Studies from the laboratory of Roger Unger presented in the current issue of Diabetes highlight the potential benefit of reducing glucagon action by examining the effects of glucagon receptor knockout (Gcgr−/−) on the phenotype of type 1 diabetes in the mouse (1). The aim of the study was to determine if glucagon action, by itself, causes the lethal consequences of insulin deficiency. Because treatment of Gcgr−/− mice with the β-cell toxin streptozotocin (STZ) previously had no effect on circulating insulin levels or pancreatic islet architecture (2), Lee et al. (1) administered a double dose of STZ to maximize β-cell destruction. Unlike STZ treated wild-type Gcgr+/+ mice, which became severely hyperglycemic, STZ-treated mice lacking glucagon signaling appeared to be in a normal state of health and were completely protected from the manifestations of diabetes (1), as shown previously by the same group in alloxan treated Gcgr−/− mice (3) and by Hancock et al. (4) in STZ-treated mice lacking glucagon because of α-cell deletion. Fasting hyperglycemia did not occur in STZ-treated Gcgr−/− mice, and astonishingly, the animals demonstrated normal or even improved glucose disposal in response to a glucose tolerance test, despite the absence of a rise in plasma insulin. These results led the authors to speculate that insulin action during glucose absorption is largely directed toward overcoming the hepatic actions of glucagon. They theorized that insulin would have little or no role in a liver not exposed to the action of glucagon because it would be in a permanent glucose storage mode.
Glucagon antagonistic peptides, neutralizing antibodies, receptor antisense oligonucleotides, and/or receptor nonpeptidyl antagonists have previously been shown to lower plasma glucose in several rodent models of diabetes (5,6). Likewise, reversal of diabetes by leptin therapy in the rodent has been attributed to a reduction in plasma glucagon (3,7), although other actions of leptin could not be ruled out. Reduction of glucagon in pancreatectomized canines caused a marked decrease in hepatic glucose production (8) and suppression of glucagon in diabetic humans improved glucose tolerance (9,10). Thus, there is strong evidence supporting a role for glucagon in contributing to diabetic hyperglycemia.
Insulin deficient glucagon receptor-null mice are functionally pancreatectomized. Thus, based on the results of Lee et al. (1), normal glucose metabolism might be expected in humans with total pancreatectomy, but this is not the case. Measurement of glucagon is complicated by nonspecific cross reacting materials (6), leading to controversy as to whether pancreatectomized patients actually lack glucagon or not. The consensus, however, appears to support the concept that glucagon is produced by the gut in such patients, but at a reduced rate relative to that produced by the pancreas in individuals with type 1 diabetes (11). This probably explains the less severe, nonketotic, form of diabetes found in this population (12). In the pancreatectomized canine, elevated levels of gut derived glucagon have been shown to contribute to the severity of the diabetic phenotype (13).
The surprise in the data of Lee et al. (1) comes not from the improvement in glycemia caused by a lack of glucagon action, but from the complete normalization of glucose tolerance that occurred. Transition from the fasted to fed state involves a reduction in glucose production by the liver and an increase in glucose disposal by insulin sensitive tissues (skeletal muscle, liver, and adipose tissue). Studies in the human and canine have indicated that following an oral glucose load of moderate size (∼1 g/kg BW), the liver and skeletal muscle are each responsible for approximately a third of glucose disposal, with noninsulin dependant tissues accounting for the remainder (14). In nondiabetic individuals, the changes in muscle and liver glucose metabolism are thought to be chiefly mediated by insulin (14). The fact that oral glucose tolerance was normal in the STZ treated Gcgr−/− mice of Lee et al. (1), despite no rise in insulin, suggests that in a net sense glucose uptake by liver and muscle was normal. Whether both tissues took up glucose normally, or one compensated for a defect in the other, is not clear. Nevertheless, this raises the question as to what is driving glucose disposal if not insulin.
Lee et al. (1) argue that insulin overcomes glucagon’s inhibitory effects on hepatic glucose uptake (HGU) and that in the absence of glucagon, the liver will take up glucose without insulin. Indeed, insulin and glucagon have opposing effects on many glucoregulatory pathways in the liver, including transcription of glucokinase and regulation of glycogen synthesis and breakdown (Fig. 1A). However, in the normal canine made acutely deficient in insulin and glucagon using somatostatin and/or pancreatectomy, the liver did not take up or store glucose when exposed to a hyperglycemic challenge, despite the lack of glucagon (15). Likewise, an acute deficiency of both insulin and glucagon in the human (16) or canine (17) led to a transient fall in glucose production followed by a return to the basal rate, despite hyperglycemia secondary to decreased muscle glucose clearance. These data could be interpreted to suggest that glucagon plays a lesser role in hepatic glucose storage in large animal models and humans than it does in the rodent. Alternatively, it is possible that the duration of glucagon lack is important to the phenotype observed by Lee et al. (1). Leptin (presumably through an effect on glucagon secretion) normalized glucose within ∼10 days of treatment in diabetic mice (3,7). In the canine, 3 days of continuous parenteral nutrition produced an adaptation in the liver which allowed for sustained net HGU even in the presence of basal insulin. Further, this glucose uptake was very sensitive to inhibition by glucagon (18,19).
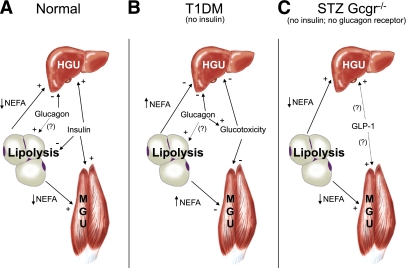
FIG. 1.
A: In the normal state, a meal-related rise in circulating glucose is efficiently cleared as the result of reciprocal changes in plasma insulin and glucagon concentrations. Increased glucose disposal is primarily accounted for by liver and skeletal muscle. Insulin increases disposal by both organs whereas glucagon opposes insulin at the liver. Insulin also inhibits lipolysis, thus reducing circulating NEFA and further promoting HGU and MGU. It is unclear what role glucagon may play in regulating lipolysis, although it appears to be minor. B: In type 1 diabetes, insulin is lacking and the effects of glucagon are unopposed. Glucose disposal is reduced as a result of the inhibitory effects of glucagon on the HGU and lack of insulin stimulated HGU and MGU. Plasma NEFAs are elevated, perhaps in part as a result of glucagon mediated stimulation of adipose tissue lipolysis in the absence of insulin. Reduced glucose clearance and increased glucose production leads to glucotoxicity, which in combination with lipotoxicity, further impairs glucose clearance. C: In STZ-treated Gcgr−/− mice, fasting NEFA levels are low, possibly because of the lack of glucagon action. GLP-1 levels are increased, and together with low NEFA, may create an adaptive state in which glucose disposal occurs normally in the absence of insulin and glucagon signaling. (A high-quality color representation of this figure is available in the online issue.)
Although the liver clearly plays an important role in glucose disposal, skeletal muscle is thought to be at least as important. In humans, muscle has been shown to account for 70% of glucose uptake during hyperinsulinemic/euglycemic clamps (20) and up to 50% after oral glucose loading (21). Because skeletal muscle is not thought to express the glucagon receptor, glucagon should not directly affect muscle glucose uptake (MGU) (Fig. 1A). Instead, insulin is the primary regulator, acting to rapidly increase muscle blood flow, stimulate transmembrane glucose transport, and activate muscle glycogen synthase (22). Why then, in the absence of glucagon receptors, might insulin no longer be required for normal MGU?
Glucagon deficiency resulting from genetic deletion of the α-cell does not appear to affect insulin’s ability to stimulate MGU because, compared with normal control animals, there was no difference in insulin stimulated glucose disposal during a hyperinsulinemic/euglycemic clamp (4). Muscle selective disruption of the insulin receptor (23) or GLUT4 (24) results in glucose intolerance in the presence of glucagon. Therefore, it is surprising that MGU would appear to have been similar in STZ and non-STZ–treated Gcgr−/− mice, despite arterial insulin levels that were elevated in one case and very low in the other (Fig. 2 in ref. 1). One possibility is that blockade of glucagon action caused the liver to take up more glucose than normal, thereby overcoming a deficit at muscle. Alternatively, adaptive changes may have occurred in muscle allowing enhanced noninsulin dependant glucose uptake to occur. For example, glucose tolerance was not altered in muscle-specific insulin receptor knockout mice (25). Likewise, compensatory insulin-independent muscle glucose transport appeared to occur in whole body GLUT4-null mice (26). How might a lack of glucagon action have triggered such a response?
Lack of glucagon may have produced indirect effects on glucose disposal in muscle through alterations in lipid homeostasis (Fig. 1C) (2,27). Adipocytes express the glucagon receptor (5) and hypoglucagonemia has been shown to reduce lipolysis and decrease nonesterified fatty acid (NEFA) production by a modest amount (28), although the extent of glucagon action on adipose tissue in vivo remains controversial (29). In any event, NEFAs have been shown to inhibit hepatic and muscle glucose uptake (30). Indeed, the fasting plasma NEFA levels in the insulin deficient Gcgr−/− mice of Lee et al. (1) were almost 70% lower than those in non-STZ–treated Gcgr−/− mice (∼0.5 vs. 1.5 nmol/L, respectively; Fig. 3 in ref. 1). Likewise, circulating NEFA levels were reduced by a third in the normal canine made acutely deficient in insulin and glucagon using somatostatin and/or pancreatectomy (15). Therefore, it is plausible that reduced NEFA availability during fasting may have caused an adaptation allowing for increased liver and muscle glucose uptake when exposed to a subsequent glucose load. Thus, by avoiding lipotoxicity and glucotoxicity, glucagon deficiency could indirectly alter glucose disposal by muscle.
It is important to remember, however, that GLP-1 levels are dramatically elevated in Gcgr−/− mice (2). GLP-1 has multiple effects on glucose metabolism, most of which are thought to be secondary to stimulation of insulin secretion, reduction in glucagon secretion, and inhibition of gastric emptying. The first two mechanisms are not relevant in STZ treated Gcgr−/− mice. Further, because glucose tolerance was apparently normal following an IPGTT in the studies of Lee et al. (1), slowed gastric emptying would not appear to explain the normal oral glucose tolerance test in these animals either, although it was a factor in improved glucose tolerance in non-STZ–treated Gcgr−/− mice (2). GLP-1 has also been shown to directly increase glycogen synthase a activity in rat hepatocytes (31) and to stimulate glucose uptake in human myocytes (32). These effects were blocked by treatment with a phosphatidylinositol 3-kinase inhibitor, suggesting crosstalk between insulin and GLP-1 receptor signaling. In vivo, GLP-1 has been shown to stimulate HGU (33) and MGU (34) independent of an effect on the β-cell. Thus, it is possible that the high GLP-1 levels seen in the Gcgr−/− mice played a role in normalization of glucose tolerance (Fig. 1C). This possibility is further supported by recent studies that demonstrated that Gcgr antagonist–mediated improvements in glycemic control were dependent on a functional pancreatic GLP-1 receptor (35). The role of GLP-1 in improving glycemia in mice lacking glucagon (4) or treated with leptin (3,7) is unclear because GLP-1 levels were not measured in those studies. Taken together, these data raise the possibility that the presence of increased GLP-1 in the STZ treated Gcgr−/− mice might explain at least part of the improved phenotype.
In summary, interfering with glucagon action (by decreasing its secretion or inhibiting its action) in the diabetic state is beneficial across species. In the rodent, complete eradication of glucagon action by deletion of the glucagon receptor is associated with a virtual elimination of the consequences of STZ induced insulin deficiency. At present it is unclear how deletion of the glucagon receptor overcomes the need for insulin to stimulate glucose uptake, presumable by the liver and muscle, but it may relate to abrogation of the hormone’s action on adipose tissue and/or the liver. The high level of GLP-1 evident in the STZ treated Gcgr−/− mice may have played a role in the response. Clearly, studies need to be carried out in humans and large animal models in which the effects of complete chronic or acute glucagon deficiency on the diabetic phenotype are examined. Further, the sites of glucose disposal in glucagon deficient STZ treated mice given an oral glucose tolerance test should be identified, and the flux rates and signaling pathways involved in glucose disposal should be determined.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 391.
REFERENCES
- 1.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes; 2011;60:391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conarello SL, Jiang G, Mu J, et al. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 2007;50:142–150 [DOI] [PubMed] [Google Scholar]
- 3.Wang MY, Chen L, Clark GO, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA 2010;107:4813–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hancock AS, Du A, Liu J, Miller M, May CL. Glucagon deficiency reduces hepatic glucose production and improves glucose tolerance in adult mice. Mol Endocrinol 2010;24:1605–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali S, Drucker DJ. Benefits and limitations of reducing glucagon action for the treatment of type 2 diabetes. Am J Physiol Endocrinol Metab 2009;296:E415–E421 [DOI] [PubMed] [Google Scholar]
- 6.Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007;28:253–283 [DOI] [PubMed] [Google Scholar]
- 7.Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA 2008;105:14070–14075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevenson RW, Williams PE, Cherrington AD. Role of glucagon suppression on gluconeogenesis during insulin treatment of the conscious diabetic dog. Diabetologia 1987;30:782–790 [DOI] [PubMed] [Google Scholar]
- 9.Dinneen S, Alzaid A, Turk D, Rizza R. Failure of glucagon suppression contributes to postprandial hyperglycaemia in IDDM. Diabetologia 1995;38:337–343 [DOI] [PubMed] [Google Scholar]
- 10.Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 2000;85:4053–4059 [DOI] [PubMed] [Google Scholar]
- 11.Boden G, Master RW, Rezvani I, Palmer JP, Lobe TE, Owen OE. Glucagon deficiency and hyperaminoacidemia after total pancreatectomy. J Clin Invest 1980;65:706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Prato S, Tiengo A, Baccaglini U, et al. Effect of insulin replacement on intermediary metabolism in diabetes secondary to pancreatectomy. Diabetologia 1983;25:252–259 [DOI] [PubMed] [Google Scholar]
- 13.Vranic M, Pek S, Kawamori R. Increased “glucagon immunoreactivity” in plasma of totally depancreatized dogs. Diabetes 1974;23:905–912 [DOI] [PubMed] [Google Scholar]
- 14.Moore MC, Cherrington AD, Wasserman DH. Regulation of hepatic and peripheral glucose disposal. Best Pract Res Clin Endocrinol Metab 2003;17:343–364 [DOI] [PubMed] [Google Scholar]
- 15.Pagliassotti MJ, Moore MC, Neal DW, Cherrington AD. Insulin is required for the liver to respond to intraportal glucose delivery in the conscious dog. Diabetes 1992;41:1247–1256 [DOI] [PubMed] [Google Scholar]
- 16.Liljenquist JE, Mueller GL, Cherrington AD, et al. Chiasson J-L Evidence for an important role of glucagon in the regulation of hepatic glucose production in normal man. J Clin Invest 1977;59:369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherrington AD, Lacy WW, Chiasson JL. Effect of glucagon on glucose production during insulin deficiency in the dog. J Clin Invest 1978;62:664–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen SS, Santomango TS, Williams PE, Lacy DB, McGuinness OP. Glucagon-mediated impairments in hepatic and peripheral tissue nutrient disposal are not aggravated by increased lipid availability. Am J Physiol Endocrinol Metab 2009;296:E1172–E1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen SS, Zhang Y, Santomango TS, Williams PE, Lacy DB, McGuinness OP. Glucagon chronically impairs hepatic and muscle glucose disposal. Am J Physiol Endocrinol Metab 2007;292:E928–E935 [DOI] [PubMed] [Google Scholar]
- 20.Yki-Järvinen H, Young AA, Lamkin C, Foley JE. Kinetics of glucose disposal in whole body and across the forearm in man. J Clin Invest 1987;79:1713–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson RA, Roshania RD, Hawa MI, Sim BM, DiSilvio L. Impact of glucose ingestion on hepatic and peripheral glucose metabolism in man: an analysis based on simultaneous use of the forearm and double isotope techniques. J Clin Endocrinol Metab 1986;63:541–549 [DOI] [PubMed] [Google Scholar]
- 22.Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab 2009;296:E11–E21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moller DE, Chang PY, Yaspelkis BB, 3rd, Flier JS, Wallberg-Henriksson H, Ivy JL. Transgenic mice with muscle-specific insulin resistance develop increased adiposity, impaired glucose tolerance, and dyslipidemia. Endocrinology 1996;137:2397–2405 [DOI] [PubMed] [Google Scholar]
- 24.Zisman A, Peroni OD, Abel ED, et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med 2000;6:924–928 [DOI] [PubMed] [Google Scholar]
- 25.Brüning JC, Michael MD, Winnay JN, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell 1998;2:559–569 [DOI] [PubMed] [Google Scholar]
- 26.Charron MJ, Gorovits N, Laidlaw JS, Ranalletta M, Katz EB. Use of GLUT-4 null mice to study skeletal muscle glucose uptake. Clin Exp Pharmacol Physiol 2005;32:308–313 [DOI] [PubMed] [Google Scholar]
- 27.Longuet C, Sinclair EM, Maida A, et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab 2008;8:359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlson MG, Snead WL, Campbell PJ. Regulation of free fatty acid metabolism by glucagon. J Clin Endocrinol Metab 1993;77:11–15 [DOI] [PubMed] [Google Scholar]
- 29.Gravholt CH, Møller N, Jensen MD, Christiansen JS, Schmitz O. Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis. J Clin Endocrinol Metab 2001;86:2085–2089 [DOI] [PubMed] [Google Scholar]
- 30.Boden G. Effects of free fatty acids (FFA) on glucose metabolism: significance for insulin resistance and type 2 diabetes. Exp Clin Endocrinol Diabetes 2003;111:121–124 [DOI] [PubMed] [Google Scholar]
- 31.Redondo A, Trigo MV, Acitores A, Valverde I, Villanueva-Peñacarrillo ML. Cell signalling of the GLP-1 action in rat liver. Mol Cell Endocrinol 2003;204:43–50 [DOI] [PubMed] [Google Scholar]
- 32.González N, Acitores A, Sancho V, Valverde I, Villanueva-Peñacarrillo ML. Effect of GLP-1 on glucose transport and its cell signalling in human myocytes. Regul Pept 2005;126:203–211 [DOI] [PubMed] [Google Scholar]
- 33.Dardevet D, Moore MC, DiCostanzo CA, et al. Insulin secretion-independent effects of GLP-1 on canine liver glucose metabolism do not involve portal vein GLP-1 receptors. Am J Physiol Gastrointest Liver Physiol 2005;289:G806–G814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson KM, Edgerton DS, Rodewald T, et al. Intraportal GLP-1 infusion increases nonhepatic glucose utilization without changing pancreatic hormone levels. Am J Physiol Endocrinol Metab 2007;293:E1085–E1091 [DOI] [PubMed] [Google Scholar]
- 35.Gu W, Winters KA, Motani AS, et al. Glucagon receptor antagonist-mediated improvements in glycemic control are dependent on functional pancreatic GLP-1 receptor. Am J Physiol Endocrinol Metab 2010;299:E624–E632 [DOI] [PubMed] [Google Scholar]