Abstract
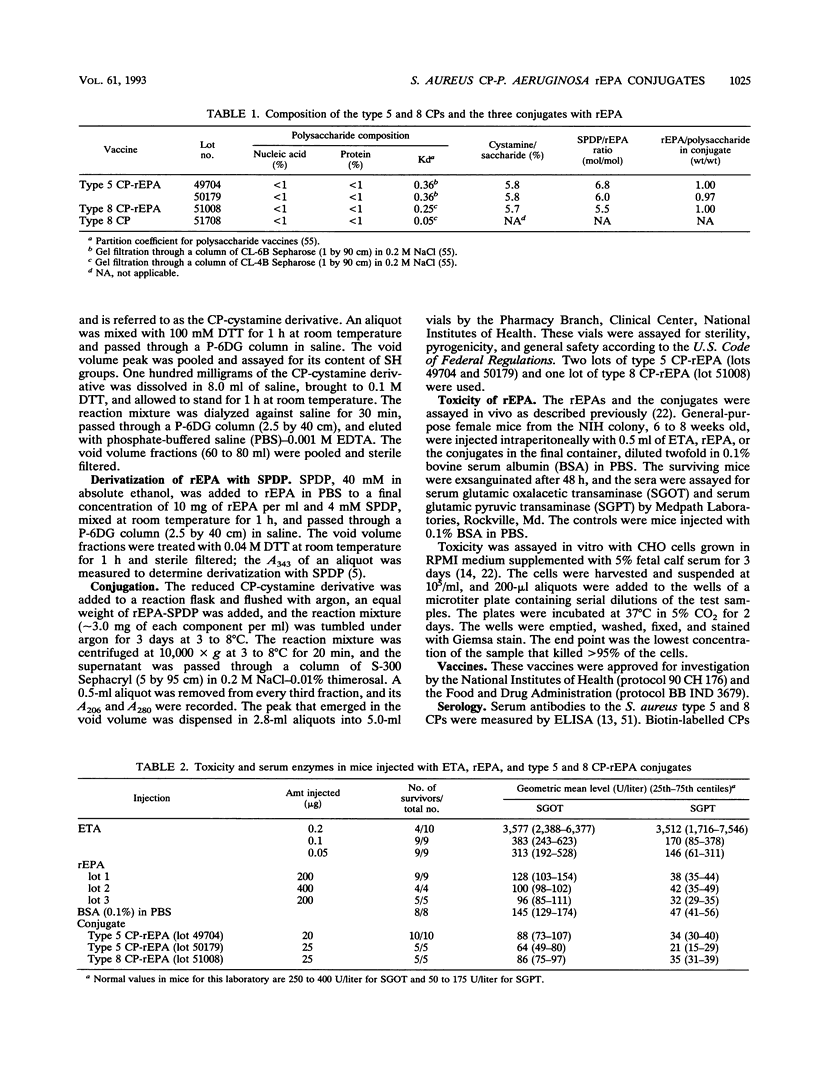
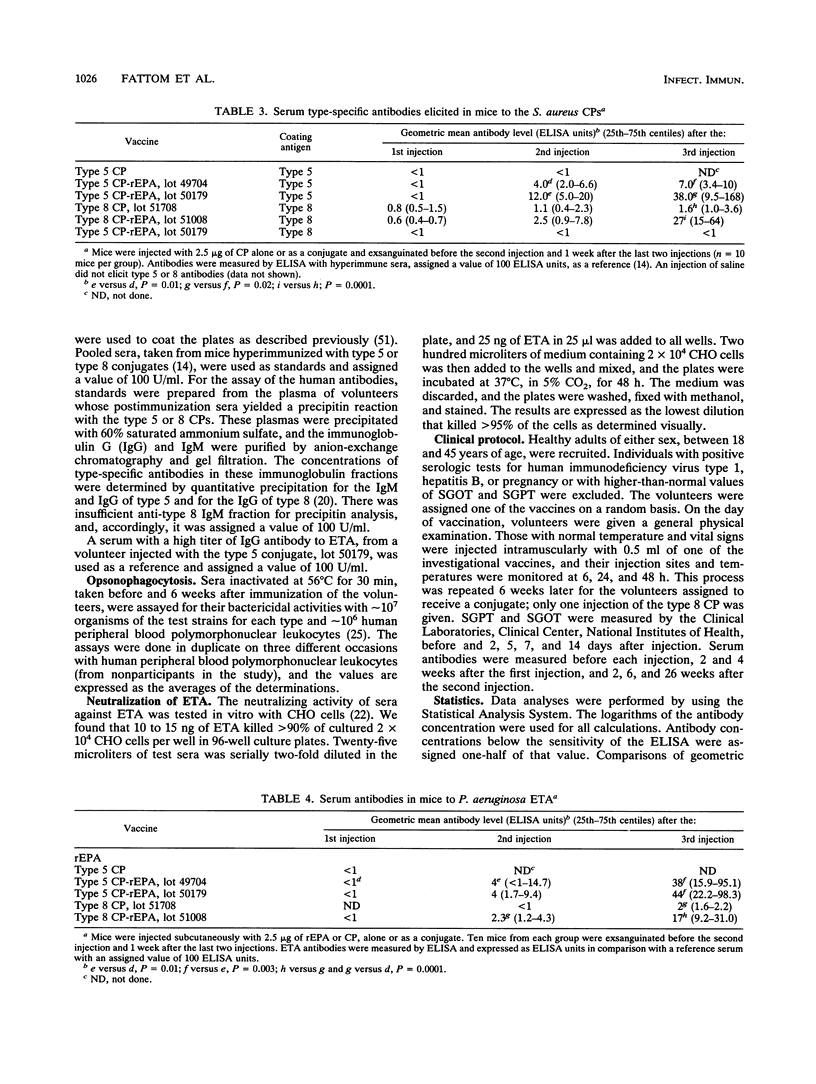
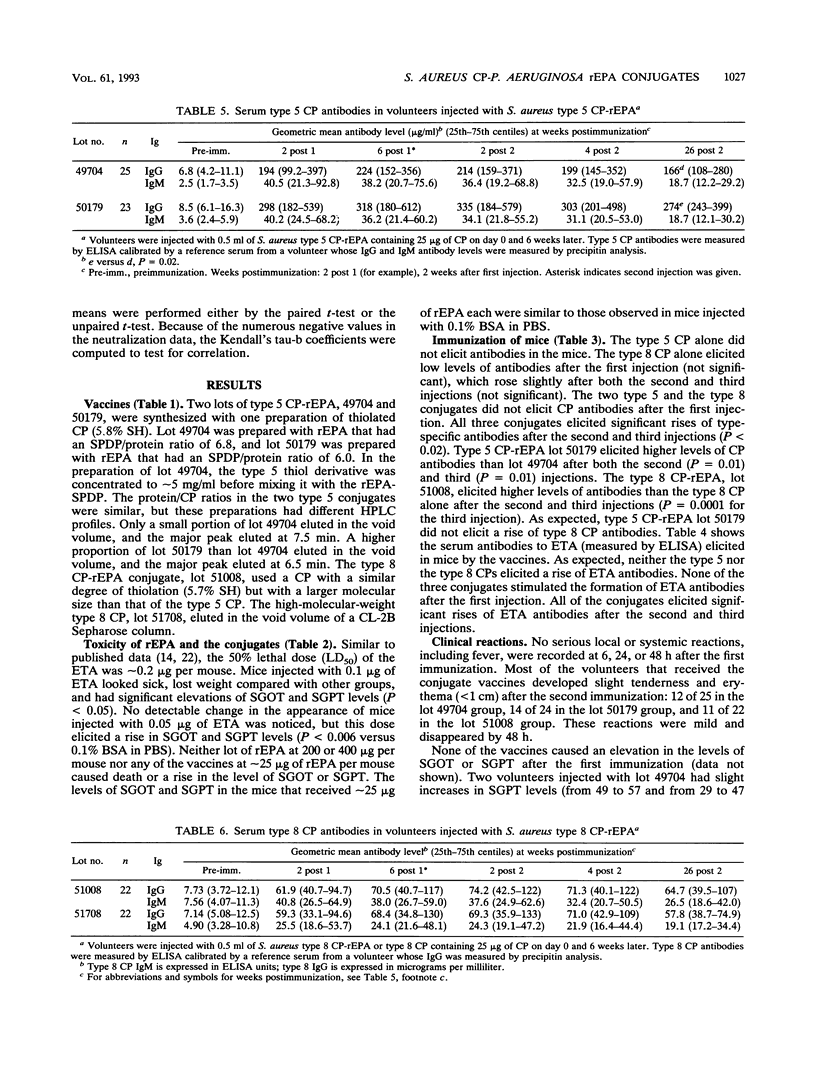
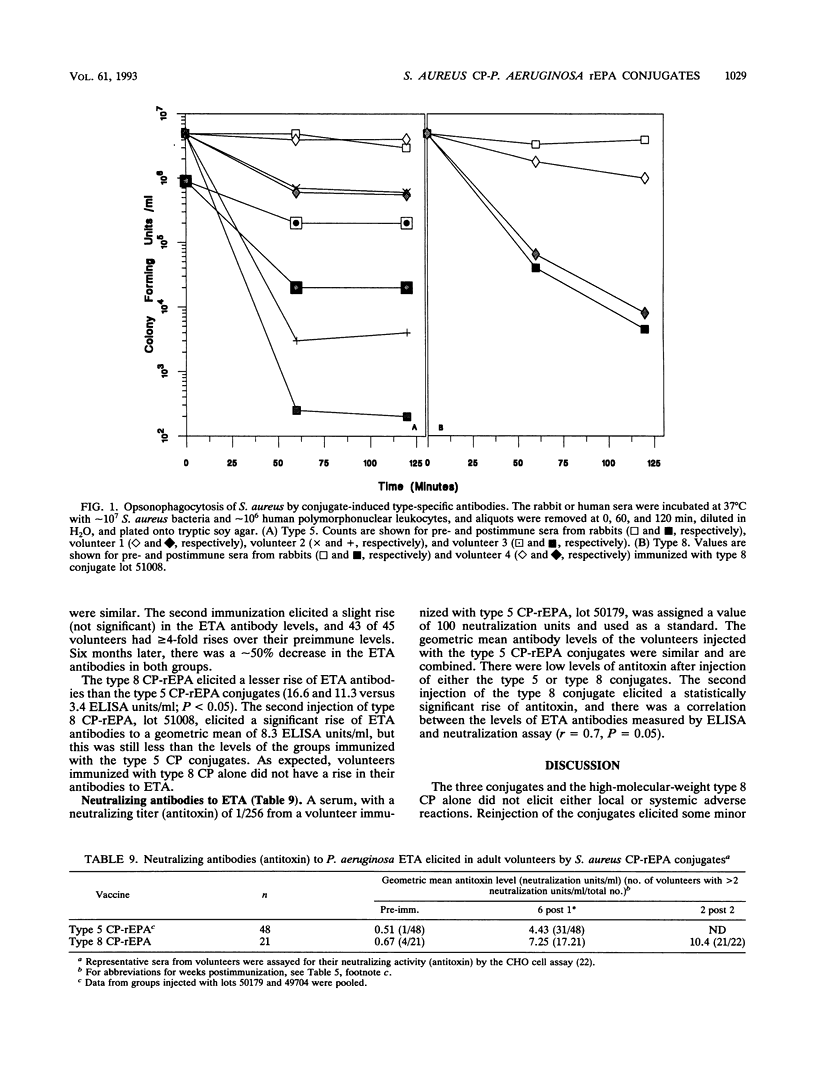
The synthesis, standardization, and immunogenicity in young outbred mice and clinical evaluation in adult volunteers of investigational vaccines designed to induce serum antibodies to the type 5 and type 8 capsular polysaccharides (CPs) of Staphylococcus aureus are described. Conjugates composed of the type 5 CP and a sonicated preparation of a high-molecular-weight type 8 CP bound to a nontoxic recombinant protein derived from Pseudomonas aeruginosa exotoxin A (rEPA) were synthesized. The conjugates were nontoxic and elicited serum CP antibodies after two subcutaneous injections into young outbred mice; a third injection elicited a booster response. The lower-molecular-weight type 8 CP was not immunogenic in the mice, and the high-molecular-weight type 8 CP elicited low levels of antibodies without a booster effect. In the volunteers, neither the conjugates nor the type 8 CP alone caused significant local reactions or fever. The conjugates elicited type-specific antibodies of both the immunoglobulin M (IgM) and IgG classes after the first injection; a second injection 6 weeks later did not stimulate a booster effect. The high-molecular-weight type 8 CP alone, injected once only, elicited levels of IgG and IgM type-specific antibodies similar to those of the conjugate. The vaccine-induced CP antibodies were mostly of the IgG1 and IgG2 subclasses and had opsonophagocytic activity. The conjugates elicited IgG antibodies to the native exotoxin A with neutralizing activity. In summary, the type 5 and type 8 conjugates were safe and elicited biologically active antibodies to both the CP and rEPA components.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeit R. D., Karakawa W. W., Vann W. F., Robbins J. B. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis. 1984 Apr;2(2):85–91. doi: 10.1016/0732-8893(84)90002-6. [DOI] [PubMed] [Google Scholar]
- Boutonnier A., Nato F., Bouvet A., Lebrun L., Audurier A., Mazie J. C., Fournier J. M. Direct testing of blood culture for detection of the serotype 5 and 8 capsular polysaccharides of Staphylococcus aureus. J Clin Microbiol. 1989 May;27(5):989–993. doi: 10.1128/jcm.27.5.989-993.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Mortality patterns--United States, 1988. MMWR Morb Mortal Wkly Rep. 1991 Jul 26;40(29):493-5, 501-2. [PubMed] [Google Scholar]
- Chaudhary V. K., Jinno Y., FitzGerald D., Pastan I. Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proc Natl Acad Sci U S A. 1990 Jan;87(1):308–312. doi: 10.1073/pnas.87.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse M. F., Ba M., Sow A. I., Samb A. Infections néonatales bacteriennes en zone tropicale. Med Trop (Mars) 1991 Oct-Dec;51(4):429–433. [PubMed] [Google Scholar]
- Claesson B. A., Trollfors B., Lagergard T., Taranger J., Bryla D., Otterman G., Cramton T., Yang Y., Reimer C. B., Robbins J. B. Clinical and immunologic responses to the capsular polysaccharide of Haemophilus influenzae type b alone or conjugated to tetanus toxoid in 18- to 23-month-old children. J Pediatr. 1988 May;112(5):695–702. doi: 10.1016/s0022-3476(88)80684-x. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Sadoff J. C., Fürer E. Immunization with a Pseudomonas aeruginosa immunotype 5 O polysaccharide-toxin A conjugate vaccine: effect of a booster dose on antibody levels in humans. Infect Immun. 1988 Jul;56(7):1829–1830. doi: 10.1128/iai.56.7.1829-1830.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin J. F., Miller M. H., Steigbigel N. H. Septicemia in patients on chronic hemodialysis. Ann Intern Med. 1978 Jan;88(1):28–33. doi: 10.7326/0003-4819-88-1-28. [DOI] [PubMed] [Google Scholar]
- Douglas C. M., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: substitution of glutamic acid 553 with aspartic acid drastically reduces toxicity and enzymatic activity. J Bacteriol. 1987 Nov;169(11):4967–4971. doi: 10.1128/jb.169.11.4967-4971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. M., Guidi-Rontani C., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: active, cloned toxin is secreted into the periplasmic space of Escherichia coli. J Bacteriol. 1987 Nov;169(11):4962–4966. doi: 10.1128/jb.169.11.4962-4966.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R., van de Walle M., Shiloach A., Joslyn A., Kaufman J., Shiloach J. Use of high density cultures of Escherichia coli for high level production of recombinant Pseudomonas aeruginosa exotoxin A. Appl Microbiol Biotechnol. 1991 Oct;36(1):65–69. doi: 10.1007/BF00164700. [DOI] [PubMed] [Google Scholar]
- Fattom A., Lue C., Szu S. C., Mestecky J., Schiffman G., Bryla D., Vann W. F., Watson D., Kimzey L. M., Robbins J. B. Serum antibody response in adult volunteers elicited by injection of Streptococcus pneumoniae type 12F polysaccharide alone or conjugated to diphtheria toxoid. Infect Immun. 1990 Jul;58(7):2309–2312. doi: 10.1128/iai.58.7.2309-2312.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattom A., Schneerson R., Szu S. C., Vann W. F., Shiloach J., Karakawa W. W., Robbins J. B. Synthesis and immunologic properties in mice of vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides conjugated to Pseudomonas aeruginosa exotoxin A. Infect Immun. 1990 Jul;58(7):2367–2374. doi: 10.1128/iai.58.7.2367-2374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattom A., Shiloach J., Bryla D., Fitzgerald D., Pastan I., Karakawa W. W., Robbins J. B., Schneerson R. Comparative immunogenicity of conjugates composed of the Staphylococcus aureus type 8 capsular polysaccharide bound to carrier proteins by adipic acid dihydrazide or N-succinimidyl-3-(2-pyridyldithio)propionate. Infect Immun. 1992 Feb;60(2):584–589. doi: 10.1128/iai.60.2.584-589.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J. Potential for vaccination against infections caused by Staphylococcus aureus. Vaccine. 1991 Apr;9(4):221–227. doi: 10.1016/0264-410x(91)90103-d. [DOI] [PubMed] [Google Scholar]
- Fournier J. M., Bouvet A., Boutonnier A., Audurier A., Goldstein F., Pierre J., Bure A., Lebrun L., Hochkeppel H. K. Predominance of capsular polysaccharide type 5 among oxacillin-resistant Staphylococcus aureus. J Clin Microbiol. 1987 Oct;25(10):1932–1933. doi: 10.1128/jcm.25.10.1932-1933.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier J. M., Vann W. F., Karakawa W. W. Purification and characterization of Staphylococcus aureus type 8 capsular polysaccharide. Infect Immun. 1984 Jul;45(1):87–93. doi: 10.1128/iai.45.1.87-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger M., Macpherson C. F. QUANTITATIVE MICRO-ESTIMATION OF ANTIBODIES IN THE SERA OF MAN AND OTHER ANIMALS. Science. 1943 Apr 30;97(2522):405–406. doi: 10.1126/science.97.2522.405. [DOI] [PubMed] [Google Scholar]
- Hochkeppel H. K., Braun D. G., Vischer W., Imm A., Sutter S., Staeubli U., Guggenheim R., Kaplan E. L., Boutonnier A., Fournier J. M. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J Clin Microbiol. 1987 Mar;25(3):526–530. doi: 10.1128/jcm.25.3.526-530.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Sadoff J. C. Toxin inhibitors of protein synthesis: production, purification, and assay of Pseudomonas aeruginosa toxin A. Methods Enzymol. 1979;60:780–793. doi: 10.1016/s0076-6879(79)60071-x. [DOI] [PubMed] [Google Scholar]
- Jinno Y., Ogata M., Chaudhary V. K., Willingham M. C., Adhya S., FitzGerald D., Pastan I. Domain II mutants of Pseudomonas exotoxin deficient in translocation. J Biol Chem. 1989 Sep 25;264(27):15953–15959. [PubMed] [Google Scholar]
- Karakawa W. W., Fournier J. M., Vann W. F., Arbeit R., Schneerson R. S., Robbins J. B. Method for the serological typing of the capsular polysaccharides of Staphylococcus aureus. J Clin Microbiol. 1985 Sep;22(3):445–447. doi: 10.1128/jcm.22.3.445-447.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakawa W. W., Sutton A., Schneerson R., Karpas A., Vann W. F. Capsular antibodies induce type-specific phagocytosis of capsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun. 1988 May;56(5):1090–1095. doi: 10.1128/iai.56.5.1090-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOURIA D. B., BLUMENFELD H. L., ELLIS J. T., KILBOURNE E. D., ROGERS D. E. Studies on influenza in the pandemic of 1957-1958. II. Pulmonary complications of influenza. J Clin Invest. 1959 Jan;38(1 Pt 2):213–265. doi: 10.1172/JCI103791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legaard P. K., LeGrand R. D., Misfeldt M. L. Lymphoproliferative activity of Pseudomonas exotoxin A is dependent on intracellular processing and is associated with the carboxyl-terminal portion. Infect Immun. 1992 Apr;60(4):1273–1278. doi: 10.1128/iai.60.4.1273-1278.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau D. F., Hash J. H. Structural analysis of the surface polysaccharide of Staphylococcus aureus M. J Bacteriol. 1977 Jul;131(1):194–200. doi: 10.1128/jb.131.1.194-200.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlam H. A., Young A. E., Berry A. J., Phillips I. The prevention of infection with Staphylococcus aureus in continuous ambulatory peritoneal dialysis. J Hosp Infect. 1989 Nov;14(4):293–301. doi: 10.1016/0195-6701(89)90069-8. [DOI] [PubMed] [Google Scholar]
- Lukac M., Pier G. B., Collier R. J. Toxoid of Pseudomonas aeruginosa exotoxin A generated by deletion of an active-site residue. Infect Immun. 1988 Dec;56(12):3095–3098. doi: 10.1128/iai.56.12.3095-3098.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. E., Jr, Barnes M. W., Finland M. Bacteremia at Boston City Hospital: Occurrence and mortality during 12 selected years (1935-1972), with special reference to hospital-acquired cases. J Infect Dis. 1975 Sep;132(3):316–335. doi: 10.1093/infdis/132.3.316. [DOI] [PubMed] [Google Scholar]
- Moreau M., Richards J. C., Fournier J. M., Byrd R. A., Karakawa W. W., Vann W. F. Structure of the type 5 capsular polysaccharide of Staphylococcus aureus. Carbohydr Res. 1990 Jul 1;201(2):285–297. doi: 10.1016/0008-6215(90)84244-o. [DOI] [PubMed] [Google Scholar]
- Neville L. O., Billington O. J., Kibbler C. C., Gillespie S. H. Methicillin resistant Staphylococcus aureus without clumping factor, protein A, and DNAse. Lancet. 1991 Aug 24;338(8765):518–518. doi: 10.1016/0140-6736(91)90595-g. [DOI] [PubMed] [Google Scholar]
- Nolan C. M., Beaty H. N. Staphylococcus aureus bacteremia. Current clinical patterns. Am J Med. 1976 Apr;60(4):495–500. doi: 10.1016/0002-9343(76)90715-4. [DOI] [PubMed] [Google Scholar]
- Pollack M., Callahan L. T., 3rd, Taylor N. S. Neutralizing antibody to Pseudomonas aeruginosa exotoxin in human sera: evidence for in vivo toxin production during infection. Infect Immun. 1976 Oct;14(4):942–947. doi: 10.1128/iai.14.4.942-947.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Young L. S. Protective activity of antibodies to exotoxin A and lipopolysaccharide at the onset of Pseudomonas aeruginosa septicemia in man. J Clin Invest. 1979 Feb;63(2):276–286. doi: 10.1172/JCI109300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poutrel B., Boutonnier A., Sutra L., Fournier J. M. Prevalence of capsular polysaccharide types 5 and 8 among Staphylococcus aureus isolates from cow, goat, and ewe milk. J Clin Microbiol. 1988 Jan;26(1):38–40. doi: 10.1128/jcm.26.1.38-40.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raad I. I., Sabbagh M. F. Optimal duration of therapy for catheter-related Staphylococcus aureus bacteremia: a study of 55 cases and review. Clin Infect Dis. 1992 Jan;14(1):75–82. doi: 10.1093/clinids/14.1.75. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Schneerson R. Polysaccharide-protein conjugates: a new generation of vaccines. J Infect Dis. 1990 May;161(5):821–832. doi: 10.1093/infdis/161.5.821. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B., Parke J. C., Jr, Bell C., Schlesselman J. J., Sutton A., Wang Z., Schiffman G., Karpas A., Shiloach J. Quantitative and qualitative analyses of serum antibodies elicited in adults by Haemophilus influenzae type b and pneumococcus type 6A capsular polysaccharide-tetanus toxoid conjugates. Infect Immun. 1986 May;52(2):519–528. doi: 10.1128/iai.52.2.519-528.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheagren J. N. Staphylococcus aureus. The persistent pathogen (first of two parts). N Engl J Med. 1984 May 24;310(21):1368–1373. doi: 10.1056/NEJM198405243102107. [DOI] [PubMed] [Google Scholar]
- Siber G. R., Weitzman S. A., Aisenberg A. C., Weinstein H. J., Schiffman G. Impaired antibody response to pneumococcal vaccine after treatment for Hodgkin's disease. N Engl J Med. 1978 Aug 31;299(9):442–448. doi: 10.1056/NEJM197808312990903. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Samra Z., Karakawa W. W., Vann W. F., Schneerson R., Malik Z. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol. 1985 Nov;22(5):828–834. doi: 10.1128/jcm.22.5.828-834.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M. C., Auerbach B. S., Nelson K. E., Vlahov D., Becker R. L., Graham N. M., Schwartz D. H., Lucas A. H., Chaisson R. E. Antibody responses to Haemophilus influenzae type B vaccines in men with human immunodeficiency virus infection. N Engl J Med. 1991 Dec 26;325(26):1837–1842. doi: 10.1056/NEJM199112263252603. [DOI] [PubMed] [Google Scholar]
- Sutra L., Mendolia C., Rainard P., Poutrel B. Encapsulation of Staphylococcus aureus isolates from mastitic milk: relationship between capsular polysaccharide types 5 and 8 and colony morphology in serum-soft agar, clumping factor, teichoic acid, and protein A. J Clin Microbiol. 1990 Mar;28(3):447–451. doi: 10.1128/jcm.28.3.447-451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutra L., Rainard P., Poutrel B. Phagocytosis of mastitis isolates of Staphylococcus aureus and expression of type 5 capsular polysaccharide are influenced by growth in the presence of milk. J Clin Microbiol. 1990 Oct;28(10):2253–2258. doi: 10.1128/jcm.28.10.2253-2258.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A., Vann W. F., Karpas A. B., Stein K. E., Schneerson R. An avidin-biotin based ELISA for quantitation of antibody to bacterial polysaccharides. J Immunol Methods. 1985 Oct 10;82(2):215–224. doi: 10.1016/0022-1759(85)90353-9. [DOI] [PubMed] [Google Scholar]
- Szu S. C., Li X. R., Schneerson R., Vickers J. H., Bryla D., Robbins J. B. Comparative immunogenicities of Vi polysaccharide-protein conjugates composed of cholera toxin or its B subunit as a carrier bound to high- or lower-molecular-weight Vi. Infect Immun. 1989 Dec;57(12):3823–3827. doi: 10.1128/iai.57.12.3823-3827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Zon G., Schneerson R., Robbins J. B. Ultrasonic irradiation of bacterial polysaccharides. Characterization of the depolymerized products and some applications of the process. Carbohydr Res. 1986 Sep 1;152:7–20. doi: 10.1016/s0008-6215(00)90283-0. [DOI] [PubMed] [Google Scholar]
- Tam A. Y., Yeung C. Y. The changing pattern of severe neonatal staphylococcal infection: a 10-year study. Aust Paediatr J. 1988 Oct;24(5):275–279. doi: 10.1111/j.1440-1754.1988.tb01361.x. [DOI] [PubMed] [Google Scholar]
- Xu S., Arbeit R. D., Lee J. C. Phagocytic killing of encapsulated and microencapsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun. 1992 Apr;60(4):1358–1362. doi: 10.1128/iai.60.4.1358-1362.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. L., Goetz A., Wagener M., Smith P. B., Rihs J. D., Hanchett J., Zuravleff J. J. Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. Efficacy of antibiotic prophylaxis. N Engl J Med. 1986 Jul 10;315(2):91–96. doi: 10.1056/NEJM198607103150204. [DOI] [PubMed] [Google Scholar]


