Abstract
OBJECTIVE
It is recognized that there is a remarkable variability in the systemic response to high-fat (HF) diets that cannot be completely explained by genetic factors. In addition, pregnancy complications leading to intrauterine growth restriction (IUGR) have been associated with an increased risk of developing metabolic syndrome (MetS) later in life. Thus, we hypothesized that offspring born with IUGR exhibit permanent metabolic changes that make them more susceptible to HF diet–induced MetS.
RESEARCH DESIGN AND METHODS
SD rats born normal (control) or with hypoxia-induced IUGR were randomized to low-fat (10% fat) or HF (45% fat) diets. After 9 weeks of feeding, physiological and molecular pathways involved in the MetS were evaluated.
RESULTS
IUGR offspring exhibited decreased energy intake and physical activity relative to controls. In offspring fed a HF diet, IUGR was associated with decreased total body fat content, a relative increase in intra-abdominal fat deposition and adipocyte size, an increase in fasting plasma concentrations of leptin, triglyceride and free fatty acids, and an increased concentration of triglycerides and ceramides in both liver and skeletal muscle. These changes in lipid homeostasis were accompanied by in vivo insulin resistance and impaired glucose tolerance and associated with increased phosphorylation of protein kinase C θ, inhibition of insulin receptor substrate 1, and a decreased activation of protein kinase B (PKB; also known as Akt) in liver and skeletal muscle in response to insulin.
CONCLUSIONS
IUGR enhances specific deleterious metabolic responses to a HF diet. Our results suggest that offspring born with IUGR may require special attention and follow-up to prevent the early onset of MetS.
The proportion of young adults with obesity has increased dramatically in recent decades. Moreover, several obesity-related pathologies are reaching record prevalence among youth and are resulting in substantial costs for the health care system (1). Although most elements of metabolic syndrome (MetS) have a complex pathophysiology, characterized by both a strong influence of inherited factors (2) and environmental variables (3), it is clear that behaviors such as decreased physical activity and consuming high-fat (HF) and hypercaloric Western diets also play a major role in their pathophysiology (4). Interestingly, there is a tremendous variability in the responses of different individuals to similar environmental, nutritional, and behavioral conditions (5) that have primarily been attributed to genetic differences (6). However, a growing body of evidence suggests that adverse environmental conditions during crucial periods of development may also predispose individuals to exhibit different components of the MetS in adulthood (7).
Intrauterine growth restriction (IUGR) is a condition in which the fetus does not reach its growth potential for a given gestational age (8). IUGR constitutes one of the most common pathological conditions diagnosed during pregnancy, affecting over 15% of all pregnancies in the U.S. (9). Beyond the well-described association between IUGR and higher rates of perinatal morbidity and mortality, being born with IUGR has also been associated with the development of most components of MetS in adulthood (10). Although the etiology of IUGR is considered multifactorial, in most instances, fetal growth is constrained by a limitation of oxygen and nutrient delivery (11). A substantial body of epidemiological and basic fundamental evidence supporting the effect of prenatal environment on the future development of obesity and other components of the MetS are based on epidemiological observations made in subjects exposed to nutritional restriction during pregnancy (12–15). Given the limitations of studying the developmental origins of MetS with clinical or epidemiological approaches, several animal models using a variety of prenatal nutritional insults (such as global food restriction, protein restriction, micronutrient restriction, and excess fat feeding) have been used (14–17). Interestingly, a recent study, in which IUGR was induced in rats by surgical ligation of the uterine arteries, demonstrated that prenatal reduction in the uterine blood supply caused insulin resistance and hyperleptinemia in offspring at 8 weeks of age (18). However, the impact of fetal hypoxia specifically on the future susceptibility to develop metabolic alterations is still unknown. This prenatal insult is relevant because many conditions that are associated with fetal hypoxia, such as placental insufficiency, preeclampsia, maternal anemia, asthma, chronic obstructive pulmonary disease, and smoking, are highly prevalent in Western societies, are strongly associated with IUGR (19), and could have fundamentally different programming mechanisms and effects.
Using a well-characterized experimental murine model of hypoxia-induced IUGR that mimics complications commonly observed during human pregnancy, we previously showed that adult IUGR offspring born from a hypoxic environment have impaired vascular function (20,21), have increased cardiac susceptibility to ischemia/reperfusion injury (22), and develop left ventricular hypertrophy (only in males) and diastolic dysfunction (both in males and females) as the rats age (20,23). Taken together, these results suggest that aging itself constitutes a stressor that affects offspring born with IUGR with higher severity (23). Moreover, during a pilot study using the hypoxia-induced IUGR model, we observed that during aging, male IUGR offspring tended to accumulate more pericardial and intra-abdominal fat relative to sex- and age-matched controls. To study potential long-term effects of prenatal fetal hypoxia, the objectives of this study were to investigate whether hypoxia-induced IUGR increases susceptibility to obesity, dyslipidemia, and insulin resistance in young adult rats exposed to a HF diet and to examine signal transduction pathways that may contribute to the long-term metabolic effects of being born from pregnancies complicated with IUGR.
RESEARCH DESIGN AND METHODS
Hypoxia-induced IUGR model.
Female SD rats were obtained at 3 months of age (Charles River, Quebec, Canada), acclimatized, and then mated within the animal facility. Throughout pregnancy, rats were given ad libitum access to water and standard laboratory murine food (LabDiet Ref. 5001; 3.02 kcal/mg, 23% protein, 4.5% fat, 6% fiber). On day 15 of pregnancy, rats were randomized to continue in normal environmental conditions (controls, n = 6) or placed inside a chamber continuously infused with nitrogen to maintain an oxygen concentration of 11.5% during the last 6 days of pregnancy (IUGR, n = 6) (20,23,24). As previously described (22), dams exposed to hypoxic environments exhibit a decrease in food intake (∼40%); however, they also exhibit a substantial decrease in physical activity. At the time of birth, litters were reduced to eight male pups, which were then weaned at 3 weeks of age and housed two per cage. All procedures in this study were approved by the University of Alberta Animal Welfare Committee.
Diet-induced obesity and MetS.
After weaning, male offspring from each experimental group (controls, n = 36; IUGR, n = 36) were randomly allocated to receive either a low-fat (LF) diet (10% fat, Research Diets D12450B) or a HF diet (45% fat, Research Diets D12451) as we and other groups (25,26) have previously described (detailed diet compositions are given in Supplementary Table 1). Therefore, four different experimental groups were created: control offspring receiving LF diet (n = 18 from six litters), control offspring receiving HF diet (n = 18 from six litters), IUGR offspring receiving LF diet (n = 18 from six litters), and IUGR offspring receiving HF diet (n = 18 from six litters). Rats were then randomly assigned to three possible experimental subgroups (designated A, B, or C) that determined the measurements performed on these animals. Because all rats included in each experimental group belonged to a different litter, we used the offspring as the unit of analysis. Rats were given ad libitum access to either diet. Nine weeks after weaning, experimental analyses were performed. The length of the nutritional intervention was selected based on previous studies in rodents that demonstrated the induction of several metabolic changes using similar nutritional insults (27).
Food intake, body weight, and body composition analyses.
After weaning, food intake and body weight were measured for all animals twice per week. After 9 weeks of nutritional intervention, body composition was determined in offspring from subgroup C using a Minispec whole-body composition analyzer (Burker Minispec LF90 II, Hamilton, Ontario, Canada).
Physical activity, oxygen consumption, and respiratory exchange ratio (subgroup A).
Oxygen consumption (Vo2), CO2 production (Vco2), and heat production were obtained by indirect calorimetry using the Comprehensive Laboratory Animal Monitoring System (Metabolic cage; Oxymax/CLAMS; Columbus Instruments, Columbus, OH). Physical activity was monitored by dual-axis detection (X, Z) using infrared photocell technology. Total physical activity was calculated by adding Z counts (rearing) to total counts associated with ambulatory movement and typical behavior.
Determination of circulating factors (subgroups B and C).
After a fasting period of 3 h, rats were anesthetized using inhaled isoflourane and killed by exsanguination. Blood samples were collected and serum was then separated by centrifugation and stored at −80°C. Circulating concentrations of insulin were determined using a radioimmunoassay kit for murine insulin (Cat. No. EZRMI-13K; Linco, St. Charles, MO). The lowest detectable level of insulin using this assay was 0.2 ng/mL. The intra-assay variability was <10%. The homeostasis model assessment (HOMA) index was determined as previously described (28). Millipore’s Biomarker Services performed a multiple adipokine determination using an Adipokine Panel 5-plex (Milliplex System, Millipore, St. Charles, MO) on a Luminex 100 machine (Millipore). The reported minimal detectable concentrations for this technique are 9.7 pg/mL leptin, 6.1 pg/mL adiponectin, 1.2 pg/mL interleukin (IL)-1β, pg/mL IL-6 8.8, and 3.2 pg/mL tumor necrosis factor (TNF)-α. The reported intra-assay variability for these determinations was <4%. All measurements were performed in duplicate in one single assay.
Pancreas insulin content (subgroup C).
After a fasting period of 3 h, rats were anesthetized as described above. The pancreas was surgically extracted, weighed, and minced in 1.0 mL acidified ethanol (75% ethanol, 1.5% 12 mmol/L HCl, and 23.5% H2O) and incubated for 24 h at 4°C to extract insulin from the tissue as previously described (29). Insulin was measured using a radioimmunoassay kit for murine insulin (Cat. No. EZRMI-13K).
Intra-abdominal fat content (subgroup C).
Intra-abdominal fat pads (mesenteric-epiploic, epididymal, retroperitoneal, and subdiaphragmatic) were surgically extracted and weighed. The left tibia was excised and measured.
Adipocyte morphometry (subgroup C).
Samples of abdominal adipose tissue from the major momentum were collected and fixed in 10% formalin. Histopathological preparations and hematoxylin/eosin staining were performed at the Alberta Diabetes Institute Histology Core (Edmonton, Canada). Under light microscopy, the internal diameters of 10 consecutive adipocytes from 10 randomly selected fields on each slide were measured using a digital micrometer under 40× magnification and averaged.
Glucose and insulin tolerance tests (subgroup B).
In a set of animals in which no other determinations/experiments were performed and after a 5-h fast, rats were injected intraperitoneally with a 50% glucose solution (2 g/kg) for the glucose tolerance test. Glucose plasma concentrations were determined using an ACCU-CHEK Advantage glucose meter (Roche Diagnostics) using blood collected from the tail in fasting conditions and after glucose injection (at 15, 30, 60, 90, and 120 min). For the insulin tolerance test, human recombinant insulin (Novolin) was used to prepare an insulin-saline solution that was injected intraperitoneally (1 mU/kg) after a 2-h fast. As described above, blood glucose from the tail was measured at baseline and after insulin injection (at 15, 30, 60, 90, and 120 min).
Determination of liver, muscle, and plasma free fatty acids; triacylglycerol; cholesterol; and cholesterol esters (subgroup B).
Plasma from nonfasted rats or from rats after a 16-h fast was collected in the presence of EDTA and immediately stored on ice to inhibit lipase activity without the use of chemical inhibition. Lipids were extracted from 200 μL plasma by the method of Folch et al. (30). Triacylglycerol, cholesterol, cholesterol ester, and free fatty acids were separated by fast protein liquid chromatography as previously described (31).
Liver and skeletal muscle tissues from nonfasted rats or from rats after a 16-h fast were homogenized, and the amount of triacylglycerol, cholesterol ester, cholesterol, and ceramides was determined by fast protein liquid chromatography as previously described (32).
Tissue homogenization and immunoblotting (subgroup B).
Rats were fasted overnight, injected with insulin (1 mU/g), and 15 min later killed as described above. Tissues were collected and frozen in liquid nitrogen and homogenated. Subsequently, frozen tissue homogenates were prepared in ice-cold sucrose homogenation buffer as previously described (32). All primary antibodies used in this study were purchased either from Cell Signaling Technology or Santa Cruz Biotechnology.
Statistical analysis.
Data are presented as mean ± SEM. Comparisons between two groups were evaluated using an unpaired t test or a Mann-Whitney test depending on the data distribution. Differences in measurements performed among four groups were analyzed using two-way ANOVA and a Bonferroni post hoc test with both diet and IUGR as sources of variation. P < 0.05 was considered statistically significant.
RESULTS
Birth characteristics, body weight gain, energy intake, and physical activity.
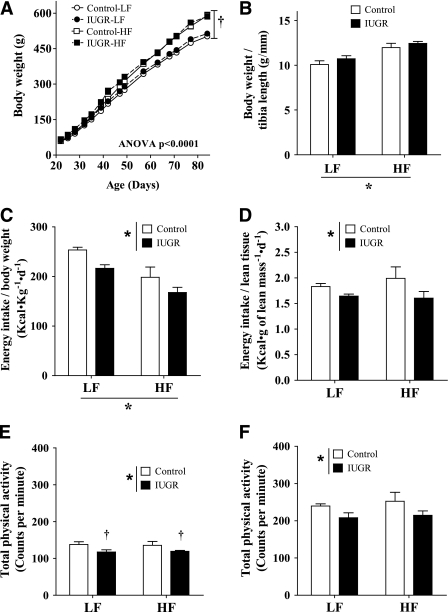
Consistent with our previous findings (20,23), the murine model of prenatal exposure to hypoxia caused a significant decrease in birth weight (control 7.06 ± 0.15 g vs. IUGR 6.46 ± 0.1 g, P < 0.01). Using our historical records including over 100 control litters, we calculated that the average birth weight of IUGR offspring was below the 15th percentile of sex-matched controls. However, the prenatal hypoxic insult had no effect on litter size (P = 0.8) or sex distribution (P = 0.6). At weaning and the start of our feeding protocol, IUGR pups had comparable weights to control pups (control 59 ± 0.5 g vs. IUGR 60.3 ± 1.1 g, P = 0.2). As expected, after 9 weeks of feeding, offspring on the HF diet gained more weight than those on the LF diet (Fig. 1A). Exposure to prenatal hypoxia had no effect on body weight gain (Fig. 1A) or body weight/tibia length ratio of the offspring (Fig. 1B). Interestingly, despite similar body weights, food intake was decreased in IUGR offspring independently of the diet received after 9 weeks of feeding (Fig. 1C and D). However, at this time, physical activity was modestly but significantly reduced in IUGR offspring independent of the diet rodents received (Fig. 1E and F). Together, these two findings could account for the lack of differences in weight gain compared with controls despite decreased energy intake.
FIG. 1.
Effect of IUGR and diet on change in body weight over time (A), body weight measured under anesthesia adjusted by tibia length (B), absolute energy intake adjusted by body weight (C), absolute energy intake adjusted by body lean tissue weight calculated by Minispec (D), and total physical activity during the light (E) and dark (F) cycles. LF diet: 10% fat, 3.85 kcal/g; HF diet: 45% fat, 4.73 kcal/g. *P values next to each label represent the significance in the effect for each source of variation (diet or IUGR) as calculated by ANOVA. †P < 0.05 in Bonferroni post hoc test comparing IUGR and control offspring receiving the same diet (n = 6 by group).
Additional assessments of physical activity and energy consumption were performed after 4 weeks of nutritional intervention; however, at this point, no statistically significant differences were observed (Supplementary Table 2).
Rats receiving HF diet had a significant decrease in the respiratory exchange ratio, indicative of greater fatty acid use for energy; however, differences were not associated with IUGR when compared with controls receiving the same diet (Supplementary Table 3). To further investigate the potential interaction between diet, IUGR, physical activity, and energy metabolism, we also measured the expression of peroxisome proliferator–activated receptor γ coactivator 1-α (PGC1-α) and 5′ AMP-activated protein kinase (AMPK) in skeletal muscle. However, no differences were observed among the groups (Supplementary Fig. 1).
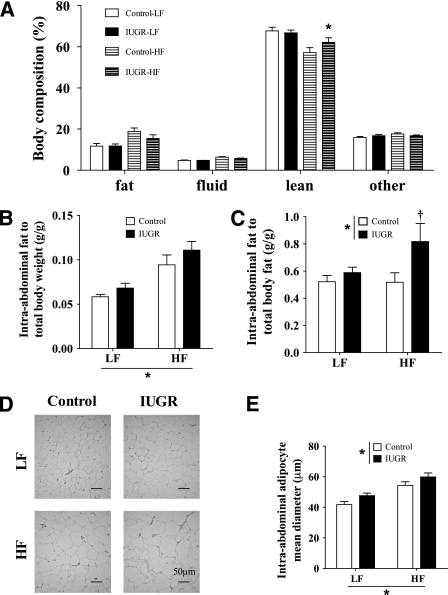
Effects of IUGR and HF diet on body composition and fat distribution.
As predicted, both control and IUGR offspring that received the HF diet had a higher proportion of total body fat mass relative to those receiving LF diet (Fig. 2A). In the LF diet group, we did not observe differences in body composition between IUGR and control offspring. Interestingly, the proportion of total body fat in the HF diet group was lower in IUGR offspring with a corresponding increase in the proportion of total lean body mass relative to controls (Fig. 2A). Despite the reduction in the proportion of body fat, IUGR offspring exhibited an increased intra-abdominal body fat (Fig. 2B and C) and a significant increase in the intra-abdominal adipocyte diameter (Fig. 2D and E).
FIG. 2.
Effect of IUGR and diet on body composition after 9 weeks of diet determined by Minispec (A), total intra-abdominal fat adjusted by body weight (B), and total fat weight estimated by Minispec (C). Representative pictures of omental fat tissue (magnification 40×) (D) and average intra-abdominal adipocyte diameter (E). LF diet: 10% fat, 3.85 kcal/g; HF diet: 45% fat, 4.73 kcal/g. *P values next to each label represent the significance in the effect for each source of variation (diet or IUGR) as calculated by ANOVA. †P < 0.05 in Bonferroni post hoc test comparing IUGR and control offspring receiving the same diet (n = 6 by group).
Effects of IUGR and HF on circulating adipokines and inflammatory markers.
Corresponding to the increased fat mass in the HF-fed groups, plasma concentrations of leptin were elevated in both control and IUGR offspring compared with their respective LF-fed controls (Table 1). Moreover, the increase in plasma leptin was more substantial in IUGR offspring fed a HF diet (1.42-fold) compared with HF-fed controls (Table 1). This finding is consistent with previous studies showing that intra-abdominal fat may be the most important source of adipokines and cytokines associated with metabolic comorbidities (33). Interestingly, no differences in circulating concentrations of adiponectin, IL-1β, and IL-6 were observed among the experimental groups (Table 1). Plasma concentrations of TNF-α were below the detectable range in most animals (data not shown).
TABLE 1.
Circulating concentrations of glucose and adipokine in control and IUGR rats fed LF and HF diets
| LF diet |
HF diet |
Two-way ANOVA |
|||||
|---|---|---|---|---|---|---|---|
| Control | IUGR | Control | IUGR | IUGR | Diet | Int | |
| Glucose (mmol/L) |
5.1 ± 0.24 |
5.2 ± 0.18 |
5.4 ± 0.17 |
5.6 ± 0.24 |
|||
| Insulin (mIU/L) |
48.1 ± 5.4 |
42.0 ± 4.9 |
59.6 ± 6.5 |
90.5 ± 7.3† |
* |
* |
* |
| HOMA index |
11.0 ± 1.2 |
10.4 ± 1.4 |
14.0 ± 1.2 |
22.7 ± 2.3† |
* |
* |
* |
| Leptin (pg/mL) |
4.03 ± 0.41 |
4.36 ± 0.65 |
9.10 ± 1.3 |
12.9 ± 1.2† |
* |
* |
|
| Adiponectin (μg/mL) |
2.74 ± 0.22 |
2.90 ± 0.17 |
3.06 ± 0.18 |
3.10 ± 0.17 |
|||
| IL-1β (pg/mL) |
145.0 ± 43.9 |
112.7 ± 47.2 |
230.8 ± 45.9 |
181.6 ± 56.2 |
|||
| IL-6 (pg/mL) | 46.2 ± 7.5 | 47.2 ± 7.8 | 47.7 ± 6.2 | 35.2 ± 8.6 | |||
Data are means ± SEM. Int, interaction.
*P < 0.05 for the respective sources of variation (IUGR, diet, or their interaction) using two-way ANOVA.
†P < 0.05 vs. controls on the same diet after a Bonferroni post hoc test (n = 6 for glucose and n = 10 for hormone and adipokine determinations).
Effects of IUGR and HF diet on circulating and tissue lipids.
As expected, HF diet increased plasma and tissue lipid concentrations in both control and IUGR rats (Table 2). However, IUGR rats displayed more severe hyperlipidemia than control rats. In addition, HF-fed IUGR rats also exhibited higher concentrations of triacylglycerol and ceramides in their livers and gastrocnemius muscles than HF-fed control offspring (Table 2).
TABLE 2.
Circulating and tissue lipid concentrations of control and IUGR rats fed LF and HF diets
| LF diet |
HF diet |
Two-way ANOVA |
|||||
|---|---|---|---|---|---|---|---|
| Control | IUGR | Control | IUGR | IUGR | Diet | Int | |
| Plasma |
|||||||
| Triglycerides (mg/dL) |
86.7 ± 18.4 |
86.7 ± 14.6 |
196.1 ± 25.2 |
298.7 ± 31.7† |
* |
* |
|
| Cholesterol esters (mg/dL) |
48.4 ± 8.9 |
55.7 ± 3.9 |
71.6 ± 4.2 |
72.9 ± 3.1 |
* |
||
| Cholesterol (mg/dL) |
29.0 ± 2.6 |
30.0 ± 1.2 |
41.5 ± 4.2 |
43.6 ± 5.6 |
* |
||
| Free fatty acid (mmol/L) |
0.53 ± 0.01 |
0.51 ± 0.01 |
0.60 ± 0.01 |
0.69 ± 0.02† |
* |
* |
* |
| Liver |
|||||||
| Triglycerides (μg/mg protein) |
47.7 ± 14.4 |
154.6 ± 26.3 |
158.2 ± 20.0* |
249.0 ± 32.1† |
* |
* |
|
| Cholesterol esters (μg/mg protein) |
80.8 ± 10.2 |
86.7 ± 2.3 |
86.2 ± 4.6 |
84.1 ± 6.3 |
|||
| Cholesterol (μg/mg protein) |
22.5 ± 3.3 |
27.0 ± 2.4 |
20.0 ± 2.0 |
27.1 ± 2.1 |
* |
||
| Ceramides (pmol/g protein) |
274.9 ± 14.3 |
271.9 ± 16.1 |
334.5 ± 21.6 |
394.4 ± 16.3† |
* |
||
| Gastrocnemius muscle |
|||||||
| Triglycerides (μg/mg protein) |
15.1 ± 3.6 |
29.5 ± 7.1 |
97.4 ± 18.7 |
132.2 ± 33.1† |
* |
* |
* |
| Cholesterol esters (μg/mg protein) |
22.0 ± 3.3 |
26.1 ± 6.7 |
39.1 ± 7.6 |
45.3 ± 10.4 |
* |
||
| Cholesterol (μg/mg protein) |
4.4 ± 0.4 |
6.98 ± 2.7 |
8.9 ± 0.5 |
8.1 ± 1.3 |
|||
| Ceramides (pmol/g protein) | 60.5 ± 4.3 | 62.2 ± 3.1 | 68.1 ± 4.6 | 86.5 ± 6.0† | * | * | |
Data are means ± SEM. Int, interaction.
*P < 0.05 for the respective sources of variation (IUGR, diet, or their interaction) using two-way ANOVA.
†P < 0.05 vs. controls on the same diet after a Bonferroni post hoc test (n = 4–6 by group).
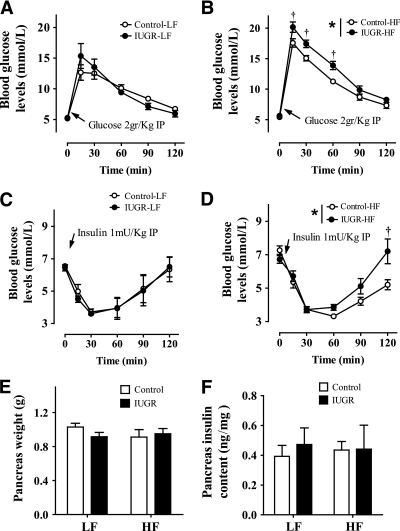
Effects of IUGR and HF diet on glucose homeostasis.
Although 9 weeks of HF diet did not result in fasting hyperglycemia in any of the experimental groups, offspring receiving a HF diet had higher fasted plasma insulin concentrations and HOMA indexes than rats fed a LF diet (Table 1). Consistent with the normal fasting plasma glucose and insulin concentrations, whole-body glucose disposal and insulin tolerance were similar in both groups of rats receiving a LF diet (Fig. 3A and C). In contrast, HF diet reduced glucose tolerance and impaired the response to insulin in both groups of rats relative to those fed a LF diet. However, it is interesting to note that impaired glucose disposal and insulin sensitivity were significantly more pronounced in rat offspring born with IUGR and receiving HF diet than in controls under similar dietary conditions (Fig. 3B and D).
FIG. 3.
Effect of IUGR on glucose tolerance test (A and B) and insulin tolerance test (C and D) in offspring fed a LF diet (10% fat, 3.85 kcal/g) or HF diet (45% fat, 4.73 kcal/g). Pancreas weight (E) and pancreas insulin content (F) are shown. *P values next to each label represent the significance in the effect for each source of variation (diet or IUGR) as calculated by ANOVA. †P < 0.05 in Bonferroni post hoc test comparing IUGR and control offspring receiving the same diet (n = 6 by group).
Although β-cell insulin content can play a role in whole-body glucose homeostasis, it is unlikely that this pathway contributed substantially in the IUGR model, since differences in the pancreas weight and insulin content were not observed among the groups (Fig. 3E and F).
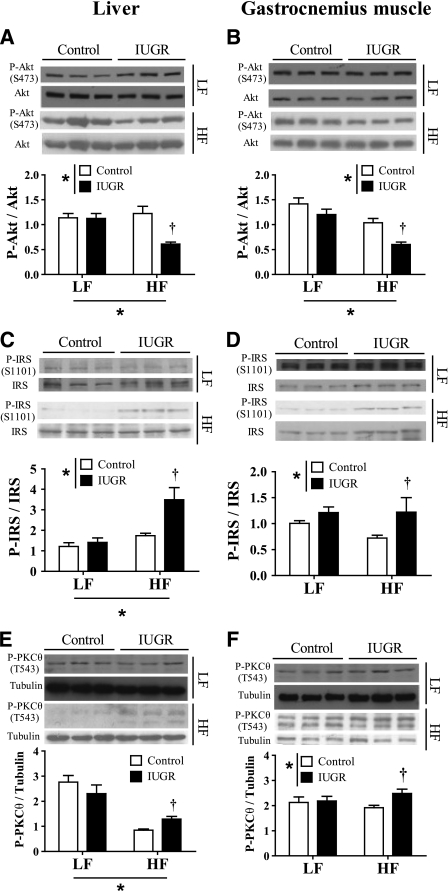
Effects of IUGR and HF diet on insulin signaling.
Although the precise signaling pathways involved in the induction of insulin resistance are incompletely understood, the major effects appear to be mediated through dysregulation of the insulin-signaling cascade (34). In agreement with the interpretation of data from glucose tolerance test and insulin tolerance test experiments, feeding a HF diet to IUGR rats but not to control rats impaired insulin-stimulated Akt phosphorylation at Ser473 (activation site) in both liver and gastrocnemius muscle (Fig. 4A and B). To further characterize the source of the signaling defect, we measured phosphorylation of upstream regulators of Akt. Because phosphorylation of insulin receptor substrate 1 (IRS-1) at Ser1101 inhibits the ability of IRS-1 to activate phosphatidylinositol 3-kinase and subsequently Akt, we measured phosphorylation at this site. Consistent with our hypothesis, phosphorylation of IRS-1 at Ser1101 in both liver and skeletal muscle was significantly higher in IUGR rats compared with control rats only when receiving HF diet (Fig. 4C and D).
FIG. 4.
Effect of IUGR and either LF or HF diets on hepatic and skeletal muscle insulin-signaling elements including phosphorylation of Akt (ratio P-Akt/Akt) (A and B), phosphorylation of IRS-1 (ratio P-IRS/IRS) (C and D), and phosphorylation of PKCθ (P-PKCθ/tubulin) (E and F). *P < 0.05 for the respective sources of variation (IUGR or diet) using two-way ANOVA. †P < 0.05 vs. controls on the same diet after a Bonferroni post hoc test (n = 6 by group).
Because phosphorylation of IRS-1 at Ser1101 was known to be directly caused by protein kinase C θ (PKCθ) (35), we measured the phosphorylation of PKCθ at Thr538, which is a requirement for PKCθ activation (36). We observed that liver and muscle levels of phosphorylated PKCθ (P-PKCθ) were increased in HF-fed IUGR rats compared with HF-fed control rats, but remained unaltered in the LF diet groups (Fig. 4E and F).
DISCUSSION
This is the first study showing that a hypoxic fetal insult resulting in IUGR can affect the postnatal response to a HF diet. Specifically, we demonstrated that hypoxia-induced IUGR increases the susceptibility to HF diet–induced alterations of fat distribution, lipid metabolism, and insulin-signaling pathways. The data presented herein provide four important findings. These include 1) IUGR significantly influences fat deposition, with HF-fed IUGR rats having significantly elevated amounts of intra-abdominal fat and larger adipocyte diameters than control rats in the absence of changes in whole-body adipose tissue mass; 2) IUGR results in a significant increase in plasma and tissue lipid concentrations in response to a HF diet compared with HF-fed controls; 3) IUGR contributes to an increased susceptibility to HF diet–induced insulin resistance by altering skeletal muscle and liver insulin-signaling pathways; and 4) IUGR results in decreased physical activity relative to control rats independent of diet.
Collectively, our findings highlight the importance of IUGR in the development of MetS and provide considerable insight into why subjects in the Western world born with IUGR may require closer clinical monitoring and could obtain greater benefit from nutritional interventions designed to reduce the prevalence of cardiovascular risk factors. The important clinical implications of these findings are discussed below.
IUGR, HF diet, fat distribution, and lipid metabolism.
Contrary to the results reported by other groups using nutritional restriction to induce IUGR (16), offspring exposed to hypoxia did not exhibit an exacerbated weight gain after birth. Moreover, feeding a HF diet to offspring born with IUGR did not cause rats to gain more weight or increase total body fat content during the early stages of their lifespan. However, relative to all other groups, rats born growth restricted exhibited a notable increase in the proportion of fat located in the intra-abdominal cavity and had a larger adipocyte diameter when fed a HF diet. Interestingly, these changes in fat distribution and morphology were associated with a significant impairment in lipid profile and glucose homeostasis, which suggests that special attention should be paid to the distribution of fat rather than body weight or total body fat content when evaluating cardiovascular and metabolic risks. Most efforts toward understanding the regulation of adipogenesis and adipose differentiation have been made ex vivo using cultures of preadipocytes (37). Based on previous studies, it was suggested that adipocyte differentiation is a complex process that can be modulated by multiple stimuli including transcription factors and hormonal signals (38). Although little is known about the mechanisms that determine adipose tissue distribution or the differential development of fat deposits (39), our data highlight the potential involvement of prenatal growth as one of the regulators of this process.
Previous studies in rats also demonstrated that, at the time of weaning, the majority of body fat is located in subcutaneous deposits, but as rats age (2 months later), the majority of body fat is located in the abdominal cavity (40). Although subcutaneous fat weight increases 6-fold between weaning and adulthood, intra-abdominal fat increases 116-fold during the same period of time (40). Because intra-abdominal fat has such a dramatic rate of increase during postnatal stages, it is possible that the catch-up growth of IUGR pups after their birth may contribute to the greater accumulation of abdominal fat and favor a later-stage metabolic dysfunction in IUGR rats. In addition, IUGR rats exposed to a hypercaloric HF diet (but not a LF diet) developed high circulating concentrations of triacylglycerol and free fatty acids, as well as an accumulation of triacylglycerols and ceramides in the liver and skeletal muscles (41). These observations demonstrate that the synergistic effects of both IUGR and diet are associated with an early onset of dyslipidemia in this model.
Effects of IUGR and HF diet on feeding behavior and physical activity.
Previous studies that evaluated the association between IUGR and the development of obesity concluded that exposure to prenatal insults that lead to IUGR and early postnatal catch-up growth produce permanent changes in neuro-endocrine appetite regulation (42). Interestingly, our study also describes an increase in circulating concentrations of leptin and reduced calorie intake in offspring born IUGR. These results suggested that, in our IUGR model, at 12 weeks of age, the appetite regulatory system and the central effects of leptin were at least partially preserved. Despite the reduced consumption of food, abdominal adiposity was still increased in HF-fed IUGR rats, together with elevated tissue and circulating levels of lipids.
Another interesting finding was that IUGR offspring had a modest decrease in physical activity relative to controls independent of the diet they were receiving. The reduced physical activity was associated with decreased oxygen consumption (Vo2). Because energy consumption was also diminished in IUGR rats, the development of intra-abdominal adiposity and other metabolic changes cannot be entirely attributed to reduced energy expenditure. However, it is well known that exercise can improve dyslipidemia and glucose tolerance (43), and it is plausible that decreased physical activity could be associated with accumulation of lipids in the tissues and impaired glucose homeostasis in IUGR offspring fed a HF diet. Although increased adiposity and reduced physical activity in adult IUGR rats may be perceived as a pathological response to the hypoxic insult, it is also reasonable to consider that these adaptations could represent compensatory mechanisms programmed during fetal growth. These compensatory mechanisms may be an adaptation aimed to prepare the developing organism to survive in an oxygen-depleted environment after birth by increasing the ability to accumulate energy and reduce energy demand through decreased physical activity. Consistent with this observation, it has been reported that teenagers born with extremely low birth weight participate much less in sports and are more sedentary than normal birth weight teenagers, despite reporting equal enjoyment of physical activity (44). Whether or not the compensatory adaptation to a growth-restricting environment could also determine the site of adipose tissue deposition is currently unknown.
IUGR, HF diet, and impaired glucose metabolism.
Insulin resistance has previously been associated with both the consumption of a HF diet (45) and being born with IUGR (46). However, in the current study, we clearly identified an interaction between these two factors by showing that IUGR increases the severity of the hyperinsulinemia and insulin resistance that resulted from the challenge of a HF diet. We also show that the changes in glucose homeostasis cannot be attributed to long-term effects of a hypoxic insult on pancreatic development, since pancreas size and pancreatic insulin content were comparable among all experimental groups. In the absence of changes in pancreatic insulin content, the increase in circulating levels of insulin could be attributed to an increase in the secretary activity of the pancreatic β-cells. Moreover, the data presented herein identified defects in the insulin-signaling cascade in relevant organs that play pivotal roles in the development of insulin resistance, such as liver and skeletal muscle. As such, these data suggest that nutritional interventions that decrease the consumption of foods high in fat and sucrose, combined with increased physical activity, could be even more beneficial in preventing or ameliorating the detrimental long-term effects of IUGR on lipid and glucose homeostasis.
Considering that inflammation has been identified as a potential mediator of obesity-induced insulin resistance (47), it is interesting that no differences were observed in inflammatory markers among the groups. Because these parameters were measured early in life after a short nutritional intervention (9 weeks of diet), it is possible that a proinflammatory phenotype had not yet been established in these animals. Interestingly, changes in insulin homeostasis were notable at this point, which suggests that the major synergistic effects of IUGR and HF diet on glucose homeostasis may not be mediated via inflammatory pathways.
One limitation of our hypoxia-induced IUGR murine model is that the hypoxic insult affects not only the fetuses, but also the dam; therefore, the potential interaction between fetal and maternal responses to hypoxia should also be considered. Despite these potential confounding variables, the hypoxia-induced IUGR murine model used in this study was extensively characterized (20,22,23,48) and is the only noninvasive technique available to induce isobaric fetal hypoxia in rodents.
In conclusion, our study provides strong evidence to support the programming effects of hypoxia-induced IUGR on metabolic function by causing a change in the adipose tissue response to a HF diet and increased the severity of diet-induced adipocyte dysfunction and insulin resistance. Our results suggest that prenatal insults causing IUGR could play a fundamental role in determining the long-term systemic response to HF diets. Indeed, our results suggest that early metabolic programming could contribute to the observed variability in the response to nutritional interventions and could further the development of cardiovascular and metabolic diseases later in life, particularly in environments where HF diets are prevalent (49).
ACKNOWLEDGMENTS
This work was supported by a research grant from the Women and Children’s Health Research Institute and the Canadian Institutes of Health Research and a personnel award from the Heart and Stroke Foundation of Canada (H&S).
C.F.R.-C., V.W.D., and J.S.M. were supported by H&S and the Alberta Heritage Foundation for Medical Research (AHFMR). C.F.R.-C. is a fellow of the Strategic Training Program in Maternal, Fetal, and Newborn Health and the Tomorrow’s Research Cardiovascular Health Professionals. S.D.P. is a H&S New Investigator. J.R.B.D. is an AHFMR Senior Scholar and a Canada Research Chair in Molecular Biology of Heart Disease and Metabolism. S.T.D. is an AHFMR Scientist and a Canada Research Chair in Women’s Cardiovascular Health.
No potential conflicts of interests relevant to this article were reported.
C.F.R.-C. and V.W.D. developed the model, performed the experiments, analyzed data, contributed to discussion, and wrote the manuscript. J.S.M. developed the model, contributed to discussion, and reviewed and edited the manuscript. S.D.P. reviewed and edited the manuscript and contributed to discussion. J.R.B.D. and S.T.D. designed the experiments, contributed to discussion, and wrote the manuscript.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1239/-/DC1.
REFERENCES
- 1.Renna F, Thakur N. Direct and indirect effects of obesity on U.S. labor market outcomes of older working age adults. Soc Sci Med 2010;71:405–413 [DOI] [PubMed] [Google Scholar]
- 2.Wardle J, Carnell S, Haworth CM, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr 2008;87:398–404 [DOI] [PubMed] [Google Scholar]
- 3.Hawkins SS, Cole TJ, Law C, Millennium Cohort Study Child Health Group An ecological systems approach to examining risk factors for early childhood overweight: findings from the UK Millennium Cohort Study. J Epidemiol Community Health 2009;63:147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y, Ma G, Zhai F, et al. Dietary patterns and glucose tolerance abnormalities in Chinese adults. Diabetes Care 2009;32:1972–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boutcher SH, Dunn SL. Factors that may impede the weight loss response to exercise-based interventions. Obes Rev 2009;10:671–680 [DOI] [PubMed] [Google Scholar]
- 6.Vaag A, Jensen CB, Poulsen P, et al. Metabolic aspects of insulin resistance in individuals born small for gestational age. Horm Res 2006;65(Suppl. 3):137–143 [DOI] [PubMed] [Google Scholar]
- 7.Shankar K, Kang P, Harrell A, et al. Maternal overweight programs insulin and adiponectin signaling in the offspring. Endocrinology 2010;151:2577–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scifres CM, Nelson DM. Intrauterine growth restriction, human placental development and trophoblast cell death. J Physiol 2009;587:3453–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ananth CV, Vintzileos AM. Distinguishing pathological from constitutional small for gestational age births in population-based studies. Early Hum Dev 2009;85:653–658 [DOI] [PubMed] [Google Scholar]
- 10.Bhuiyan AR, Chen W, Srinivasan SR, Azevedo MJ, Berenson GS. Relationship of low birth weight to pulsatile arterial function in asymptomatic younger adults: the Bogalusa Heart Study. Am J Hypertens 2010;23:168–173 [DOI] [PubMed] [Google Scholar]
- 11.Marsál K. Intrauterine growth restriction. Curr Opin Obstet Gynecol 2002;14:127–135 [DOI] [PubMed] [Google Scholar]
- 12.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 1976;295:349–353 [DOI] [PubMed] [Google Scholar]
- 13.Ross MG, Desai M. Gestational programming: population survival effects of drought and famine during pregnancy. Am J Physiol Regul Integr Comp Physiol 2005;288:R25–R33 [DOI] [PubMed] [Google Scholar]
- 14.Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol 2005;288:R91–R96 [DOI] [PubMed] [Google Scholar]
- 15.Vickers MH. Developmental programming and adult obesity: the role of leptin. Curr Opin Endocrinol Diabetes Obes 2007;14:17–22 [DOI] [PubMed] [Google Scholar]
- 16.Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol 2007;92:287–298 [DOI] [PubMed] [Google Scholar]
- 17.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem 1995;270:17513–17520 [DOI] [PubMed] [Google Scholar]
- 18.Heltemes A, Gingery A, Soldner EL, et al. Chronic placental ischemia alters amniotic fluid milieu and results in impaired glucose tolerance, insulin resistance and hyperleptinemia in young rats. Exp Biol Med (Maywood) 2010;235:892–899 [DOI] [PubMed] [Google Scholar]
- 19.Cogswell ME, Yip R. The influence of fetal and maternal factors on the distribution of birthweight. Semin Perinatol 1995;19:222–240 [DOI] [PubMed] [Google Scholar]
- 20.Morton JS, Rueda-Clausen CF, Davidge ST. Mechanisms of endothelium-dependent vasodilation in male and female, young and aged offspring born growth restricted. Am J Physiol Regul Integr Comp Physiol 2010;298:R930–R938 [DOI] [PubMed] [Google Scholar]
- 21.Williams SJ, Hemmings DG, Mitchell JM, McMillen IC, Davidge ST. Effects of maternal hypoxia or nutrient restriction during pregnancy on endothelial function in adult male rat offspring. J Physiol 2005;565:125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Williams SJ, O’Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J 2006;20:1251–1253 [DOI] [PubMed] [Google Scholar]
- 23.Rueda-Clausen CF, Morton JS, Davidge ST. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res 2009;81:713–722 [DOI] [PubMed] [Google Scholar]
- 24.Williams SJ, Campbell ME, McMillen IC, Davidge ST. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol 2005;288:R360–R367 [DOI] [PubMed] [Google Scholar]
- 25.Ussher JR, Koves TR, Jaswal JS, et al. Insulin-stimulated cardiac glucose oxidation is increased in high-fat diet-induced obese mice lacking malonyl CoA decarboxylase. Diabetes 2009;58:1766–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Haschimi K, Pierroz DD, Hileman SM, Bjørbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 2000;105:1827–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buettner R, Schölmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 2007;15:798–808 [DOI] [PubMed] [Google Scholar]
- 28.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 29.Suarez-Pinzon WL, Power RF, Yan Y, Wasserfall C, Atkinson M, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes 2008;57:3281–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 31.Christie WW. Rapid separation and quantification of lipid classes by high performance liquid chromatography and mass (light-scattering) detection. J Lipid Res 1985;26:507–512 [PubMed] [Google Scholar]
- 32.Koonen DP, Jacobs RL, Febbraio M, et al. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes 2007;56:2863–2871 [DOI] [PubMed] [Google Scholar]
- 33.Esposito K, Giugliano G, Giugliano D. Metabolic effects of liposuction: yes or no? N Engl J Med 2004;351:1354–1357 [DOI] [PubMed] [Google Scholar]
- 34.Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol 2002;90:11G–18G [DOI] [PubMed] [Google Scholar]
- 35.Griffin ME, Marcucci MJ, Cline GW, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes 1999;48:1270–1274 [DOI] [PubMed] [Google Scholar]
- 36.Sparatore B, Passalacqua M, Pedrazzi M, et al. Role of the kinase activation loop on protein kinase C theta activity and intracellular localisation. FEBS Lett 2003;554:35–40 [DOI] [PubMed] [Google Scholar]
- 37.Mur C, Arribas M, Benito M, Valverde AM. Essential role of insulin-like growth factor I receptor in insulin-induced fetal brown adipocyte differentiation. Endocrinology 2003;144:581–593 [DOI] [PubMed] [Google Scholar]
- 38.Tremblay F, Gagnon A, Veilleux A, Sorisky A, Marette A. Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3-L1 and human adipocytes. Endocrinology 2005;146:1328–1337 [DOI] [PubMed] [Google Scholar]
- 39.Giorgino F, Laviola L, Eriksson JW. Regional differences of insulin action in adipose tissue: insights from in vivo and in vitro studies. Acta Physiol Scand 2005;183:13–30 [DOI] [PubMed] [Google Scholar]
- 40.Caluwaerts S, Lambin S, van Bree R, Peeters H, Vergote I, Verhaeghe J. Diet-induced obesity in gravid rats engenders early hyperadiposity in the offspring. Metabolism 2007;56:1431–1438 [DOI] [PubMed] [Google Scholar]
- 41.Jacob S, Machann J, Rett K, et al. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes 1999;48:1113–1119 [DOI] [PubMed] [Google Scholar]
- 42.Coupé B, Amarger V, Grit I, Benani A, Parnet P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology 2010;151:702–713 [DOI] [PubMed] [Google Scholar]
- 43.Tuomilehto J. Nonpharmacologic therapy and exercise in the prevention of type 2 diabetes. Diabetes Care 2009;32(Suppl. 2):S189–S193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitfield MF, Grunau RE. Teenagers born at extremely low birth weight. Paediatr Child Health (Oxford) 2006;11:275–277 [PMC free article] [PubMed] [Google Scholar]
- 45.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 1997;46:3–10 [PubMed] [Google Scholar]
- 46.Simmons RA. Developmental origins of diabetes: the role of epigenetic mechanisms. Curr Opin Endocrinol Diabetes Obes 2007;14:13–16 [DOI] [PubMed] [Google Scholar]
- 47.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87–91 [DOI] [PubMed] [Google Scholar]
- 48.Hemmings DG, Williams SJ, Davidge ST. Increased myogenic tone in 7-month-old adult male but not female offspring from rat dams exposed to hypoxia during pregnancy. Am J Physiol Heart Circ Physiol 2005;289:H674–H682 [DOI] [PubMed] [Google Scholar]
- 49.Benson L, Baer HJ, Kaelber DC. Trends in the diagnosis of overweight and obesity in children and adolescents: 1999–2007. Pediatrics 2009;123:e153–e158 [DOI] [PubMed] [Google Scholar]