Abstract
OBJECTIVE
To examine the role of AMP-activated protein kinase (AMPK) in the control of glucoprivic feeding by hindbrain catecholamine neurons.
RESEARCH DESIGN AND METHODS
Micropunched hindbrain samples were collected from control and 2-deoxy-d-glucose (2DG)-injected rats for Western blot analysis of phosphorylated (activated) AMPK (pAMPK). Samples also were collected from 2DG-injected rats pretreated with anti-dopamine-β-hydroxylase conjugated to saporin to lesion hindbrain catecholamine neurons. In a second experiment, rats were given a fourth-ventricle injection of compound C (CC) or 5-aminoimidazole-4-carboxyamide ribonucleoside (AICAR), an inhibitor and activator of AMPK, to identify a role for AMPK in hindbrain neurons required for elicitation of 2DG-induced feeding.
RESULTS
Systemic 2DG stimulated food intake in controls but not in catecholamine-lesioned rats. In controls, but not catecholamine-lesioned rats, 2DG also increased phosphorylated Thr172 at AMPKα subunits (pAMPKα) in hindbrain micropunches containing catecholaminergic cell groups A1 through the middle region of C1 (A1–C1m). Increased pAMPKα was not observed in the adjacent noncatecholaminergic ventromedial medulla or in the A2–C2 catecholamine cell groups in the dorsal hindbrain. Fourth-ventricle injection of CC attenuated 2DG-induced feeding during the first 2 h of the test, and AICAR alone increased food intake only during the first 60 min of the 4-h test.
CONCLUSIONS
Results indicate that AMPK in catecholaminergic A1–C1m neurons is activated by glucoprivation. Therefore, AMPK may contribute to the glucose-sensing mechanism by which these neurons detect and signal a glucose deficit in the service of systemic glucoregulation.
A continuous supply of glucose is essential for the function and survival of neurons in the brain. Maintaining the brain’s glucose supply depends on a number of glucoregulatory responses elicited by a glucose deficit. Systemic injection of 2-deoxy-d-glucose (2DG), a competitive inhibitor of intracellular glucose utilization (1), induces cellular glucoprivation and triggers a number of glucoregulatory responses. Increased food intake is one of the most crucial responses to a glucose deficit. Studies using a selective retrogradely transported catecholamine immunotoxin, anti-dopamine-β-hydroxylase (DBH)-saporin (DSAP), have demonstrated that catecholamine neurons innervating the medial hypothalamus are required for elicitation of glucoprivic feeding as well as for glucoprivation-induced corticosterone secretion and suppression of estrous cycles (2–4) and for glucoprivation-induced increases in arcuate nucleus neuropetide Y (Npy) and agouti-related protein (Agrp) mRNAs (5). A separate population that projects spinally mediates the adrenal medullary response (2,6). Additional work using a number of different approaches has shown that the hindbrain contains cells capable of detecting a glucose deficit and eliciting feeding and other glucoregulatory responses (7–11) and suggests the possibility that certain catecholamine neurons may themselves be glucoreceptive.
A number of findings indicate that the specific catecholamine neurons required for glucoprivic feeding are located in the ventrolateral hindbrain area containing the rostral portion of cell group A1 and the caudal two-thirds of group C1 (A1–C1). The A1–C1 neurons are potently activated by glucoprivation (12–14), and they innervate the hypothalamic areas involved in the control of metabolism and appetite (15). Furthermore, glucoprivic feeding is blocked by simultaneous silencing in A1–C1 neurons of the genes encoding the catecholamine biosynthetic enzyme, DBH, and the colocalized peptide, NPY, respectively (16).
AMP-activated protein kinase (AMPK) is a highly conserved serine/threonine kinase that is activated by an increase of the cellular AMP-to-ATP ratio and is considered to be a sensor of cellular energy status (17–20). Upon activation, AMPK inhibits ATP-consuming pathways and stimulates ATP-generating pathways to restore cellular energy balance. AMPK is expressed in brain regions involved in the regulation of feeding behavior (21,22), and a number of studies have shown that brain AMPK contributes to the control of food intake and glucose homeostasis. Food deprivation increases AMPK activity in the hypothalamus, whereas refeeding inhibits it (23). A third-ventricle injection of the AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) or overexpression of AMPK in the hypothalamus increases feeding and body weight (23,24). Activation of AMPK requires phosphorylation of catalytic α-subunits at Thr172 (pAMPKα). Reports have shown that pAMPKα in the hypothalamus is upregulated by insulin-induced hypoglycemia or 2DG-induced glucoprivation (25–27) and that 2DG enhances pAMPKα expression through decreasing cellular ATP levels in cultured neuronal cells (28). Thus, we hypothesized that AMPK in the hindbrain also contributes to 2DG-induced glucoprivic feeding and may be a part of the glucose-sensing transduction mechanism. As a first step in testing this hypothesis, we measured pAMPKα in the hindbrain catecholamine cell group A1–C1 after 2DG injection in control rats and in rats with selective retrograde immunotoxin lesion of these neurons. In addition, we examined the effect of enhancement or blockade of AMPK activity in the hindbrain on glucoprivic feeding.
RESEARCH DESIGN AND METHODS
Male SD rats (Simonsen Laboratories, Gilroy, CA) were housed individually and maintained on a 12-h light/dark cycle (lights on at 0600 h) with ad libitum access to pelleted rodent food (F6 diet; Harlan Teklad, Madison, WI) and tap water. All experimental procedures were approved by the Washington State University Institutional Animal Care and Use Committee, which conforms to National Institutes of Health guidelines.
Cannula implantation and fourth-ventricle injections.
For the fourth-ventricle cannula implantation, rats were anesthetized using 1.0 mL/kg body wt of a ketamine/xylazine/acepromazine cocktail (5 mL ketamine HCl, 100 mg/mL, Fort Dodge Animal Health, Fort Dodge, IA; 2.5 mL xylazine, 20 mg/mL, Vedco, St. Joseph, MO; 1 mL acepromazine, 10 mg/mL, Vedco; and 1.5 mL 0.9% saline solution) and placed in a stereotaxic device. A 26G cannula (15.0 mm long) was implanted 1.55 mm rostral to the occipital suture, ±0 mm lateral to midline and 6.3–6.4 mm ventral to the skull surface (29). Experiments were initiated 1 week later. Fourth-ventricle injections were delivered over a 3- to 5-min period using a PB-600 repeating dispenser (Hamilton, Reno, NV). The position of the fourth-ventricle cannula for each rat was confirmed after the experiments by staining hindbrain sections with cresyl violet. Only rats with a cannula correctly inserted into the fourth ventricle were used for data analysis.
Feeding tests.
For glucoprivic feeding tests, rats were injected subcutaneously with 2DG (200 mg/kg body wt in 0.9% sterile saline; Sigma-Aldrich, St. Louis, MO) or with 0.9% sterile saline as the control. Food was removed at 0800 h, 2 h before the injections. Food intake was measured beginning immediately after the injection and at various intervals thereafter, as indicated. The AMPK inhibitor, compound C (CC; Sigma-Aldrich), was dissolved in DMSO (3 μg/μL) and was injected into the fourth ventricle at a volume of 1 μL per rat 10–15 min before 2DG injection. At this concentration and volume, nonspecific effects of DMSO on feeding were not observed. The AMPK activator AICAR (Sigma-Aldrich) was dissolved in saline and injected into the fourth ventricle at a dose of 20, 100, 300 (or 500) nmoL per rat in 3 (or 5) μL saline.
DSAP microinjection and DBH immunohistochemistry.
The immunotoxin DSAP (82 ng/200 nL; Advanced Targeting Systems, San Diego, CA) or control unconjugated saporin (SAP) (17.2 ng/200 nL), dissolved in 0.1 mol/L PBS (pH 7.4), was infused bilaterally through a pulled-glass capillary pipette (30-μm tip diameter) positioned stereotaxically just dorsal to the targeted site in the paraventricular hypothalamic nucleus (PVH). Solutions were delivered using a picospritzer (Parker, Cleveland, OH). Stereotaxic coordinates for the PVH injection site were 1.8 mm caudal and ± 0.45 mm lateral to bregma and 7.3–7.4 mm ventral to dura mater (29). The amount of unconjugated SAP in the control solution approximated the amount of SAP present in the DSAP conjugate (21%), as indicated in the manufacturer’s product information. Previous work comparing SAP and uninjected controls demonstrated that SAP did not produce behavioral or significant histological signs of toxicity (2). Glucoprivic feeding induced by 2DG (200 mg/kg) was tested 3 weeks after DSAP or SAP injections. One week later, expression of pAMPKα and total AMPKα in hindbrain after the 2DG injection was measured. The DSAP lesion was evaluated by Western blot analysis of tyrosine hydroxylase (TH) expression in hindbrain sites of interest and in a separate group of lesioned rats using immunohistochemistry (IHC) staining to detect DBH-positive cell bodies. Two methods were used to confirm the DSAP lesion because we knew that Western blot analysis would detect the TH present in the punched sample from terminals and processes of unlesioned catecholamine neurons not targeted by PVH DSAP injection. Thus, Western blots of TH would not reflect the destruction of catecholamine cell bodies in the lesion site as accurately as IHC.
For IHC, brains were sectioned coronally into four serial sets (40-μm thickness) and stained using standard avidin-biotin-peroxidase IHC techniques (13). Cell bodies expressing DBH were detected by murine anti-DBH antibody (1:10,000; Millipore, Billerica, MA) and counted in three consecutive sections (29) at each of four hindbrain regions.
Micropunch and dissection of hindbrain tissues.
Tissue sites containing catecholamine cell groups were micropunched or dissected from hindbrain for Western blot. Catecholamine cell groups are defined according to the Paxinos and Watson (1998) rat brain atlas (29). However, we refer to the middle portion of C1 as C1m, which extends from 12.72 to 12.30 mm caudal to bregma.
At designated times after treatment, rats were anesthetized deeply with halothane (Halocarbon Products, North Augusta, SC) and were quickly decapitated. The brain was removed and frozen on dry ice. In 2DG-induced AMPK experiments, a 2-mm-thick coronal section was cut, using obex (defined here as the caudal border of the area postrema) to locate the caudal border of the slice (0–2.0 mm rostral to obex). Then, the desired regions from this 2-mm-thick slice were micropunched with a stainless steel sample corer that had a 1.0-mm inner diameter (Fine Science Tools, Foster City, CA). Tissue punches were collected from three areas (from A1–C1m, from an [noncatecholamine] area medial to A1–C1m [ventromedial medulla (VMM)], and from A2–C2 in the nucleus of the solitary tract [NTS]).
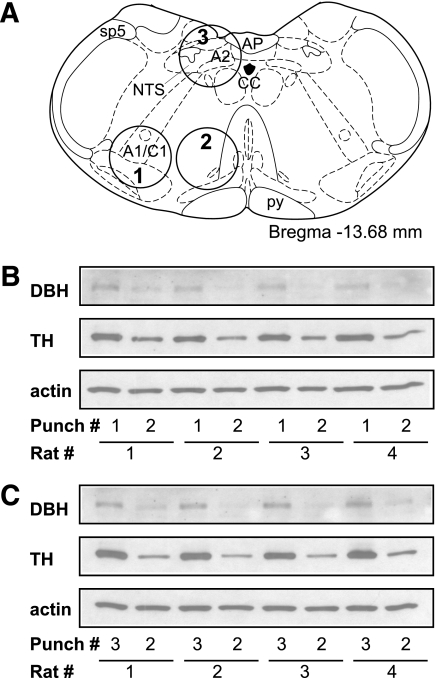
Micropunched samples were quickly placed into a radioimmunoprecipitation assay (RIPA) buffer for protein isolation. The accuracy of the punches was confirmed by Western blot using antibodies against enzymes for catecholamine synthesis (DBH and TH). Representative images of DBH and TH Western blots for A1–C1m, VMM, and A2–C2 tissue punches were shown in Fig. 1. For all rats, both DBH and TH expression were expressed more strongly (more than three times) in A1–C1m and A2–C2 samples than in the VMM samples, indicating the accuracy of the punches.
FIG. 1.
Tissue micropunches from the hindbrain slices. A: Coronal section of rat hindbrain (Paxinos and Watson [29]) showing the positions of each 1.0-mm-diameter circular punches used for Western blots. Punches 1, 2, or 3 included A1–C1m, VMM, or A2–C2, respectively. B and C: Immunoblots of DBH, TH, and β-actin from different tissue micropunches are indicated in A. Higher expression levels of DBH and TH were found in punch 1 (A1–C1m) tissue (B) or punch 3 (A2–C2) tissue (C) than in the adjacent punch 2 (VMM) tissue in all individual rats. AP, area postrema; CC, central canal; py, pyramidal tract; sp5, spinal trigeminal tract.
In AICAR-induced AMPK experiments, two 2-mm-thick coronal sections were cut, using obex to locate the position of the cuts (0–2.0 and 2.0–4.0 mm rostral to obex, respectively). Then, the two slices were divided into the dorsal and ventral parts. Slice 1, the dorsal part, included A2, C2, and the NTS. Slice 1, the ventral part, included A1–C1m. Slice 2, the dorsal part, included A2 and C2. Slice 2, the ventral part, included C1r and A5. Tissue sections were quickly placed into RIPA buffer for protein isolation and then used for Western blot.
Western blot.
The method was modified from our previous reports (30,31). Hindbrain tissues were first incubated with RIPA buffer (50 mmol/L Tris, pH 8.0; 150 mmol/L NaCl; 1.0% (Octylphenoxy)polyethoxyethanol; 0.5% sodium deoxycholate; and 0.1% SDS), with 1 mmol/L sodium ortho-vanadate, 1 μmol/L okadiac acid, and 1/10 (vol/vol) protease inhibitor cocktail (Roche Diagnostics, Penzberg, Germany) for 30 min on ice. After centrifugation (14,000 rpm for 15 min at 4°C), an equivalent amount of protein (20 μg per lane) for each sample was separated by SDS-PAGE. The resolved proteins on the gel were transferred to a nitrocellulose membrane (Amersham, Piscataway, NJ). The membranes were blocked in 5% nonfat dry milk in Tris-buffered saline/0.05% Tween 20 and incubated with one of the following primary antibodies in blocking solution for 16–18 h at 4°C. The membranes were then washed with Tris-buffered saline/0.05% Tween 20, incubated in goat anti-rabbit IgG, goat anti-murine IgG, or donkey anti-goat IgG antibody conjugated with peroxidase (1:4,000; DakoCytomation, Glostrup, Denmark) and developed with the enhanced chemiluminescence method (Amersham) and exposed to X-OMAT film (Kodak, Rochester, NY). The following antibodies were used: murine anti–β-actin (1:10,000; Sigma-Aldrich), rabbit anti-AMPKα (1:1,000; Santa Cruz Biotech, Santa Cruz, CA), rabbit anti-pAMPKα (Thr172) (1:1,000; Millipore), goat anti-DBH (1:600; Santa Cruz Biotech), and murine anti-TH (1:6,000; Millipore). Densitometric analysis of blots was performed using Scion Image software (Scion, Frederick, MD). The intensity of each band for each protein was quantified and normalized with housekeeping gene β-actin expression in the same membrane. The levels of total AMPKα or pAMPKα were expressed as a percentage over that in the rats injected with saline.
Statistical analysis.
All results are presented as means ± SEM. For statistical analysis of data, we used unpaired t test or one-way ANOVA using SigmaStat (Systat Software, San Jose, CA). After significance was determined by ANOVA, multiple comparisons between individual groups were tested using a post hoc Fisher protected least significant difference (PLSD) test. P = 0.05 or P < 0.05 was considered to be statistically significant.
RESULTS
2DG increased pAMPKα expression in A1–C1m.
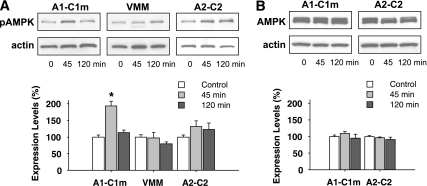
As shown in Fig. 2A, pAMPKα was significantly increased by 2DG in ventrolateral medulla punches, which included A1–C1m, 45 min after the injection (193.6 ± 13.8% in the 2DG group vs. 100.0 ± 6.1% in the saline group; P < 0.001) and returned to near-control values 2 h after the injection (113.1 ± 8.1% of control). This enhancement was site specific. In the VMM, a region adjacent to the A1–C1m, pAMPKα was unchanged by 2DG (Fig. 2A). In the A2–C2 region, pAMPKα was somewhat increased at both 45 min and 2 h after 2DG injection, but the increased levels did not differ significantly from control values (P > 0.4 between groups). Total AMPKα expression was not altered by 2DG in any of the sampled regions at the time points tested (Fig. 2B).
FIG. 2.
Total and phosphorylated forms of AMPKα in three hindbrain regions after 2DG injection. Tissue homogenates were obtained 45 or 120 min after saline (control) or 2DG (200 mg/kg s.c.) injection. Equivalent amounts of protein were subjected to SDS-PAGE and immunoblotted with anti-pAMPKα, anti-total AMPKα, or anti–β-actin antibody. A: Upper panel: immunoblots of pAMPKα and β-actin in A1–C1m, VMM, and A2–C2 regions with saline or 2DG injection. Lower panel: quantitative data. B: Upper panel: immunoblots of total AMPKα and β-actin in A1–C1m and A2–C2 regions. Lower panel: quantitative data. To compare expression levels of these proteins, the intensity of each band was quantified and normalized with β-actin and expressed as the percentage of saline control rats. *P < 0.01 vs. the control saline-injected rats. Fisher PLSD test after one-way ANOVA (n = eight rats for each group).
Lesion of hindbrain neurons by DSAP abolished 2DG-induced pAMPKα expression.
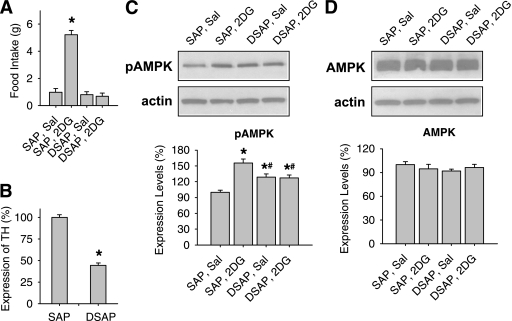
After PVH DSAP injections, catecholamine neurons in the hindbrain were greatly reduced, as indicated by the attenuation of 2DG-induced glucoprivic feeding (Fig. 3A) and decreased TH expression (Fig. 3B) in the hindbrain. SAP rats ate fivefold more food during the 4-h period after 2DG injection than after the saline injection (P < 0.001), whereas DSAP rats ate almost the same amount of food in the two tests (P > 0.1). In a separate experiment, food intake was measured at both 2 and 4 h after 2DG injection. Between 0 and 2 h, SAP rats ate 4.8 ± 1.1 g vs. 0.2 ± 0.1 g for DSAP rats, whereas between 2 and 4 h, DSAP rats ate 0.4 ± 0.1 g vs. 0.5 ± 0.1 g for SAP rats (n = 5 for each group). 2DG increased the pAMPKα expression in A1–C1m in the SAP rats at 45 min after 2DG injection (P < 0.01), whereas 2DG had no effect on pAMPKα expression in the DSAP rats (Fig. 3C). DSAP rats, treated with saline or 2DG, had slightly higher pAMPKα expression levels than SAP saline rats, but there was no significant difference between the two DSAP groups. No significant changes were found in the total AMPKα expression levels between SAP and DSAP rats, regardless of whether they were injected with saline or 2DG (Fig. 3D).
FIG. 3.
Glucoprivic feeding and AMPK expression in DSAP- or SAP-injected rats. A: Food intake during the 4-h period after 2DG (200 mg/kg s.c.) or saline (Sal) injection in SAP and DSAP rats. *P < 0.001 vs. SAP rats with saline injection (n = 16 rats per group). B: TH expression levels in A1–C1m regions in SAP and DSAP rats, measured by Western blot. The level of TH in SAP rats, normalized to β-actin, was used as 100%. *P < 0.001 vs. SAP rats (n = 16 rats per group). C: Upper panel: immunoblots of pAMPKα and β-actin in A1–C1m regions 45 min after saline or 2DG injection. Lower panel: quantitative pAMPKα data, normalized to β-actin. *P < 0.05 vs. saline-injected SAP rats; #P < 0.05 vs. 2DG-injected SAP rats. Fisher PLSD test after one-way ANOVA (n = eight rats per group). D: Upper panel: immunoblots of total AMPKα and β-actin in A1–C1m regions 45 min after saline or 2DG injection. Lower panel: quantitative data, normalized to β-actin (n = eight rats per group).
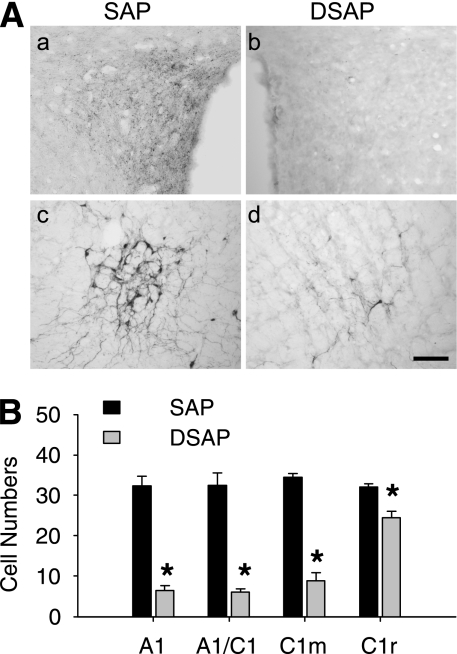
In DSAP rats, TH expression in A1–C1m, by Western blot, was decreased to 44.2% of the level in SAP controls (P < 0.001) (Fig. 3B). TH expression did not differ for A1–C1m in SAP saline and non–SAP saline rats (100.0 ± 10.2% vs. 94.3 ± 4.6%; n = 6–7 rats; P > 0.6), indicating that SAP injections did not retrogradely lesion hindbrain catecholamine neurons. Parallel IHC analysis of the lesion (Fig. 4) showed that PVH DSAP injection eliminated DBH-positive fibers in the PVH and significantly reduced the number of DBH-positive cells in the A1–C1m, which heavily innervate the PVH, as shown previously (15,32). The number of DBH-positive cells was reduced in the A1–C1m to 21.5% of the number present in SAP rats.
FIG. 4.
Lesion effect of DSAP on hindbrain catecholamine neurons. A: Representative IHC images of DBH staining in a SAP-injected (a and c) or DSAP-injected (b and d) rat in PVH (a and b) or the hindbrain A1/C1 region (c and d). Scale bar = 0.5 mm. B: DBH-positive cells in each of the hindbrain regions. *P < 0.01 vs. SAP rats (n = five rats in each group).
Inhibition of AMPK activity in the hindbrain impaired glucoprivic feeding.
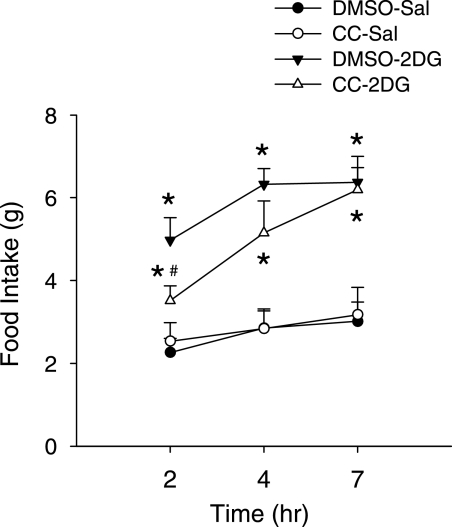
Preinjection of CC significantly suppressed glucoprivic feeding during the first 2 h after 2DG injection to 70.6% of the amount consumed by rats injected with 2DG alone (P < 0.05) but did not suppress 2DG-induced feeding at 4 h (81%, P > 0.1) or 7 h (97.1%, P > 0.8) after injection (Fig. 5).
FIG. 5.
Effect of CC, an AMPK inhibitor, on 2DG-induced feeding. CC or the control solvent, DMSO, was injected into the fourth ventricle 10–15 min before 2DG (200 mg/kg s.c.) or saline (Sal) injection. Food intake was measured for the next 7 h. *P < 0.05 vs. DMSO-Sal group; #P < 0.05 vs. DMSO-2DG group. Fisher PLSD test after one-way ANOVA (n = eight rats in each group).
Effect of hindbrain activation of AMPK on food intake.
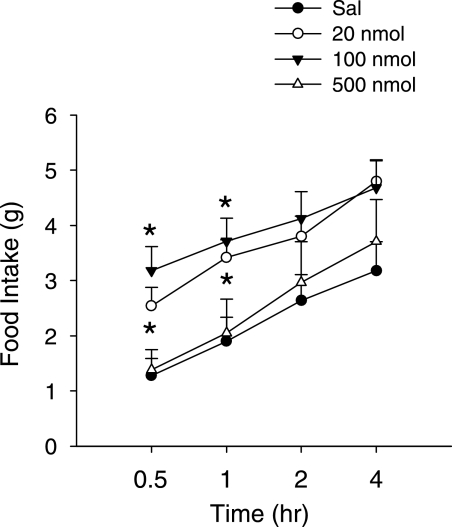
To reveal whether activation of hindbrain AMPK can induce feeding, an AMPK activator, AICAR, was injected into the fourth ventricle. Figure 6 shows that AICAR at 20 and 100 nmol per rat increased feeding only during the first 60 min after the injection (all P = 0.05 or P < 0.05). The higher dose, 500 nmol, did not appear to alter food intake at any time point. The time course of the AICAR response was much more transient than that produced by 2DG or insulin-induced hypoglycemia. Western blot was performed to check whether such doses of AICAR efficiently increase AMPK activity in the hindbrain. As shown in Table 1, AICAR doses ≥100 nmol per rat significantly increased pAMPKα expression in the hindbrain 35–45 min later. AICAR was more effective in increasing pAMPKα in the ventral than in the dorsal hindbrain (Table 1), in both slice 1 (0–2.0 mm rostral to obex) and slice 2 (2.0–4.0 mm rostral to obex).
FIG. 6.
Effect of hindbrain AMPK activation on food intake. Saline (Sal) or different doses of the AMPK activator, AICAR, were injected into the fourth ventricle. Food intake was measured after the injection. *P = 0.05 or P < 0.05 vs. the saline group. Fisher PLSD test after one-way ANOVA (n = 5–8 rats per group).
TABLE 1.
Expression levels of pAMPKα and total AMPKα in the hindbrain after AICAR injection
| Dose of AICAR (nmol per rat) | ||||
|---|---|---|---|---|
| Region | 0 | 20 | 100 | 300–500 |
| Slice 1, dorsal part | ||||
| pAMPKα | 100.0 ± 2.6 | 100.3 ± 15.8 | 116.0 ± 22.5 | 100.3 ± 12.8 |
| Total AMPKα | 100.0 ± 1.5 | 111.3 ± 3.1 | 124.3 ± 6.3 | 111.1 ± 8.6 |
| Slice 1, ventral part | ||||
| pAMPKα | 100.0 ± 5.1 | 115.4 ± 8.6 | 159.5 ± 19.1* | 191.8 ± 24.8† |
| Total AMPKα | 100.0 ± 1.4 | 101.6 ± 10.3 | 108.3 ± 6.5 | 106.2 ± 5.8 |
| Slice 2, dorsal part | ||||
| pAMPKα | 100.0 ± 4.2 | 87.3 ± 18.5 | 111.3 ± 8.7 | 120.0 ± 17.0 |
| Total AMPKα | 100.0 ± 7.3 | 104.6 ± 13.2 | 118.9 ± 7.5 | 107.8 ± 2.3 |
| Slice 2, ventral part | ||||
| pAMPKα | 100.0 ± 5.9 | 111.4 ± 8.1 | 143.9 ± 11.7* | 159.5 ± 17.4† |
| Total AMPKα | 100.0 ± 0.6 | 103.0 ± 2.3 | 110.0 ± 2.6 | 105.6 ± 5.2 |
Data are means ± SEM. Expression levels of pAMPKα and total AMPKα (both normalized to β-actin expression) quantified by Western blot from dissected hindbrain tissues collected 35–45 min after an injection of AICAR (0, 20, 100, or 300–500 nmol per rat in saline) into the fourth ventricle.
*P < 0.05;
†P < 0.01 vs. the control saline-injected rats (as 100%) by Fisher PLSD test after one-way ANOVA (n = 4 rats per group).
DISCUSSION
In the current study, we found that systemic 2DG treatment increased hindbrain AMPK phosphorylation at the Thr172 of the α-subunit detected by our antibody. pAMPKα expression was significantly elevated only in the A1–C1m region of the ventrolateral medulla but not in neighboring noncatecholaminergic VMM. Expression of pAMPKα also tended to increase in A2–C2, but there the increase was not statistically significant. The fact that pAMPKα expression levels were not increased by 2DG in the A1–C1m region in rats in which catecholamine neurons in this region were lesioned retrogradely by PVH DSAP injection suggests that the AMPK phosphorylation induced by 2DG in SAP controls occurs in A1–C1m catecholamine neurons. The fact that this same DSAP lesion also abolished the glucoprivic feeding response is consistent with a functional role for AMPK in mediation of glucoprivic feeding.
AMPK is a universal cellular energy sensor, present in both peripheral tissues and throughout the brain. Therefore, one question that immediately arises from our results is why increased pAMPK induced by systemic glucoprivation was apparent only in the A1–C1m tissue punch. One would expect that ubiquitous reduction in glucose utilization after systemic 2DG treatment would activate AMPK in many neurons. However, based on Fos expression as an indicator of activation, relatively few neurons are activated by glucoprivation. Furthermore, increased Fos in many other brain areas is reduced or abolished when hindbrain catecholamine neurons are destroyed by DSAP (2). Hence, it is clear that A1–C1 neurons are more responsive to glucoprivation than other brain neurons, including those that are downstream in the glucoregulatory circuitry. Therefore, a potential answer to this question is that catecholamine neurons in the A1–C1m region are more sensitive to a glucoprivic challenge than most neurons. This possibility is consistent with previous reports showing that A1–C1m neurons are more sensitive than most other catecholamine neurons to glucoprivation-induced increases in expression of c-Fos, Npy, and Dbh mRNA (2,12,13). These results suggest that AMPK may be phosphorylated at a higher glucose level in these catecholamine neurons than in many other neurons in part because their activity is increased, as opposed to reduced or not altered, by a glucose deficit, causing them to generate an ATP deficit more rapidly.
The above question is important in considering whether the pAMPK is a consequence of increased neuronal activity during glucoprivation or whether this signal is a component of the glucoreceptive transduction mechanism. The effects of CC on glucoprivic feeding suggest that the elevated pAMPK may be related more to activation of the neurons than to the transduction mechanism for sensing glucoprivation. CC-induced attenuation of 2DG-induced feeding had a transient effect, and rats completely compensated for the brief reduction of food intake during the ensuing hours of the feeding test. If AMPK phosphorylation represented an adequate stimulus for detecting glucoprivation, one might have expected a reversal or attenuation of feeding that was uncompensated. Thus, it appears as if the CC did not block a transduction process or reduce glucoprivic signal strength but rather reduced the rate of translation to a behavioral response, possibly by reducing the cellular metabolic rate of the essential catecholamine neurons. Likewise, a fourth-ventricle injection of AICAR stimulated feeding only during the first 60 min after injection. The brevity of these effects may be attributable to differing pharmacokinetics of the 2DG, CC, and AICAR. However, this explanation does not account for the fact that 2DG injection produces long-lasting effects on feeding but produced a relatively transient activation of pAMPKα expression in the A1–C1m region. Indeed, it is known that the feeding response and increased activation of hindbrain catecholamine neurons (as measured by hypothalamic norepinephrine turnover) persists even after the return of normoglycemia, >6 h after 2DG- or insulin-induced hypoglycemia, if nutrients are withheld during the glucoprivic episode (33–36). Additional work will be required to determine how AMPK phosphorylation and its downstream effects in A1–C1m and other neurons are related to the stimulation and time course of glucoprivic feeding.
Although the results of pharmacological AMPK manipulations do not provide strong support for a role in transduction of the glucoprivic signal, several experimental observations have linked AMPK activation with transcriptional changes appropriate to increasing food intake. First, overexpression of the dominant-negative AMPKα subunits in the ventromedial hypothalamus suppresses food intake, reduces body weight, and decreases expression of the orexigenic neuropetide genes Npy and Agrp in the arcuate nucleus, whereas expression of constitutively active AMPKα subunits in that region increases feeding, body weight, and enhances the fasting-induced increase of Npy and Agrp in the arcuate nucleus (23). Injection of AICAR into the lateral ventricle enhances Npy expression in the hypothalamus, as shown by Northern blot (37). Our previous work is consistent with the possibility that Npy gene expression also is a target of AMPK in the hindbrain. Studies have shown that Dbh and Npy are colocalized in A1–C1 catecholamine neurons (15,32) and are both required for glucoprivic feeding (2,3,12–14,38). Glucoprivation enhances expression of these genes in A1–C1 neurons (12,13). Simultaneous silencing Npy and Dbh by injection of small-interfering mRNA into the A1–C1 region blocks feeding in response to systemic glucoprivation but fails to block feeding when either of these genes is blocked separately (16). Npy in A1–C1 catecholamine neurons would be a feasible target of AMPK because it appears to be in the hypothalamus.
In the current study, we found a significant enhancement of pAMPKα by 2DG in the ventral (including A1, A1/C1 overlap, and C1m), but not in dorsal, hindbrain micropunches (including A2, C2, and the NTS). The neurons in the ventral hindbrain regions also were more sensitive to AMPK activation because AICAR injection into the fourth ventricle increased pAMPKα expression in the ventral but not the dorsal part of the hindbrain. In contrast, Hayes et al. (39) have reported an enhancement of pAMPKα in rat NTS after 24–48 h of food deprivation. They did not report results from ventral hindbrain samples. However, the fact that their dorsal hindbrain results differ from ours deserves comment. First, the differences may be attributed to the differing treatments used in these two studies. Food deprivation for 24–48 h does not necessarily produce glucoprivation. Food deprivation over this period alters nutrient and gastrointestinal signals known to act on the vagus nerve and/or the NTS (40) and are a likely stimulus for AMPK activation, whereas the major responses to glucoprivation occur by direct effects of glucoprivation within the brain. In addition, differences between these studies may be attributed to tissue sampling. Our dorsal hindbrain tissue punches included the entire extent of the NTS in order to sample both the A2 and C2 cell groups. Hayes et al. (39) found, however, that the caudal NTS at the level of the area postrema was sensitive to CC exposure, whereas the rostral NTS was not. If this differential response also occurs during glucoprivation, then our sampling method may have diluted the pAMPK signal to a nonsignificant level. In support of this, we found that pAMPKα expression was increased by ~30% in the A2–C2 micropunches at both 45 min and 2 h after the 2DG treatment.
Basal levels of pAMPKα in DSAP rats were slightly enhanced in the A1–C1m compared with SAP rats. The mechanisms underlying this apparent slight enhancement and the cell types contributing to this increase were unclear in the current study. One possibility is that activated glia cells, which persist for prolonged periods after neuronal destruction, were present in the A1–C1 area as a correlate of catecholamine neuron degeneration and may have contributed to enhancement of basal AMPK phosphorylation levels. Regardless of the cell type responsible, it is clear from the current study that this enhanced basal pAMPKα did not enhance, and therefore presumably was not related to, glucoprivic feeding.
In summary, we found that 2DG-induced glucoprivation activated pAMPKα in the hindbrain region containing A1–C1m catecholamine neurons. This activation was site specific (it did not occur in adjacent noncatecholaminergic tissue). Both 2DG-induced glucoprivic feeding and the enhancement of pAMPKα in the A1–C1m were completely abolished by retrograde DSAP lesion of A1–C1m neurons, suggesting a major contribution of these neurons to the pAMPK enhancement. Activation of AMPK by fourth ventricular AICAR induced a brief feeding response, and preinhibition of hindbrain AMPK activity by CC briefly impaired the feeding response to 2DG. These results suggest that AMPK may contribute to the functions of A1–C1 catecholamine neurons during glucoprivation and may indicate a role for AMPK as a glucosensory transduction mechanism for the initiation of systemic glucoregulatory responses.
ACKNOWLEDGMENTS
This work was supported by Public Health Service Grants DK040498 and DK00345072 (to S.R.).
No potential conflicts of interest relevant to this article were reported.
A.-J.L. and S.R. researched data, contributed to discussion, and wrote and reviewed the manuscript. Q.W. researched data and contributed to discussion.
REFERENCES
- 1.Brown J. Effects of 2-deoxyglucose on carbohydrate metablism: review of the literature and studies in the rat. Metabolism 1962;11:1098–1112 [PubMed] [Google Scholar]
- 2.Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol 2001;432:197–216 [DOI] [PubMed] [Google Scholar]
- 3.Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology 2003;144:1357–1367 [DOI] [PubMed] [Google Scholar]
- 4.I’Anson H, Sundling LA, Roland SM, Ritter S. Immunotoxic destruction of distinct catecholaminergic neuron populations disrupts the reproductive response to glucoprivation in female rats. Endocrinology 2003;144:4325–4331 [DOI] [PubMed] [Google Scholar]
- 5.Fraley GS, Dinh TT, Ritter S. Immunotoxic catecholamine lesions attenuate 2DG-induced increase of AGRP mRNA. Peptides 2002;23:1093–1099 [DOI] [PubMed] [Google Scholar]
- 6.Madden CJ, Stocker SD, Sved AF. Attenuation of homeostatic responses to hypotension and glucoprivation after destruction of catecholaminergic rostral ventrolateral medulla neurons. Am J Physiol Regul Integr Comp Physiol 2006;291:R751–R759 [DOI] [PubMed] [Google Scholar]
- 7.Flynn FW, Grill HJ. Insulin elicits ingestion in decerebrate rats. Science 1983;221:188–190 [DOI] [PubMed] [Google Scholar]
- 8.Darling RA, Ritter S. 2-Deoxy-D-glucose, but not mercaptoacetate, increases food intake in decerebrate rats. Am J Physiol Regul Integr Comp Physiol 2009;297:R382–R386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 1981;213:451–452 [DOI] [PubMed] [Google Scholar]
- 10.Ritter S, Dinh TT, Zhang Y. Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res 2000;856:37–47 [DOI] [PubMed] [Google Scholar]
- 11.Andrew SF, Dinh TT, Ritter S. Localized glucoprivation of hindbrain sites elicits corticosterone and glucagon secretion. Am J Physiol Regul Integr Comp Physiol 2007;292:R1792–R1798 [DOI] [PubMed] [Google Scholar]
- 12.Li AJ, Ritter S. Glucoprivation increases expression of neuropeptide Y mRNA in hindbrain neurons that innervate the hypothalamus. Eur J Neurosci 2004;19:2147–2154 [DOI] [PubMed] [Google Scholar]
- 13.Li AJ, Wang Q, Ritter S. Differential responsiveness of dopamine-beta-hydroxylase gene expression to glucoprivation in different catecholamine cell groups. Endocrinology 2006;147:3428–3434 [DOI] [PubMed] [Google Scholar]
- 14.Ritter S, Llewellyn-Smith I, Dinh TT. Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-D-glucose induced metabolic challenge. Brain Res 1998;805:41–54 [DOI] [PubMed] [Google Scholar]
- 15.Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol 1985;241:138–153 [DOI] [PubMed] [Google Scholar]
- 16.Li AJ, Wang Q, Dinh TT, Ritter S. Simultaneous silencing of Npy and Dbh expression in hindbrain A1/C1 catecholamine cells suppresses glucoprivic feeding. J Neurosci 2009;29:280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem 1998;67:821–855 [DOI] [PubMed] [Google Scholar]
- 18.Kim MS, Lee KU. Role of hypothalamic 5′-AMP-activated protein kinase in the regulation of food intake and energy homeostasis. J Mol Med 2005;83:514–520 [DOI] [PubMed] [Google Scholar]
- 19.Ramamurthy S, Ronnett GV. Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. J Physiol 2006;574:85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol 2006;574:73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem 1999;72:1707–1716 [DOI] [PubMed] [Google Scholar]
- 22.Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci 2001;17:45–58 [DOI] [PubMed] [Google Scholar]
- 23.Minokoshi Y, Alquier T, Furukawa N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004;428:569–574 [DOI] [PubMed] [Google Scholar]
- 24.Andersson U, Filipsson K, Abbott CR, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 2004;279:12005–12008 [DOI] [PubMed] [Google Scholar]
- 25.Kim MS, Park JY, Namkoong C, et al. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med 2004;10:727–733 [DOI] [PubMed] [Google Scholar]
- 26.Han SM, Namkoong C, Jang PG, et al. Hypothalamic AMP-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia 2005;48:2170–2178 [DOI] [PubMed] [Google Scholar]
- 27.Alquier T, Kawashima J, Tsuji Y, Kahn BB. Role of hypothalamic adenosine 5′-monophosphate-activated protein kinase in the impaired counterregulatory response induced by repetitive neuroglucopenia. Endocrinology 2007;148:1367–1375 [DOI] [PubMed] [Google Scholar]
- 28.Lee K, Li B, Xi X, Suh Y, Martin RJ. Role of neuronal energy status in the regulation of adenosine 5′-monophosphate-activated protein kinase, orexigenic neuropeptides expression, and feeding behavior. Endocrinology 2005;146:3–10 [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA, Academic Press, 1998 [Google Scholar]
- 30.Li AJ, Suzuki M, Suzuki S, Ikemoto M, Imamura T. Differential phosphorylation at serine sites in glutamate receptor-1 within neonatal rat hippocampus. Neurosci Lett 2003;341:41–44 [DOI] [PubMed] [Google Scholar]
- 31.Li AJ, Ritter S. Functional expression of neuropeptide Y receptors in human neuroblastoma cells. Regul Pept 2005;129:119–124 [DOI] [PubMed] [Google Scholar]
- 32.Everitt BJ, Hökfelt T, Terenius L, Tatemoto K, Mutt V, Goldstein M. Differential co-existence of neuropeptide Y (NPY)-like immunoreactivity with catecholamines in the central nervous system of the rat. Neuroscience 1984;11:443–462 [DOI] [PubMed] [Google Scholar]
- 33.Bellin SI, Ritter S. Disparate effects of infused nutrients on delayed glucoprivic feeding and hypothalamic norepinephrine turnover. J Neurosci 1981;1:1347–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellin SI, Ritter S. Insulin-induced elevation of hypothalamic norepinephrine turnover persists after glucorestoration unless feeding occurs. Brain Res 1981;217:327–337 [DOI] [PubMed] [Google Scholar]
- 35.Granneman J, Friedman MI. Feeding after recovery from 2-deoxyglucose injection: cerebral and peripheral factors. Am J Physiol 1983;244:R383–R388 [DOI] [PubMed] [Google Scholar]
- 36.Ritter RC, Roelke M, Neville M. Glucoprivic feeding behavior in absence of other signs of glucoprivation. Am J Physiol 1978;234:E617–E621 [DOI] [PubMed] [Google Scholar]
- 37.Kim EK, Miller I, Aja S, et al. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem 2004;279:19970–19976 [DOI] [PubMed] [Google Scholar]
- 38.Hudson B, Ritter S. Hindbrain catecholamine neurons mediate consummatory responses to glucoprivation. Physiol Behav 2004;82:241–250 [DOI] [PubMed] [Google Scholar]
- 39.Hayes MR, Skibicka KP, Bence KK, Grill HJ. Dorsal hindbrain 5′-adenosine monophosphate-activated protein kinase as an intracellular mediator of energy balance. Endocrinology 2009;150:2175–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ritter RC. Gastrointestinal mechanisms of satiation for food. Physiol Behav 2004;81:249–273 [DOI] [PubMed] [Google Scholar]