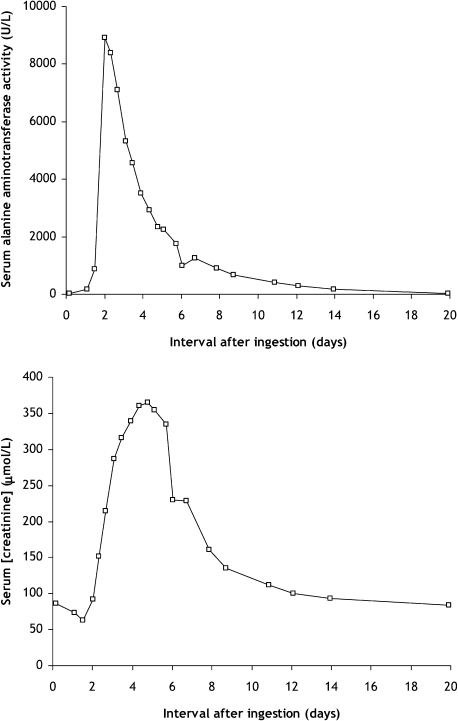
A standardised regimen of intravenous acetylcysteine 250 mg/kg was administered, based on body weight 78 kg. After treatment, the patient was asymptomatic and investigations showed serum creatinine 74 μmol/litre, alanine transaminase activity 166 U/litre and the INR was 2.1. In view of the prolonged INR, acetylcysteine infusion was continued. Despite treatment, acute hepatic and renal impairment occurred; peak serum alanine transaminase activity occurred at 2 days after ingestion, and peak serum creatinine concentration was at 5 days after overdose (fig 1). Metformin was withheld and acetylcysteine infusion was administered until 9 days after ingestion. Supportive care was given to ensure adequate hydration and correct any electrolyte imbalance. Biochemical markers of hepatic and renal function were restored to normal at 14–20 days after overdose.
Figure 1.
Serum alanine aminotransferase activity and serum creatinine concentration at various timepoints after acute paracetamol overdose.
OUTCOME AND FOLLOW-UP
After the acute hepatic and renal dysfunction had recovered, the patient was transferred back to the psychiatric unit for continuing care, and metformin was reintroduced. Laboratory investigations 2 months after the overdose showed serum creatinine 77 μmol/litre and alanine transaminase activity 20 U/litre.
DISCUSSION
This patient ingested a substantial quantity of paracetamol, confirmed by serum paracetamol concentrations higher than the “200 line” standard treatment nomogram. No additional risk factors for hepatotoxicity were present. Despite prompt administration of acetylcysteine, the patient developed acute liver injury, and additional treatment was administered. Serum creatinine concentrations increased above baseline values only after more than 2 days had elapsed. Acute renal failure was detectable in this case only because the patient was observed for longer than the standard duration of acetylcysteine administration. Peak creatinine concentrations occurred 5 days after drug overdose, and slowly recovered to baseline values at 14–20 days.
Paracetamol-induced nephrotoxicity is thought due to acute tubular necrosis, and may occur even in the absence of detectable hepatic injury.6,7 Distinct mechanisms are responsible for hepatic and renal injury after paracetamol dose. For example, glutathione depletion is an important mechanism of hepatotoxicity but is not observed in the kidney.8 Alternative pathways are implicated in paracetamol-induced nephrotoxicity. For example, caspase-mediated apoptosis and oxidative stress, which can evoke acute tubular dysfunction and cortical interstitial inflammation.9,10 Acetylcysteine administration may not lessen the risk of paracetamol-induced nephrotoxicity, which further emphasises the distinct mechanisms underlying hepatic and renal injury.11 Clinical markers of paracetamol nephrotoxicity have been explored, for example urinary protein concentrations and γ glutamyltransferase activity, but none has been found to offer a reliable early marker of injury.12,13
Clinical management of paracetamol-induced nephrotoxicity normally consists of supportive care. Appropriate secondary investigations should be considered in cases where there is diagnostic uncertainty or where an additional cause of acute renal failure is suspected. Hypokalaemia is a dose-dependent toxic effect of paracetamol, and potassium supplements may be required to maintain normal electrolyte status.14 Hydration and urinary volumes should be carefully observed and, in the presence of severe oliguria, then temporary haemodialysis may need to be considered.6
A limitation is that the patient’s regular medications might have contributed to the duration or extent of hepatic and renal injury. However, biochemical indices were entirely normal while the patient was receiving the same medicines after recovery from the acute effects of the overdose. It is uncertain whether the extended acetylcysteine regimen had any therapeutic effect in this single case. Nonetheless, no adverse effects were encountered, and prolonged treatment may improve outcome in patients with established paracetamol-induced hepatotoxicity.2
LEARNING POINTS
Renal function should be checked daily in patients that develop hepatotoxicity after paracetamol overdose.
Determination of liver biochemistry and international normalised ratio (INR) after a standard acetylcysteine infusion allows early detection of hepatotoxicity but might not detect patients with isolated paracetamol nephrotoxicity.
Footnotes
Competing interests: None.
Patient consent: Patient/guardian consent was obtained for publication.
REFERENCES
- 1.Thanacoody HK, Good AM, Waring WS, et al. Survey of cases of paracetamol overdose in the UK referred to National Poisons Information Service (NPIS) consultants. Emerg Med J 2008; 25: 140–3 [DOI] [PubMed] [Google Scholar]
- 2.Buckley N, Eddleston M. Paracetamol (acetaminophen) poisoning. Clin Evid 2005; 14: 1738–44 [PubMed] [Google Scholar]
- 3.Waring WS, Stephen AF, Robinson OD, et al. Serum urea concentration and the risk of hepatotoxicity after paracetamol overdose. QJM 2008; 101: 359–63 [DOI] [PubMed] [Google Scholar]
- 4.Brok J, Buckley N, Gluud C. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev 2006; 2: CD003328. [DOI] [PubMed] [Google Scholar]
- 5.Waring WS, Jamie H, Leggett GE. Delayed onset of acute renal failure after significant paracetamol overdose: a case series. Hum Exp Toxicol. In press [DOI] [PubMed] [Google Scholar]
- 6.Mour G, Feinfeld DA, Caraccio T, et al. Acute renal dysfunction in acetaminophen poisoning. Ren Fail 2005; 27: 381–3 [PubMed] [Google Scholar]
- 7.Boutis K, Shannon M. Nephrotoxicity after acute severe acetaminophen poisoning in adolescents. J Toxicol Clin Toxicol 2001; 39: 441–5 [DOI] [PubMed] [Google Scholar]
- 8.Möller-Hartmann W, Siegers CP. Nephrotoxicity of paracetamol in the rat: mechanistic and therapeutic aspects. J Appl Toxicol 1991; 11: 141–6 [DOI] [PubMed] [Google Scholar]
- 9.Lorz C, Justo P, Sanz A, Subirá D, et al. Paracetamol-induced renal tubular injury: a role for ER stress. J Am Soc Nephrol 2004; 15: 380–9 [DOI] [PubMed] [Google Scholar]
- 10.Isik B, Bayrak R, Akcay A, et al. Erdosteine against acetaminophen induced renal toxicity. Mol Cell Biochem 2006; 287: 185–91 [DOI] [PubMed] [Google Scholar]
- 11.Slitt AL, Dominick PK, Roberts JC, et al. Standard of care may not protect against acetaminophen-induced nephrotoxicity. Basic Clin Pharmacol Toxicol 2004; 95: 247–8 [DOI] [PubMed] [Google Scholar]
- 12.Benhalim S, Leggett GE, Jamie H, et al. Proteinuria is unrelated to the extent of acute acetaminophen overdose: a prospective study. J Med Toxicol 2008; 4: 232–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitić-Zlatković M, Stefanović V. Acute effects of acetaminophen on renal function and urinary excretion of some proteins and enzymes in patients with kidney disease. Ren Fail 1999; 21: 525–32 [DOI] [PubMed] [Google Scholar]
- 14.Waring WS, Stephen AF, Malkowska AM, et al. Acute acetaminophen overdose is associated with dose-dependent hypokalaemia: a prospective study of 331 patients. Basic Clin Pharmacol Toxicol 2008; 102: 325–8 [DOI] [PubMed] [Google Scholar]