β-Cells are the core effector of glycemic control, and when largely lost in the course of type 1 diabetes, glycemic control is no longer guaranteed. Although glycemic control can be established by insulin injections, diabetic patients still suffer from microvascular (nephropathy, retinopathy, neuropathy) and macrovascular (ischemic heart disease, cerebrovascular disease, peripheral vascular disease) complications, resulting in a lower life expectancy (1). Therefore, islet transplantation has been implemented as one therapeutic means to achieve a more physiologic form of glycemic control. Although high rates of insulin independence can be achieved shortly after transplantation, the rate of insulin independence after longer time intervals remains suboptimal, especially in view of the potential complications of immunosuppressive treatment (2). One of the reasons for the limited clinical success of islet transplantation is the exposure of the islets to stress prior to and in the first weeks after transplantation. The liver may actually not be an optimal transplantation site (although it connects the islets to the blood flow from the portal vein). Also, it has been shown that islet grafts will only be fully vascularized after 1–2 weeks, reaching the final state of vascularization even later (3). As a consequence of the lack of nutrients and hypoxia, together with other factors such as hyperglycemia and immunosuppressant toxicity, to name only two of them, a considerable proportion of the transplanted β-cells will undergo apoptosis, further limiting the success of islet transplantation.
In order to optimize the treatment of patients after islet transplantation, a methodology has been sought that would allow for in vivo transplanted islets to be followed noninvasively. The first method that allowed for imaging of transplanted islets in vivo was described in 2004 by Jirák et al. (4). They demonstrated that β-cells could be labeled with superparamagnetic iron oxide particles (SPIOs) and detected by MRI in vivo in a rodent model. It was shown later that islets prelabeled with SPIOs could be followed for 6 months after transplantation (5) and that the signal was specific for islets. Although false-positive results due to macrophaged SPIOs outside of the transplanted islets remain a concern, the authors did not find evidence for this. Another major concern associated with this technology has always been the potential damage to the β-cells by the transfection procedure and the toxicity of the SPIOs themselves. Although it has been demonstrated that β-cells can be labeled with SPIOs without functional impairment, it seems to remain a challenge to load the β-cells with the right amount of SPIOs to guarantee in vivo detectability by MRI and at the same time avoid toxic effects. It appears that the labeling protocols need to be followed thoroughly (6). Therefore, alternative methods have been established for visualization of transplanted β-cells. Although techniques that would use genetically modified β-cells for transplantation are at this point in time not suitable for imaging in humans (7,8) (and also have been dependent on the insulin promoter giving limited extra information on top of insulin production), specific radiolabeled tracer molecules can be used for imaging of transplanted islets by PET (positron emission tomography) or SPECT (single photon emission computed tomography). It has been demonstrated that islet grafts can be visualized in vivo by targeting VMAT2 (vasoactive monoamine transporter 2) or the GLP-1 receptor (glucagon-like peptide-1) (9–11). These approaches have the advantage that they can be used at any time point by injecting the tracer molecule and do not require prelabeling of the β-cells prior to transplantation. So, why should one continue to monitor islet transplants in vivo by MRI using the rather complicated prelabeling strategy?
A number of therapeutic strategies have been used to increase the survival of freshly transplanted β-cells. Transfection of β-cells with siRNA (small interfering RNA) silencing caspase-3 has been shown to result in inhibition of apoptosis, but the effect was limited to a few days; an adenoviral vector was therefore used to guarantee stable expression of the silencing RNA (12). However, transfecting β-cells by means of an adenovirus may also cause apoptosis (13) and is a problem when it comes to clinical β-cell transplantation in humans. So, other therapeutic approaches seem to be necessary, although gene suppression by means of silencing RNA appears to be a very elegant, attractive method.
The group of Anna Moore (5,6,14) is taking the field of β-cell imaging a great stride forward (Fig. 1). Although not avoiding the challenging prelabeling strategy for MR imaging of transplanted islets, the group effectively takes advantage of the therapeutic potential of nanoparticles used as a contrast agent. By conjugating the nanoparticles to the siRNA targeting human caspase-3, the silencing RNA remains inside the cell without the necessity of genetically modifying the β-cells. By this elegant approach, a prolonged effect of the siRNA can be obtained, and as shown by MR imaging, the volume of the siRNA-treated graft was significantly larger than the untreated control graft, while the rate of apoptosis was lower. Although the long-term effects on islet survival and glycemic control remain to be elucidated, the proof of principal for the use of this combined diagnostic and therapeutic approach has been demonstrated. Although delivery of therapeutic nanoparticles labeled with SPIOs for tumor targeting in vivo has previously been described (14), we must keep in mind that imaging of β-cells is much more of a challenge than imaging of tumors. Tumors are focally growing, metabolically hyperactive cell clusters that overexpress many receptors and transport proteins as potential targets for imaging, rendering targeting much less of a challenge than in the case of β-cells/islets. The results of this article are in line with those of another recently published study showing that the use of fluorinated alginate microcapsules increased the insulin secretion rate of human islets and at the same time provided a signal for detection by MRI and contrast for ultrasound and CT imaging (15). It therefore appears that the first steps toward therapeutic molecular imaging of islets have been taken.
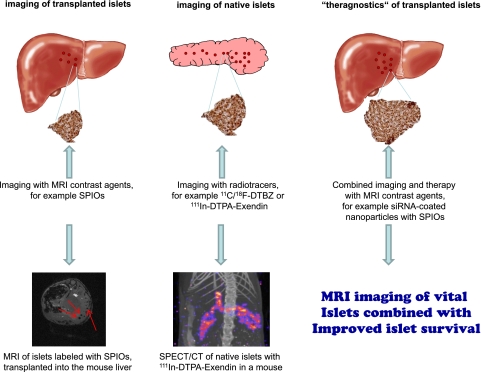
FIG. 1.
This figure shows the main approaches to imaging of β-cells at this point in time. SPIOs have been used for labeling of islets prior to transplantation since 2004 (left). Other MRI contrast agents are currently under investigation and potentially also allow for quantification by MRI spectroscopy. For the determination of the pancreatic β-cell mass, PET and SPECT imaging with highly specific radiotracers seem to be the techniques of choice today (middle). MRI imaging of native islets using the calcium analog Mg++ as contrast agent has successfully been performed for imaging of the functional pancreatic β-cells. The approach using siRNA-coated nanoparticles, providing contrast for diagnostics (MRI) and at the same time exerting therapeutic effects that increase the islet survival, is taking β-cell imaging to a new level (right). (A high-quality digital representation of this figure is available in the online issue.)
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying original article, p. 565.
REFERENCES
- 1.Borchers AT, Uibo R, Gershwin ME. The geoepidemiology of type 1 diabetes. Autoimmun Rev 2010;9:A355–A365 [DOI] [PubMed] [Google Scholar]
- 2.CITR Research Group 2007 update on allogeneic islet transplantation from the Collaborative Islet Transplant Registry (CITR). Cell Transplant 2009;18:753–767 [DOI] [PubMed] [Google Scholar]
- 3.Speier S, Nyqvist D, Cabrera O, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med 2008;14:574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jirák D, Kríz J, Herynek V, et al. MRI of transplanted pancreatic islets. Magn Reson Med 2004;52:1228–1233 [DOI] [PubMed] [Google Scholar]
- 5.Evgenov NV, Medarova Z, Dai G, Bonner-Weir S, Moore A. In vivo imaging of islet transplantation. Nat Med 2006;12:144–148 [DOI] [PubMed] [Google Scholar]
- 6.Medarova Z, Evgenov NV, Dai G, Bonner-Weir S, Moore A. In vivo multimodal imaging of transplanted pancreatic islets. Nat Protoc 2006;1:429–435 [DOI] [PubMed] [Google Scholar]
- 7.Lu Y, Dang H, Middleton B, et al. Noninvasive imaging of islet grafts using positron-emission tomography. Proc Natl Acad Sci USA 2006;103:11294–11299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SJ, Doudet DJ, Studenov AR, et al. Quantitative micro positron emission tomography (PET) imaging for the in vivo determination of pancreatic islet graft survival. Nat Med 2006;12:1423–1428 [DOI] [PubMed] [Google Scholar]
- 9.Witkowski P, Sondermeijer H, Hardy MA, et al. Islet grafting and imaging in a bioengineered intramuscular space. Transplantation 2009;88:1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pattou F, Kerr-Conte J, Wild D. GLP-1-receptor scanning for imaging of human beta cells transplanted in muscle. N Engl J Med 2010;363:1289–1290 [DOI] [PubMed] [Google Scholar]
- 11.Andrałojć K, Brom M, Joosten L, Oyen W, Boerman C, Gotthardt M. In vivo visualization of transplanted islets in rat by SPECT imaging with 111In-Exendin. Diabetologia 2010;53(Suppl. 1):S196 [Google Scholar]
- 12.Cheng G, Zhu L, Mahato RI. Caspase-3 gene silencing for inhibiting apoptosis in insulinoma cells and human islets. Mol Pharm 2008;5:1093–1102 [DOI] [PubMed] [Google Scholar]
- 13.Langlois A, Bietiger W, Sencier MC, et al. Adenoviral infection or deferoxamine? Two approaches to overexpress VEGF in beta-cell lines. J Drug Target 2009;17:415–422 [DOI] [PubMed] [Google Scholar]
- 14.Kumar M, Yigit M, Dai G, Moore A, Medarova Z. Image-guided breast tumor therapy using a small interfering RNA nanodrug. Cancer Res 2010;70:7553–7561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnett BP, Ruiz-Cabello J, Hota P, et al. Fluorocapsules for improved function, immunoprotection, and visualization of cellular therapeutics with MR, US, and CT imaging. Radiology 2011;258:182–191 [DOI] [PMC free article] [PubMed]