Abstract
OBJECTIVE
To determine the role of glucagon action in the metabolic phenotype of untreated insulin deficiency.
RESEARCH DESIGN AND METHODS
We compared pertinent clinical and metabolic parameters in glucagon receptor-null (Gcgr−/−) mice and wild-type (Gcgr+/+) controls after equivalent destruction of β-cells. We used a double dose of streptozotocin to maximize β-cell destruction.
RESULTS
Gcgr+/+ mice became hyperglycemic (>500 mg/dL), hyperketonemic, polyuric, and cachectic and had to be killed after 6 weeks. Despite comparable β-cell destruction in Gcgr−/− mice, none of the foregoing clinical or laboratory manifestations of diabetes appeared. There was marked α-cell hyperplasia and hyperglucagonemia (∼1,200 pg/mL), but hepatic phosphorylated cAMP response element binding protein and phosphoenolpyruvate carboxykinase mRNA were profoundly reduced compared with Gcgr+/+ mice with diabetes—evidence that glucagon action had been effectively blocked. Fasting glucose levels and oral and intraperitoneal glucose tolerance tests were normal. Both fasting and nonfasting free fatty acid levels and nonfasting β-hydroxy butyrate levels were lower.
CONCLUSIONS
We conclude that blocking glucagon action prevents the deadly metabolic and clinical derangements of type 1 diabetic mice.
The development of a radioimmunoassay for glucagon (1) established the hormonal status of this peptide (2), originally considered to be a contaminant of the insulin extraction process. Glucagon was immunocytochemically localized to pancreatic α-cells (3) and shown to be secreted in response to increased glucose need, as in starvation and exercise (4). By 1973, it was recognized that α-cell function was grossly abnormal in diabetes, particularly in type 1 diabetes (5). Here, β-cells are largely replaced by α-cells, and, without the inhibitory action of insulin, their secretion of glucagon is unrestrained, and glucagon action on the liver is unopposed. The result is a lethal hypercatabolic state. In 1973, the discovery of somatostatin (6), a potent inhibitor of glucagon secretion, made it possible to demonstrate the essential role of glucagon in the metabolic phenotype of type 1 diabetes (7–10).
Those studies led to a search for a therapeutic suppressor of diabetic hyperglucagonemia or blocker of its action on the liver. In the 37 years since the discovery of somatostatin, only one other potent glucagon-suppressing substance, leptin, has been identified (11,12). By contrast, inactivators of glucagon have been less elusive. High affinity antiglucagon antibodies have benefited diabetic animals (13), as have a variety of molecules that block binding of glucagon to the glucagon receptor and/or block its signaling (14–18). Diabetic mice with glucagon receptor knockout (19) or mice treated with Gcgr antisense oligonucleotide similarly benefit from the elimination of glucagon action (20,21). Although all of the foregoing reports demonstrate that abrogation of glucagon action reduces hepatic overproduction of glucose, a potential therapeutic asset in diabetes management, none of the foregoing diabetic models studied thus far have been totally insulin-deficient, as in type 1 diabetes. In type 1 diabetes, islets are virtually devoid of β-cells, and are largely made up of hyperplastic α-cells. In contrast to normal α-cells, which are restrained by the high local concentrations of intraislet insulin (22), type 1 diabetic α-cells are unregulated, which results in inappropriate hyperglucagonemia (5). Moreover, with no insulin to oppose the hepatic actions of the hyperglucagonemia, unrestrained glycogenolysis, gluconeogenesis, ketogenesis, and hypercatabolism lead rapidly to ketoacidosis, cachexia, coma, and death.
An essential role of hyperglucagonemia in the pathogenesis of this lethal syndrome has long been suspected but never fully proven. Recent studies of adenovirally induced hyperleptinemia in type 1 diabetic mice (12) indicate that glucagon suppression normalizes all metabolic parameters for more than a month despite a total absence of insulin. More recently, the same antidiabetic efficacy has been demonstrated with recombinant leptin (11,23). However, leptin-induced actions other than suppression of glucagon, such as increased insulin-like growth factor-1 (IGF-1) (12) and insulin-like growth factor-binding protein-2 (IGFBP-2) (23), may have contributed. To obtain unassailable proof that glucagon action by itself causes the lethal consequences of insulin deficiency, we induced insulin deficiency in glucagon receptor-null (Gcgr−/−) mice. Gcgr−/− mice were treated with streptozotocin (STZ), the most commonly used β-cytotoxins in rodents, in an effort to achieve complete insulin deficiency in the complete absence of glucagon activity. We compared the metabolic phenotype of complete β-cell destruction in mice in which glucagon action had been transgenically abrogated by knockout of the glucagon receptor (24). Because Gcgr−/− mice are resistant to STZ-induced β-cell destruction (24), it was necessary to use a double dose of the β-cell poison STZ. Despite β-cell destruction equivalent to the wild-type (Gcgr+/+) mice, Gcgr−/− remained free of all manifestations of insulin deficiency.
RESEARCH DESIGN AND METHODS
Male mice with global glucagon receptor knockout and wild-type mice (19) were provided by M.J.C. They were housed in individual cages with constant temperature and 12 h of light alternating with 12 h of darkness and fed Teklad 6% mouse/rat diet (Teklad, Madison, WI) with free access to water. The protocol was approved by the Institutional Animal Care and Research Advisory Committee of the University of Texas Southwestern Medical Center and the Institutional Animal Care and Use Committee at the North Texas Veterans Administration Medical Center.
Chemical destruction of β-cells.
β-cells were destroyed in 10- to 12-week-old Gcgr−/− and Gcgr+/+ mice with two intravenous injections of STZ (100 mg/kg body weight followed in 7 days by 80 mg/kg). Food intake, body weight, and blood glucose were measured weekly for 6 weeks.
Plasma measurements.
Blood glucose levels were measured with a glucose meter (Bayer Healthcare, Mishawka, IN). Plasma glucagon levels were measured using a Linco glucagon RIA kit (Linco Research, St. Charles, MO). Plasma insulin levels were measured using a rat/mouse insulin ELISA kit (Crystal Chem, Downers Grove, IL), and IGF-1 levels were measured using an IGF-1 ELISA kit (Immunodiagnostic Systems Ltd., Boldon, UK). Free fatty acids (FFAs) were measured with a nonesterified fatty acid (NEFA) kit (Waco Pure Chemical Industries, Osaka, Japan).
Oral glucose tolerance test.
After a 16 h fast, glucose (2 g/kg) was administered by gavage to the Gcgr mice. Blood was taken at 0, 15, 30, 45, 60, 90, and 120 min intervals, and the blood glucose and plasma insulin were analyzed.
Immunoblotting.
Livers were lysed in radioimmunoprecipitation (RIPA) buffer (0.15mM NaCl/0.05mM TrisHCl, pH,7.2/1% Triton-X100/1% sodium deoxycholate/0.1% SDS) with 10 μg/mL leupeptin and 10 μg/mL aprotinin. Proteins were resolved by sulfate-polyacrylamide gel electrophoresis (SDS/PAGE) and transferred to a membrane (GE Healthcare, Piscataway, NJ). The blotted membrane was blocked in 1 × Tris-buffered saline (TBS) containing 0.1% tween 20 (TBST) and 5% nonfat dry milk for 1 h at room temperature. After incubation with primary antibodies to cAMP-responsive element binding (CREB) and phospho-CREB (P-CREB) (1:1000; Cell Signaling Technology, Danvers, MA) or anti–γ-tubulin at 4°C overnight, the membrane was washed with TBST buffer. Membranes were incubated with goat anti-rabbit or anti-mouse horseradish peroxidase–conjugated IgG for 1 h. The proteins were detected by using enhanced chemiluminescence detection system (GE Healthcare).
Real-time quantitative PCR.
Total RNA was extracted from the liver by Trizol isolation method (Life Technologies, Rockville, MD). All PCR reactions were performed in triplicate as previously described (25). The mRNA level was calculated by using standard curve method. Primer sequences are shown in Supplementary Table 1.
Amino acid analysis of livers.
Amino acids were analyzed in liver homogenates by tandem mass spectrometry (MS/MS). All MS analyses used stable-isotope dilution with internal standards from Isotec, Cambridge Isotope Laboratories, and CDN Isotopes.
Immunohistochemistry and β-cell mass measurement.
The distal 20% of pancreata dissected from adult mice were fixed in Bouin’s solution (Sigma, St. Louis, MO) and dehydrated prior to paraffin embedding. Five micrometer contiguous paraffin sections were prepared on a Leica RM2155 rotary microtome for insulin and glucagon immunohistochemical staining, as previously described (26). Morphometric analysis for insulin cells was performed using Image-J image analysis software and particle analysis macro (Scion, Frederick, MD). The area of insulin staining in three sections of pancreas from 4–6 animals, relative to total sectional area examined, was quantified by monochromatic thresholding.
Statistical analysis.
Results are reported as means ± SEM. Statistical analysis of the data was performed with Student t test.
RESULTS
Assessing completeness of β-cell destruction by STZ.
Conarello et al. (24) reported that Gcgr−/− mice are resistant to the β-cytotoxic action of STZ and without complete destruction of β-cells, it would be impossible to evaluate the benefits of glucagon blockade in total insulin deficiency states. Because increased β-cell survival in Gcgr−/− mice could explain the lack of hyperglycemia in response to a β-cytotoxin, we administered a double dose, 100 mg/kg of body weight of STZ, followed 7 days later with an 80 mg/kg dose. We quantified the surviving β-cells with STZ-treated Gcgr+/+ mice by immunostaining for insulin and glucagon. Morphometry of immunocytochemically stained insulin-positive cells revealed 90% β-cell destruction in sections of pancreata from Gcgr−/− mice, not significantly different from the 94% destruction Gcgr+/+ mice (Table 1). After STZ treatment, α-cell area of Gcgr+/+ mice increased 10-fold. The α-cell area of Gcgr−/− mice before STZ was almost 7-fold greater than that of untreated Gcgr+/+ mice and after STZ it was 39-fold greater (Table 1). Plasma insulin levels were reduced by 90% after STZ in both groups (Table 1). Finally, the most sensitive and specific indicator of β-cell activity, pancreatic preproinsulin mRNA, was below the detectable range of quantitative real-time PCR (qRT-PCR) in both Gcgr+/+ and Gcgr/- mice treated with STZ (Table 1). These findings indicate that the STZ-resistance of β-cells of Gcgr−/− mice could be overcome by doubling the dose of the β-cytotoxin. This made it possible to determine the role of diabetic hyperglucagonemia on the phenotype of complete insulin deficiency.
TABLE 1.
Comparison residual insulin production in nonfasting Gcgr+/+ and Gcgr−/− mice treated with STZ (n = 6)
| Nondiabetic |
STZ-diabetic |
|||
|---|---|---|---|---|
| Gcgr+/+ | Gcgr−/− | STZ Gcgr+/+ | STZ Gcgr−/− | |
| β-cell area (μm2) | 23,279 ± 5,614 | 15,760 ± 1,413* | 1,548 ± 132 | 1,618 ± 147 |
| α-cell area (μm2) | 4,431 ± 873 | 27,825 ± 4,358 | 44,927 ± 5.488 | 17,4081 ± 26,103 |
| Plasma insulin (ng/mL) | 1.86 ± 0.11 | 1.82 ± 0.16 | 0.19 ± 0.15 | 0.21 ± 0.07 |
| Preproinsulin (AU) | 0.106 ± 0.0081 | 0.215 ± 0.0530 | undetectable | undetectable |
Data are means ± SEM.
*P < 0.05.
AU, arbitrary unit.
Completeness of glucagon inactivation.
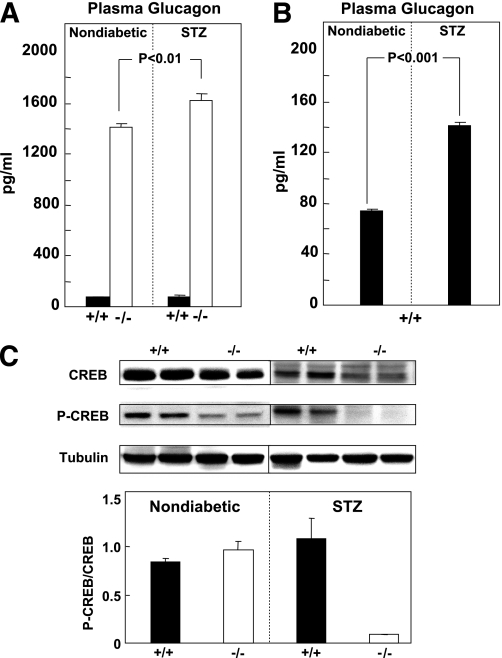
A second prerequisite of the study was that glucagon be completely inactivated. Plasma glucagon levels in Gcgr−/− mice were ∼14-fold higher than in Gcgr+/+ mice prior to STZ treatment, confirming the report of Gelling et al. (19), and ∼16-fold higher after STZ treatment (Fig. 1A and B). To assess the completeness of glucagon inactivation in the Gcgr−/− mice, we compared the hepatic levels of P-CREB (27), a key protein in its signal transduction pathway, with those of wild-type controls. Given the marked hyperglucagonemia, hepatic P-CREB should be increased if any functionally intact glucagon receptor was present in liver. However, P-CREB could not be detected in livers of STZ-diabetic Gcgr−/− mice (Fig. 1C). Total CREB protein was also reduced. These results are consistent with those previously reported by Gelling et al. (19) that confirm a complete blockade of glucagon action in the liver of Gcgr−/− mice.
FIG. 1.
A: Comparison of plasma glucagon levels in Gcgr+/+ (■) and Gcgr−/− (□) mice before and 6 weeks after STZ induction of β-cell destruction. B: Glucagon in Gcgr+/+ mice before and after STZ. C: Representative immunoblots for CREB and P-CREB in livers of Gcgr+/+ (■) and Gcgr−/− (□) mice after β-cell destruction by treatment with STZ and in untreated controls (upper panel). The P-CREB/CREB ratio in densitometric units in six Gcgr+/+ (■) and six Gcgr−/− (□) mice after β-cell destruction by treatment with STZ (lower panel).
Comparison of clinical parameters.
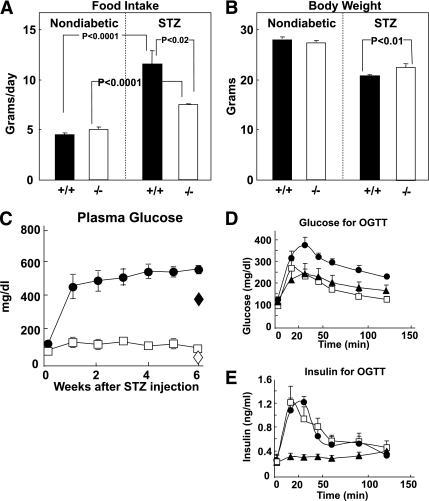
The lethal phenotype that developed rapidly in Gcgr+/+ mice after the large dose of STZ did not appear in Gcgr−/− mice treated with the same STZ dose. Food intake rose in the Gcgr+/+ diabetic mice from 4.5 g/d before the diabetes to 9.3 g/d after STZ treatment but increased by only 3.0 g/d in the Gcgr−/− mice (Fig. 2A). The severe polyuria and polydipsia of the Gcgr+/+ mice did not occur in the STZ-treated Gcgr−/− mice (data not shown). The diabetic wild-type mice became progressively sicker and had to be killed, whereas the Gcgr−/− mice seemed to remain in their normal state of health throughout the 6-week study despite the lack of insulin and were apparently healthy when finally killed at 12 weeks after STZ.
FIG. 2.
A: Comparison of food intake in nondiabetic and STZ-treated Gcgr+/+ (■) and Gcgr−/− (□) mice (n = 6). B: Comparison of body weight in Gcgr+/+ (■) and Gcgr−/− (□) before and after STZ induction of β-cell destruction. C: Comparisons of weekly nonfasting glucose levels in Gcgr+/+ (●) and Gcgr−/− (□) after STZ-induced β-cell destruction, and overnight fasting glucose levels for Gcgr+/+ (♦) and Gcgr−/− (◇) at the end of the study (n = 6). D: Glucose values for oral glucose tolerance test (OGTT) (2 g/kg) performed after a 16-h fast in normal Gcgr+/+ (●), Gcgr−/− (☐), and STZ-treated Gcgr−/− (▲) mice (n = 4). E: Insulin levels for OGTT in normal Gcgr+/+ (●), Gcgr−/− (☐), and STZ-treated Gcgr−/− (▲) mice (n = 4).
Body weight of the mice before and 6 weeks after STZ treatment appears in Fig. 2B.
Comparison of glucoregulatory parameters.
All Gcgr+/+ mice became severely hyperglycemic after treatment with STZ, their fed and fasting blood glucose levels approaching 500 mg/dL within a week after administration of the STZ and increasing slightly thereafter. By contrast, in the Gcgr−/− mice fed and fasting glucose levels after STZ-treatment remained in a perfectly normal range throughout the 6-week study and for at least 6 weeks thereafter (Fig. 2C).
The ablation of glucagon action was expected to prevent fasting hyperglycemia by reducing hepatic glucose production but was not expected to improve the glucose tolerance test, which is generally considered to be, in large part, an index of insulin-mediated glucose disposal. Surprisingly, comparison of oral glucose tolerance of Gcgr−/− mice with and without STZ-induced destruction of β-cells revealed normal curves in both groups. In other words, the presence or absence of β-cells had no apparent effect on the glucose curves of the Gcgr−/− mice, all of which were well within normal limits (Fig. 2D). To exclude a difference in glucose absorption in the STZ-treated mice, we performed intraperitoneal glucose tolerance tests in Gcgr−/− mice with and without intact β-cells. Again, glucose tolerance was normal in both groups (data not shown).
To determine if these unexpected results could be explained by an increase in insulin from a few surviving β-cells in the STZ-treated Gcgr−/− mice, we compared insulin levels in the two groups. The STZ-treated Gcgr−/− mice exhibited no increase in insulin levels above the basal value, whereas the insulin response of untreated knockout mice rose to over 1.2 ng/mL—about the same as in normal Gcgr+/+ mice (Fig. 2E). Thus, insulin was not involved in the normal glucose tolerance curves of Gcgr−/− mice.
IGF-1.
To determine if an insulin-like growth factor might have normalized glucose tolerance in the absence of insulin, we measured fasting and postprandial IGF-1 levels. In Gcgr−/− mice, fasting (65 ± 27 vs. 256 ± 48 ng/mL) and nonfasting (130 ± 20 vs. 303 ± 23 ng/mL) IGF-1 levels were significantly below those of Gcgr+/+ controls (P < 0.001). After STZ treatment, in Gcgr+/+ mice fasting IGF-1 levels varied widely, averaging 284 ± 90 vs. 140 ± 10 ng/mL (not significant) in Gcgr−/− mice. Nonfasting IGF-1 levels, which could not be detected in Gcgr+/+ mice after STZ treatment, averaged 109 ± 17 ng/mL in STZ-treated Gcgr−/− mice. We concluded that IGF-1 could not be the cause of the normal glucose tolerance observed in the absence of both insulin and glucagon action.
Comparison of FFAs and ketones.
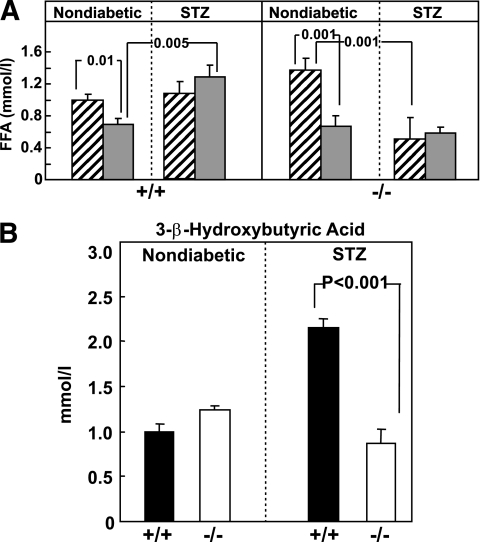
Fasting and FFA levels were significantly higher in normal Gcgr−/− mice and Gcgr+/+ controls. They were not suppressed by food in either group (Fig. 3A). But both fasting and nonfasting levels were significantly lower in STZ-treated Gcgr−/− mice than in Gcgr+/+ controls, consistent with a lipolytic role for glucagon when insulin is very low. The plasma β-hydroxybutyrate levels of Gcgr−/− mice were significantly below the elevated levels of STZ-treated Gcgr+/+ controls (P < 0.001) (Fig. 3B), evidence that glucagon action is required for ketogenesis, as proposed by Meier et al. (28).
FIG. 3.
Comparison of fasting (▨) and nonfasting FFAs ( ) (A) and nonfasting β-OH butyrate levels (B) in STZ-treated Gcgr+/+ (■) and Gcgr−/− (□) mice and untreated controls (N = 6).
) (A) and nonfasting β-OH butyrate levels (B) in STZ-treated Gcgr+/+ (■) and Gcgr−/− (□) mice and untreated controls (N = 6).
Phosphoenolpyruvate carboxykinase mRNA and lactate levels in insulin-deficient Gcgr+/+ and Gcgr−/− mice.
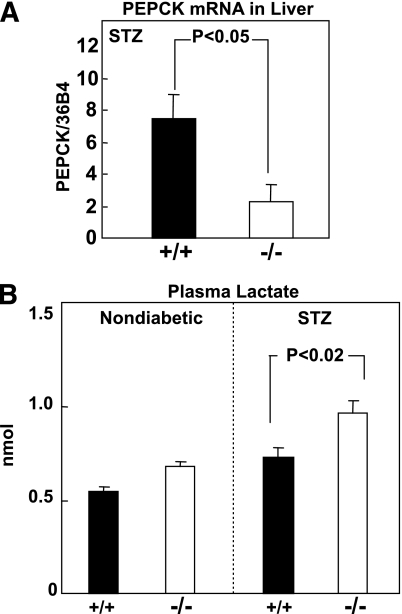
Uncontrolled insulin deficiency with hyperglucagonemia is characterized by increased gluconeogenesis associated with upregulation of the gluconeogenic enzyme, phosphoenolpyruvate carboxykinase (PEPCK) (29,30). The expression of this enzyme was therefore compared in Gcgr+/+ and Gcgr−/− mice treated with STZ. Figure 4A shows the expected increase in PEPCK mRNA in STZ-induced insulin deficiency of Gcgr+/+ mice. It was substantially reduced in Gcgr−/− mice with STZ-induced insulin deficiency. This reduction was associated with a significant increase in lactate, a gluconeogenic precursor (Fig. 4B).
FIG. 4.
Comparison of the ratio of PEPCK mRNA to 36B4 mRNA (A) and plasma lactate levels (B) in STZ-treated Gcgr+/+ (■) and Gcgr−/− (□) mice (N = 6).
Amino acid levels in normal and insulin-deficient Gcgr+/+ and Gcgr−/− mice.
Boden et al. (31) have reported that in normal subjects, hyperglucagonemia lowers plasma amino acids through increased urinary excretion and that somatostatin-induced hypoglucagonemia has the opposite effect. This prompted a metabolomic analysis of amino acid profiles in the liver of both normal and insulin-deficient Gcgr+/+ and Gcgr−/− mice (Table 2). Of the 16 amino acids measured, 9 were significantly (P < 0.01) increased in normal Gcgr−/− mice, but only 1 was elevated in the STZ-treated insulin-deficient Gcgr−/− mice. This suggests that in the presence of normal insulin action, glucagon action is required to prevent hepatic overaccumulation of amino acids. When insulin is absent, however, amino acid levels are relatively normal despite lack of glucagon action.
TABLE 2.
Liver amino acid levels (μM) in Gcgr+/+ and Gcgr−/− mice
| Normal |
Insulin deficiency |
|||
|---|---|---|---|---|
| Gcgr+/+ | Gcgr−/− | Gcgr+/+ STZ | Gcgr−/− STZ | |
| Ala | 246.10 ± 51.36* | 487.51 ± 58.13* | 359.31 ± 123.78 | 327.45 ± 42.27 |
| Ser | 145.86 ± 4.77* | 232.16 ± 11.87*‡ | 158.80 ± 14.46 | 195.02 ± 9.07‡ |
| Glx | 232.33 ± 30.40* | 554.39 ± 62.84* | 308.27 ± 30.97† | 526.43 ± 23.00† |
| Pro | 21.64 ± 2.01* | 30.59 ± 1.32*‡ | 30.54 ± 5.47 | 25.79 ± 0.100‡ |
| Phe | 20.01 ± 0.91* | 26.48 ± 1.86* | 26.88 ± 4.59 | 30.42 ± 1.44 |
| Asx | 53.63 ± 8.73 * | 115.39 ± 10.78*‡ | 51.81 ± 4.62 | 55.76 ± 4.95‡ |
| Met | 7.97 ± 0.35* | 12.33 ± 0.64* | 9.05 ± 1.44 | 10.79 ± 0.53 |
| Arg | 5.12 ± 0.22* | 15.19 ± 2.25* | 9.33 ± 3.09 | 16.75 ± 1.02 |
| Orn | 27.31 ± 1.50* | 73.22 ± 43.79* | 74.97 ± 11.22 | 64.05 ± 8.75 |
Data are means ± SEM. Ala, alanine; Arg, arginine; Asx, aspartic acid and asparagines; Glx, glutamic acid and glutamine; Met, methionine; Orn, ornithine; Phe, phenylalanine; Pro, proline; Ser, serine.
Gcgr+/+ vs. Gcgr−/−; *P < 0.01.
Gcgr+/+ STZ vs. Gcgr−/− STZ; †P < 0.001.
Gcgr−/− vs. Gcgr−/− STZ; ‡P < 0.05.
DISCUSSION
The purpose of this study was to assess the role of the hyperglucagonemia of total insulin deficiency in the lethal catabolic consequences of the disease and to determine if beneficial effects of glucagon blockade are sufficient and safe enough to qualify as a potential therapeutic target in human type 1 diabetes. This idea was proposed previously (9,10) when somatostatin, the first glucagon-suppressing agent to be discovered (6), was found to prevent the acute metabolic consequences of complete insulin lack (7,8). The search for more practical suppressors or antagonists of glucagon has been reenergized by the recent demonstration that leptin can suppress diabetic hyperglucagonemia and reverse the catabolic effects of complete insulin deficiency for extended periods without apparent negative consequences (11,12).
Although leptin is very effective in suppressing the hyperglucagonemia and in reversing the metabolic manifestations of type 1 diabetes (11,12), the many other actions of the adipocyte hormone make it impossible to assume that suppression of glucagon by itself accounts for the remarkable remission of all clinical and laboratory manifestations of total insulin deficiency. The Gcgr−/− mice of Gelling et al. (24) provided an ideal model in which to assess the immediate and longer-term benefits and risks of permanent elimination of glucagon action without other actions of leptin unrelated to α-cell suppression, such as food restriction and lipopenia. We were able to overcome the previously reported resistance of Gcgr−/− mice to STZ destruction of β-cells by using higher doses of STZ (24). The β-cell destruction was as complete as in Gcgr+/+ controls, as evidenced by insulin assays of plasma, morphometric comparison of pancreata for insulin-positive cells, and qRT-PCR measurements of pancreatic preproinsulin mRNA. This enabled an assessment of the effects of complete elimination of glucagon action on the phenotype of mice with total insulin deficiency.
Whereas all STZ-treated Gcgr+/+ diabetic mice developed severe hyperglycemia, ketosis, and cachexia and had to be killed for humane reasons ∼30 days after induction of diabetes, none of the insulin-deficient Gcgr−/− mice developed any laboratory or clinical evidence of insulin deficiency. Without exception, all STZ-treated Gcgr−/− mice appeared to be in a seemingly normal state of health after the high dose of β-cytotoxin for at least 6 weeks. This indicates that in the absence of glucagon action, insulin deficiency in mice is a silent disorder without overt metabolic or clinical manifestations, confirming an earlier preliminary observation in alloxan-treated mice (11). It also suggests that glucagon suppression and/or antagonism may be an extremely useful strategy to treat type 1 diabetes.
Other than fasting hypoglycemia, no significant undesirable side effects of chronic blockade of glucagon action were observed in this brief study. The marked α-cell hyperplasia observed in Gcgr−/− pancreata (19) has led to concerns that long-term glucagon receptor blockade could predispose to glucagonoma. However, thus far glucagonoma has not been observed in any of the Gcgr−/− mice followed for up to 2 years (M.J.C., unpublished observation). Another theoretical hazard is the possibility of predisposition to lactic acidosis based on the high blood lactate levels in the Gcgr−/− mice. This is attributed to reduced gluconeogenesis secondary to downregulation of gluconeogenic genes. A third potential issue relates to the role of glucagon in amino acid homeostasis. Amino acid deficiency has been described in glucagonoma patients (33,34), raising the possibility that Gcgr−/− mice might have high amino acid levels, as reported by Boden et al. (31) in humans in whom glucagon was suppressed by somatostatin. For this reason, we measured amino acid levels in the livers of our normal and STZ-treated Gcgr+/+ and Gcgr−/− mice. As shown in Table 2, eight of the nine hepatic amino acids measured were significantly higher in Gcgr−/− mice with intact β-cells than in normal Gcgr+/+ mice. But no such differences occurred in STZ-treated mice, suggesting that insulin action, when unopposed by glucagon, was responsible for the increase.
By far the most surprising result of the study was the normal glucose tolerance of insulin-deficient Gcgr−/− mice. Currently, it is believed that it is the action of glucose-stimulated insulin secretion on hepatic glucose balance and glucose uptake in muscle and fat that is the essential determinant of glucose tolerance, with glucagon suppression playing a supporting role. Here we find that both oral and intraperitoneal glucose tolerance in Gcgr−/− mice with β-cell destruction is as normal as in Gcgr+/+mice with intact islets, even though no rise in insulin was detected in peripheral plasma of the former. Although we did not measure portal vein insulin levels, the lack of preproinsulin mRNA in the mice that received double-dose STZ argues against undetected insulin reaching the liver. The presence of residual granules in STZ-treated pancreata does not necessarily signify a residual insulin source because insulin granules may persist in apoptotic β-cells or in macrophages after all functioning β-cells have been destroyed. Certainly in wild-type mice, neither the “residual” insulin in the pancreas nor the low levels of “plasma insulin” were able to prevent a ketoacidotic death. The lack of preproinsulin mRNA detectable by qRT-PCR, which is far more sensitive and specific than either of the immunologic techniques, suggests that the STZ-treated mice were unable to produce insulin.
It seemed possible that the insulin-like responses were caused by an increase in a hormone with insulinometic activity. But neither leptin (not shown) (11) nor IGF-1 rose significantly in response to the glucose challenge. Alternatively, the elevated glucagon-like peptide 1 levels might have increased muscle glucose uptake (32).
Recent studies by Meyer et al. (35) indicate that in normal humans 90% of ingested glucose is taken up by liver, muscle, brain, and kidney, and hepatic glucose release declines 82%. Except for the brain, red cells, and possibly the kidney, glucose uptake is considered to be insulin-mediated. To account for the perfectly normal glucose tolerance in insulin-deficient mice with congenital lack of glucagon action, one can posit that insulin action during glucose absorption is largely directed toward overcoming the hepatic actions of glucagon during the preglucose fast. In this case, if glucagon action on the liver is absent, there is little, if anything, for insulin to do because the liver is already in a permanent storage mode. Therefore, when the liver has never experienced the action of glucagon, as in the Gcgr−/− mice, glucose disposition without insulin is no different than when both hormones are normally active (36). It should also be noted that fasting and fed FFA levels (Fig. 3A) were both lower in insulin-deficient Gcgr−/− mice than in insulin-deficient Gcgr+/+ mice, suggesting that, at least when unopposed by insulin, glucagon may have lipolytic action in adipocytes, as it does in hepatocytes.
Finally, a persistent but fallacious argument against an essential role of glucagon in diabetes is the occurrence of diabetes in totally depancreatized humans and animals. It is not widely appreciated that when totally depancreatized animals or humans are deprived of insulin, there is an increased production of glucagon from extrapancreatic α-cells (37) in the stomach and upper small bowel (38–43). Suppression of the extrapancreatic hyperglucagonemia restores hyperglycemia to normal (44).
Taken together these findings indicate in mice that type 1 diabetes can be converted into an asymptomatic, benign, noncatabolic, insulin-independent disorder by elimination of glucagon action. These studies support the clinical utility of the development of potent Gcgr antagonists and/or glucagon suppressors capable of eliminating the lethal glucagon-dependent component of type 1 diabetes (45).
ACKNOWLEDGMENTS
This work was supported by intramural funds and two private donations and the High Impact Grant Fund from the University of Texas (UT) Southwestern Medical School, by the VA North Texas Health Care System, and by Amylin Pharmaceuticals (San Diego, CA). The laboratory of M.J.C. was funded by National Institutes of Health Grant DK081194 and American Diabetes Association Mentor-Based Postdoctoral Fellowship.
No potential conflicts of interest relevant to this article were reported.
Y.L. carried out the individual experiments and calculated the data. M.-Y.W. provided the primers for qRT-PCR and performed oral glucose tolerance tests. X.Q.D. and M.J.C. provided the glucagon-receptor knockout mice and wild-type controls. R.H.U. designed the study and wrote the manuscript.
The authors are grateful to Christopher Newgard, Ph.D. and James Bain, Ph.D., from the Sarah W. Stedman Nutrition and Metabolism Center and Department of Pharmacology and Cancer Biology at Duke University Medical Center for the amino acid analyses. Sara K. McCorkle of the Dallas VA North Texas Care System contributed expert technical help and designed the figures. The authors thank Christie Fisher of UT Southwestern Medical Center for outstanding editorial work.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0426/-/DC1.
See accompanying commentary, p. 377.
REFERENCES
- 1.Unger RH, Eisentraut AM, McCall MS, Leller S, Lanz HC, Madison LL. Glucagon antibodies and their use for immunoassay for glucagon. Proc Soc Exp Biol Med 1959;102:621–623 [DOI] [PubMed] [Google Scholar]
- 2.Unger RH, Eisentraut AM, McCall MS, Madison LL. Measurements of endogenous glucagon in plasma and the influence of blood glucose concentration upon its secretion. J Clin Invest 1962;41:682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baum J, Simons BE, Jr, Unger RH, Madison LL. Localization of glucagon in the alpha cells in the pancreatic islet by immunofluorescent technics. Diabetes 1962;11:371–374 [PubMed] [Google Scholar]
- 4.Unger RH, Eisentraut AM, Madison LL. The effects of total starvation upon the levels of circulating glucagon and insulin in man. J Clin Invest 1963;42:1031–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller WA, Faloona GR, Unger RH. Hyperglucagonemia in diabetic ketoacidosis. Its prevalence and significance. Am J Med 1973;54:52–57 [DOI] [PubMed] [Google Scholar]
- 6.Rivier J, Brazeau P, Vale W, et al. Solid-phase total synthesis of a tetradecapeptide having the chemical and biological properties of somatostatine. C R Acad Sci Hebd Seances Acad Sci D 1973;276:2737–2740 [PubMed] [Google Scholar]
- 7.Dobbs R, Sakurai H, Sasaki H, et al. Glucagon: role in the hyperglycemia of diabetes mellitus. Science 1975;187:544–547 [DOI] [PubMed] [Google Scholar]
- 8.Gerich JE, Lorenzi M, Bier DM, et al. Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N Engl J Med 1975;292:985–989 [DOI] [PubMed] [Google Scholar]
- 9.Raskin P, Unger RH. Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. N Engl J Med 1978;299:433–436 [DOI] [PubMed] [Google Scholar]
- 10.Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1975;1:14–16 [DOI] [PubMed] [Google Scholar]
- 11.Wang MY, Chen L, Clark GO, et al. Leptin therapy in insulin-deficient type I diabetes. Proc Natl Acad Sci USA 2010;107:4813–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci USA 2008;105:14070–14075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brand CL, Jørgensen PN, Svendsen I, Holst JJ. Evidence for a major role for glucagon in regulation of plasma glucose in conscious, nondiabetic, and alloxan-induced diabetic rabbits. Diabetes 1996;45:1076–1083 [DOI] [PubMed] [Google Scholar]
- 14.Djuric SW, Grihalde N, Lin CW. Glucagon receptor antagonists for the treatment of type II diabetes: current prospects. Curr Opin Investig Drugs 2002;3:1617–1623 [PubMed] [Google Scholar]
- 15.Johnson DG, Goebel CU, Hruby VJ, Bregman MD, Trivedi D. Hyperglycemia of diabetic rats decreased by a glucagon receptor antagonist. Science 1982;215:1115–1116 [DOI] [PubMed] [Google Scholar]
- 16.Parker JC, McPherson RK, Andrews KM, et al. Effects of skyrin, a receptor-selective glucagon antagonist, in rat and human hepatocytes. Diabetes 2000;49:2079–2086 [DOI] [PubMed] [Google Scholar]
- 17.Petersen KF, Sullivan JT. Effects of a novel glucagon receptor antagonist (Bay 27-9955) on glucagon-stimulated glucose production in humans. Diabetologia 2001;44:2018–2024 [DOI] [PubMed] [Google Scholar]
- 18.Qureshi SA, Rios Candelore M, Xie D, et al. A novel glucagon receptor antagonist inhibits glucagon-mediated biological effects. Diabetes 2004;53:3267–3273 [DOI] [PubMed] [Google Scholar]
- 19.Gelling RW, Du XQ, Dichmann DS, et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci USA 2003;100:1438–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang Y, Osborne MC, Monia BP, et al. Reduction in glucagon receptor expression by an antisense oligonucleotide ameliorates diabetic syndrome in db/db mice. Diabetes 2004;53:410–417 [DOI] [PubMed] [Google Scholar]
- 21.Sloop KW, Cao JX, Siesky AM, et al. Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J Clin Invest 2004;113:1571–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH. Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 1984;74:2296–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedbacker K, Birsoy K, Wysocki RW, et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab 2010;11:11–22 [DOI] [PubMed] [Google Scholar]
- 24.Conarello SL, Jiang G, Mu J, et al. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 2007;50:142–150 [DOI] [PubMed] [Google Scholar]
- 25.Wang MY, Orci L, Ravazzola M, Unger RH. Fat storage in adipocytes requires inactivation of leptin’s paracrine activity: implications for treatment of human obesity. Proc Natl Acad Sci USA 2005;102:18011–18016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orci L, Baetens D, Rufener C, et al. Hypertrophy and hyperplasia of somatostatin-containing D-cells in diabetes. Proc Natl Acad Sci USA 1976;73:1338–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beale E, Andreone T, Koch S, Granner M, Granner D. Insulin and glucagon regulate cytosolic phosphoenolpyruvate carboxykinase (GTP) mRNA in rat liver. Diabetes 1984;33:328–332 [DOI] [PubMed] [Google Scholar]
- 28.Meier JM, McGarry JD, Faloona GR, Unger RH, Foster DW. Studies of the development of diabetic ketosis in the rat. J Lipid Res 1972;13:228–233 [PubMed] [Google Scholar]
- 29.Christ B, Nath A, Jungermann K. Mechanism of the inhibition by insulin of the glucagon-dependent activation of the phosphoenolpyruvate carboxykinase gene in rat hepatocyte cultures. Action on gene transcription, mRNA level and -stability as well as hysteresis effect. Biol Chem Hoppe Seyler 1990;371:395–402 [DOI] [PubMed] [Google Scholar]
- 30.Herzig S, Hedrick S, Morantte I, Koo SH, Galimi F, Montminy M. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-gamma. Nature 2003;426:190–193 [DOI] [PubMed] [Google Scholar]
- 31.Boden G, Rezvani I, Owen OE. Effects of glucagon on plasma amino acids. J Clin Invest 1984;73:785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ayala JE, Bracy DP, James FD, Julien BM, Wasserman DH, Drucker DJ. The glucagon-like peptide-1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its incretin action. Endocrinology 2009;150:1155–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almdal TP, Heindorff H, Bardram L, Vilstrup H. Increased amino acid clearance and urea synthesis in a patient with glucagonoma. Gut 1990;31:946–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norton JA, Kahn CR, Schiebinger R, Gorschboth C, Brennan MF. Amino acid deficiency and the skin rash associated with glucagonoma. Ann Intern Med 1979;91:213–215 [DOI] [PubMed] [Google Scholar]
- 35.Meyer C, Dostou JM, Welle SL, Gerich JE. Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am J Physiol Endocrinol Metab 2002;282:E419–E427 [DOI] [PubMed] [Google Scholar]
- 36.Holste LC, Connolly CC, Moore MC, Neal DW, Cherrington AD. Physiological changes in circulating glucagon alter hepatic glucose disposition during portal glucose delivery. Am J Physiol 1997;273:E488–E496 [DOI] [PubMed] [Google Scholar]
- 37.Ravazzola M, Unger RH, Orci L. Demonstration of glucagon in the stomach of human fetuses. Diabetes 1981;30:879–882 [DOI] [PubMed] [Google Scholar]
- 38.Bajorunas DR, Fortner JG, Jaspan JB. Glucagon immunoreactivity and chromatographic profiles in pancreatectomized humans. Paradoxical response to oral glucose. Diabetes 1986;35:886–893 [DOI] [PubMed] [Google Scholar]
- 39.Del Prato S, Riva F, Devidé A, Nosadini R, Fedele D, Tiengo A. Glucagon levels and ketogenesis in human diabetes following total or partial pancreatectomy and severe chronic pancreatitis. Acta Diabetol Lat 1980;17:111–118 [DOI] [PubMed] [Google Scholar]
- 40.Mashiter K, Harding PE, Chou M, et al. Persistent pancreatic glucagon but not insulin response to arginine in pancreatectomized dogs. Endocrinology 1975;96:678–693 [DOI] [PubMed] [Google Scholar]
- 41.Matsuyama T, Wider MD, Tanaka R, et al. A-Cell and gut glucagon in normal and depancreatized dogs. Inhibition by somatostatin and insulin. Diabete Metab 1979;5:141–147 [PubMed] [Google Scholar]
- 42.Sudo T, Suzuki T, Tobe T. Changes in plasma glucagon after total pancreatectomy in man. Gastroenterol Jpn 1980;15:464–468 [DOI] [PubMed] [Google Scholar]
- 43.Tanjoh K, Tomita R, Fukuzawa M, Hayashi N. Peculiar glucagon processing in the intestine is the genesis of the paradoxical rise of serum pancreatic glucagon in patients after total pancreatectomy. Hepatogastroenterology 2003;50:535–540 [PubMed] [Google Scholar]
- 44.Ross G, Lickley L, Vranic M. Extrapancreatic glucagon in control of glucose turnover in depancreatized dogs. Am J Physiol 1978;234:E213–E219 [DOI] [PubMed] [Google Scholar]
- 45.Rivera N, Everett-Grueter CA, Edgerton DS, et al. A novel glucagon receptor antagonist, NNC 25-0926, blunts hepatic glucose production in the conscious dog. J Pharmacol Exp Ther 2007;321:743–752 [DOI] [PubMed] [Google Scholar]