Abstract
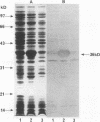
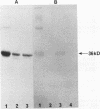
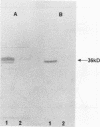
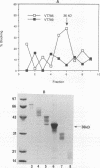
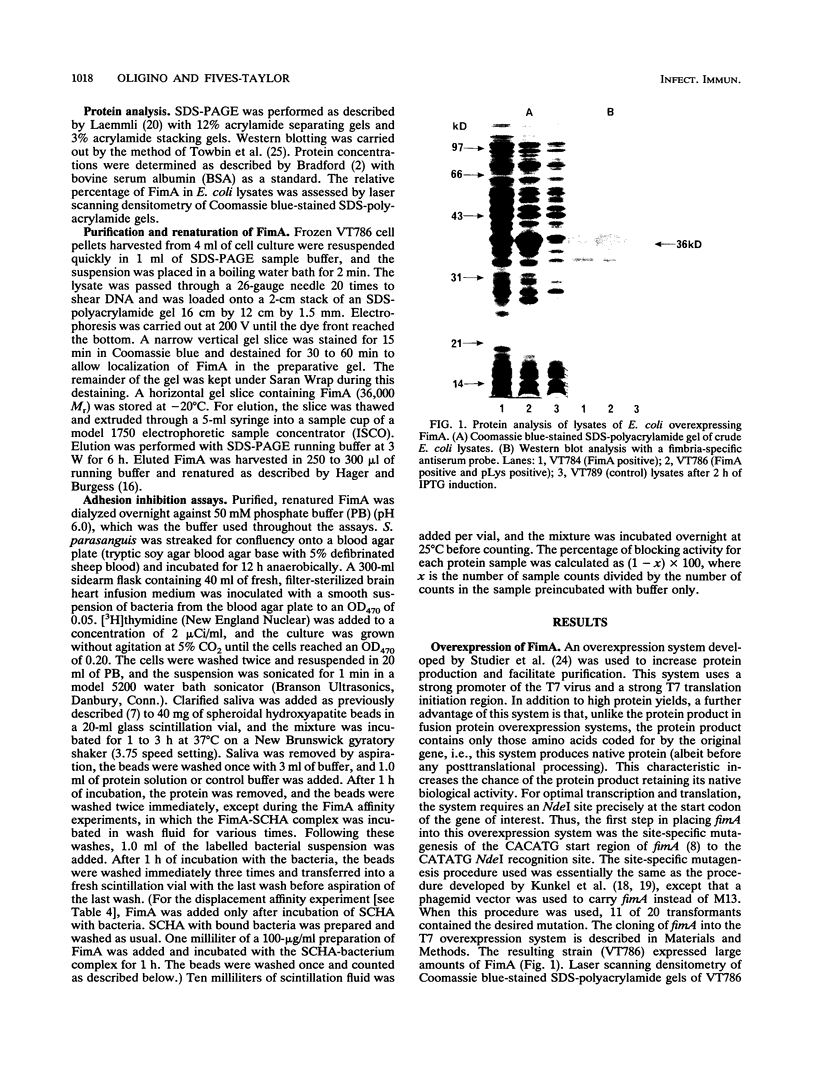
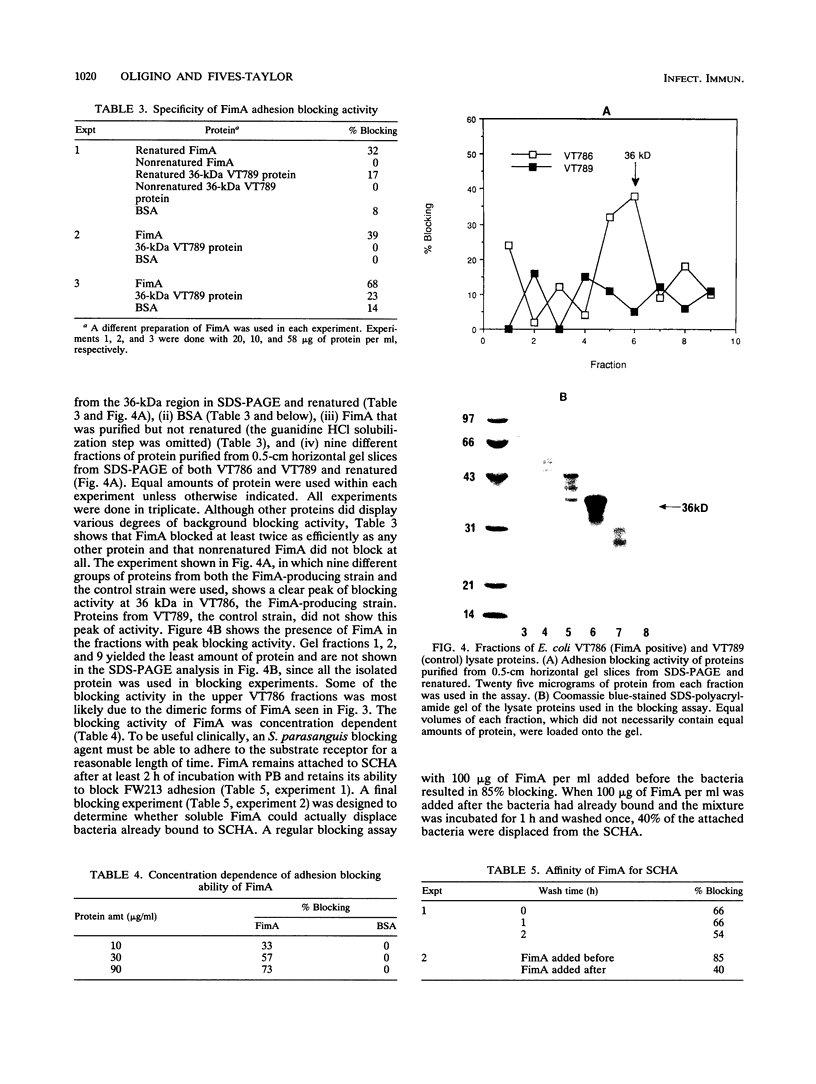
A Streptococcus parasanguis adhesin that blocks the attachment of S. parasanguis FW213 to saliva-coated hydroxyapatite (SCHA) has been purified. Previous work demonstrated that the attachment of FW213 to SCHA is mediated by fimbriae and that one component associated with fimbriae is a 36-kDa protein (FimA) that reacts with antifimbria serum in Western blots (immunoblots) and is not present in afimbriated mutants. To obtain amounts of FimA sufficient for adhesion blocking assays, we cloned the gene coding for FimA into an Escherichia coli T7 overexpression system. The resulting strain produced large amounts of FimA, as much as 50% of the total cell protein. FimA was purified by elution from sodium dodecyl sulfate-polyacrylamide gels, and its native conformation was reestablished by sodium dodecyl sulfate removal, resolubilization in guanidine hydrochloride, and 50-fold dilution. Some refolded FimA aggregated into dimers and trimers. Preincubation of SCHA with 100 micrograms of purified, renatured FimA per ml blocked 85% of the binding of FW213. The FimA-SCHA complex was quite stable and could be washed continuously for at least 2 h with only a slight loss of FimA blocking activity. When FimA was added to preformed bacterium-SCHA complexes, it displaced 40% of the bacteria already bound to SCHA. The results suggest that FimA is an adhesin with a high substrate affinity and may prove useful in the development of a therapeutic agent for the prevention of plaque formation and endocarditis initiated by the sanguis streptococci.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Grahnén H., Jonsson G., Wikner S. Establishment of Streptococcus sanguis in the mouths of infants. Arch Oral Biol. 1970 Dec;15(12):1143–1148. doi: 10.1016/0003-9969(70)90005-1. [DOI] [PubMed] [Google Scholar]
- Cisar J. O., Barsumian E. L., Siraganian R. P., Clark W. B., Yeung M. K., Hsu S. D., Curl S. H., Vatter A. E., Sandberg A. L. Immunochemical and functional studies of Actinomyces viscosus T14V type 1 fimbriae with monoclonal and polyclonal antibodies directed against the fimbrial subunit. J Gen Microbiol. 1991 Aug;137(8):1971–1979. doi: 10.1099/00221287-137-8-1971. [DOI] [PubMed] [Google Scholar]
- Fachon-Kalweit S., Elder B. L., Fives-Taylor P. Antibodies that bind to fimbriae block adhesion of Streptococcus sanguis to saliva-coated hydroxyapatite. Infect Immun. 1985 Jun;48(3):617–624. doi: 10.1128/iai.48.3.617-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno J. C., LeBlanc D. J., Fives-Taylor P. Nucleotide sequence analysis of a type 1 fimbrial gene of Streptococcus sanguis FW213. Infect Immun. 1989 Nov;57(11):3527–3533. doi: 10.1128/iai.57.11.3527-3533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fives-Taylor P. M., Macrina F. L., Pritchard T. J., Peene S. S. Expression of Streptococcus sanguis antigens in Escherichia coli: cloning of a structural gene for adhesion fimbriae. Infect Immun. 1987 Jan;55(1):123–128. doi: 10.1128/iai.55.1.123-128.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fives-Taylor P. M., Thompson D. W. Surface properties of Streptococcus sanguis FW213 mutants nonadherent to saliva-coated hydroxyapatite. Infect Immun. 1985 Mar;47(3):752–759. doi: 10.1128/iai.47.3.752-759.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen E. V., Pedrazzoli V., Kilian M. Ecology of viridans streptococci in the oral cavity and pharynx. Oral Microbiol Immunol. 1991 Jun;6(3):129–133. doi: 10.1111/j.1399-302x.1991.tb00466.x. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J., SOCRANSKY S. S., DEARAUJO W. C., VANHOUTE J. STUDIES OF THE PREDOMINANT CULTIVABLE MICROBIOTA OF DENTAL PLAQUE. Arch Oral Biol. 1964 May-Jun;9:365–370. doi: 10.1016/0003-9969(64)90069-x. [DOI] [PubMed] [Google Scholar]
- Ganeshkumar N., Hannam P. M., Kolenbrander P. E., McBride B. C. Nucleotide sequence of a gene coding for a saliva-binding protein (SsaB) from Streptococcus sanguis 12 and possible role of the protein in coaggregation with actinomyces. Infect Immun. 1991 Mar;59(3):1093–1099. doi: 10.1128/iai.59.3.1093-1099.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshkumar N., Song M., McBride B. C. Cloning of a Streptococcus sanguis adhesin which mediates binding to saliva-coated hydroxyapatite. Infect Immun. 1988 May;56(5):1150–1157. doi: 10.1128/iai.56.5.1150-1157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marinus M. G. DNA methylation in Escherichia coli. Annu Rev Genet. 1987;21:113–131. doi: 10.1146/annurev.ge.21.120187.000553. [DOI] [PubMed] [Google Scholar]
- Marston F. A. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem J. 1986 Nov 15;240(1):1–12. doi: 10.1042/bj2400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]