Abstract
OBJECTIVE
Obesity is characterized by elevated levels of proinflammatory cytokines, including interleukin (IL)-1β, that contribute to the development of insulin resistance. In this study, we set out to investigate whether hyperglycemia drives IL-1β production and caspase-1 activation in murine and human adipose tissue, thus inducing insulin resistance.
RESEARCH DESIGN AND METHODS
ob/ob animals were used as a model to study obesity and hyperglycemia. Human adipose tissue fragments or adipocytes were cultured in medium containing normal or high glucose levels. Additionally, the role of thioredoxin interacting protein (TXNIP) in glucose-induced IL-1β production was assessed.
RESULTS
TXNIP and caspase-1 protein levels were more abundantly expressed in adipose tissue of hyperglycemic ob/ob animals as compared with wild-type mice. In human adipose tissue, high glucose resulted in a 10-fold upregulation of TXNIP gene expression levels (P < 0.01) and a 10% elevation of caspase-1 activity (P < 0.05), together with induction of IL-1β transcription (twofold, P < 0.01) and a significant increase in IL-1β secretion. TXNIP suppression in human adipocytes, either by a small interfering RNA approach or a peroxisome proliferator–activated receptor-γ agonist, counteracted the effects of high glucose on bioactive IL-1 production (P < 0.01) mainly through a decrease in transcription levels paralleled by reduced intracellular pro-IL-1β levels.
CONCLUSIONS
High glucose activates caspase-1 in human and murine adipose tissue. Glucose-induced activation of TXNIP mediates IL-1β mRNA expression levels and intracellular pro-IL-1β accumulation in adipose tissue. The concerted actions lead to enhanced secretion of IL-1β in adipose tissue that may contribute to the development of insulin resistance.
Obesity is associated with a low-grade systemic inflammation, with elevated levels of proinflammatory cytokines, such as interleukin (IL)-1β, tumor necrosis factor-α (TNF-α), and IL-6, that contribute to the development of insulin resistance and progression to type 2 diabetes mellitus (1,2). The mechanisms that trigger the development of inflammation are currently unknown, although the adipose tissue has been viewed upon as the instigator of these effects (3,4). Earlier studies have shown that high glucose promotes the production of acute phase reactants (5) and proinflammatory cytokines by adipocytes (6). In addition, several studies have shown that hyperglycemia induces the production of IL-1β in different cell types including endothelial cells, monocytes, β-cells, and other pancreatic islet cells (7–9). Although short time exposure to low concentrations of IL-1β enhances β-cell function, high glucose-induced islet IL-1β secretion is known to negatively interfere with insulin signaling and has cytotoxic effects on β-cells leading to impaired insulin secretion (8,10). IL-1β is produced via cleavage of pro-IL-1β by caspase-1, a cysteine protease (11) that is activated by a protein complex named the inflammasome (12). Until recently, the mechanism by which high glucose induces IL-1β remained largely unknown. However, in a recent study Zhou et al. demonstrated a crucial role for thioredoxin interacting protein (TXNIP or vitamin D3 upregulated protein 1 [VDUP1]) during high glucose-mediated caspase-1 activation in murine β-cells, by direct interaction with the nucleotide-binding oligomerization domain (NOD)-like receptor (NLR)-3 (NLRP3)-inflammasome (13). TXNIP is expressed in a wide variety of cell types including skeletal myocytes, pancreatic β-cells, endothelial cells, and adipocytes and acts as an endogenous inhibitor of the reactive oxygen species (ROS) scavenging protein thioredoxin (14,15). TXNIP levels are elevated in subjects with type 2 diabetes mellitus (15), and its expression is induced by glucose-6-phosphate through an intracellular transcriptional complex of MondoA and Max-like protein X (16).
Here we investigate whether high glucose levels also drive TXNIP and caspase-1 activation in human adipocytes and intact adipose tissue and whether this may contribute to the production of IL-1β.
RESEARCH DESIGN AND METHODS
Animal experiments.
Blood glucose levels of five 8-week-old male ob/ob C57/Bl6 and wild-type C57/Bl6 animals (Jackson Laboratory) were determined after 4 h of fasting. A glucose tolerance test and insulin tolerance test were performed in five 8-week-old male caspase-1−/− C57/Bl6 mice (average body wt: 23.6 g) and five age- and weight-matched wild-type C57/Bl6 animals. Epididymal white adipose tissue was used to analyze protein levels of caspase-1 and TXNIP.
In vitro and ex vivo experiments with human adipose tissue.
Intact adipose tissue fragments and cultured human preadipocytes from subcutaneous adipose tissue (n = 6), obtained during plastic surgery, and the SGBS cell line were used to study the effects of high glucose levels. The protocol was approved by the hospital ethics committee, and the tissue samples were collected after written informed consent. Adipose tissue fragments were directly cultured for 48 h in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum containing normal glucose levels (5 mM) or high glucose levels (25 mM). Preadipocytes were isolated from the adipose tissue according to the procedure described by Rodbell (17). Primary or SGBS preadipocytes were differentiated toward mature adipocytes using a standard adipogenic protocol (18). After 12 days of differentiation adipocytes were cultured under 5 or 25 mM glucose conditions. Mannose was added to cells cultured in the presence of 5 mM glucose.
RNA isolation and PCR analysis.
RNA was extracted from total adipose tissue or adipocytes using TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA synthesis was performed using the iScript cDNA Synthesis Kit (Bio–Rad Laboratories, Hercules, CA). Real-time PCR was done using Power-SYBR Green master mix and the 7300 Real-Time PCR system (Applied Biosystem, Warrington, U.K.). Expression of genes was normalized to β−2M or 36B4 gene expression levels. Primer sequences are available upon request.
Protein analysis.
Protein expression of TXNIP, caspase-1, β-actin, and GAPDH were measured by Western blotting. Antibodies were from Santa Cruz (caspase-1), Zymed (TXNIP), Abcam (NLRP3), Sigma-Aldrich (β-actin), and Calbiochem (GAPDH). Bioactive IL-1 secretion was quantified in a bioassay using the murine thymoma cell line EL4/NOB 1 that produces IL-2 in response to bioactive IL-1 (19). IL-2 and intracellular human pro-IL-1β levels were measured by Elisa (R&D). In short, medium collected from adipocytes or total adipose tissue cultured in the presence of 5 or 25 mM of glucose was added to NOB-1 cells. After 24 h of incubation, medium was used for IL-2 measurements. The specificity of the NOB-1 assay to produce IL-2 in response to IL-1β was confirmed in this study (Supplementary Fig. 1).
Caspase-1 activity assay.
Caspase-1 activity in adipocyte lysates was determined with a caspase-1 fluorometric kit (Biovision) following the cleavage of 50 μmol/L peptide YVAD-AFC. The fluorescence of the cleaved substrate was measured every 90 s using a fluorometer (Polarstar BMG, fluostar galaxy).
Small interfering RNA.
To specifically suppress TXNIP expression in differentiated adipocytes, cells were transfected (X-tremeGENE siRNA Transfection Reagent, Roche) with small interfering (si)RNA against TXNIP (Thermo Scientific). As a nonspecific control, scrambled siRNA (Thermo Scientific) was used.
Statistical analysis.
Variables are expressed as means ± SD. One-way ANOVA, the Wilcoxon rank test, and Student paired t test were used to analyze statistical significance. Two-tailed P < 0.05 was considered significant. All statistical analyses were performed using SPSS software (version 16.0; SPSS, Chicago, IL).
RESULTS
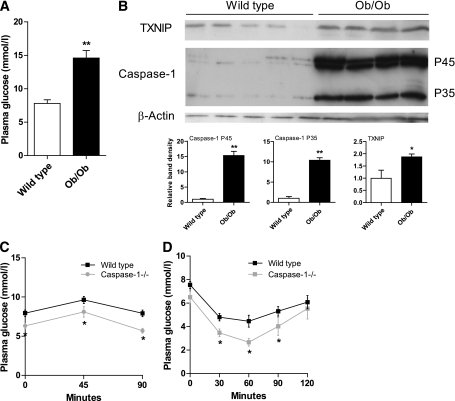
To determine whether hyperglycemic conditions induce caspase-1 and TXNIP in adipose tissue in vivo, we used the ob/ob animal model, which is characterized by obesity, insulin resistance, and hyperglycemia (Fig. 1A). Both the inactive procaspase-1 (p45) and the active caspase-1 (p35) protein levels were upregulated in ob/ob mice compared with the normoglycemic wild-type animals (Fig. 1B). In parallel with caspase-1 activation, TXNIP protein levels were elevated in adipose tissue of ob/ob animals. Interestingly, the absence of caspase-1 led to an improvement of glucose tolerance (Fig. 1C) and insulin sensitivity (Fig. 1D). Supported by the observation that TXNIP−/− animals are more insulin sensitive (20), these in vivo results strongly suggest a link between TXNIP and caspase-1 activation during hyperglycemic conditions in adipose tissue that contributes to the development of insulin resistance.
FIG. 1.
TXNIP and caspase-1 protein levels are increased in the adipose tissue of ob/ob mice. A: Plasma glucose levels in fasted wild-type mice and ob/ob mice (n = 5 per group). B: Western blot images and quantification of TXNIP, procaspase-1 (p45), and active caspase-1 (p35) protein levels in the epididymal adipose tissue of wild-type mice and ob/ob mice (n = 5 per group). C: A glucose tolerance test was done using fasted wild-type and caspase-1−/− animals (n = 5 animals per group). D: An insulin tolerance test was performed in fasted wild-type and caspase-1−/− animals (n = 5 animals per group). *P < 0.05; **P < 0.01 using a Student t test.
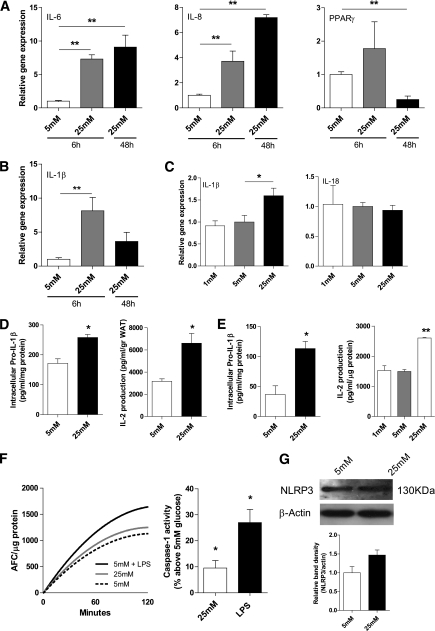
We next studied the effects of high glucose on caspase-1 activation and the production of IL-1β in human adipose tissue. Treatment of adipose tissue with 25 mM glucose increased the gene expression levels of proinflammatory mediators including IL-6 and IL-8 (P < 0.01) together with a significant fourfold reduction in PPAR-γ expression levels (Fig. 2A). Moreover, IL-1β gene expression levels were also elevated upon short- or long-term stimulation of adipose tissue and adipocytes with 25 mM of glucose (7.5-fold, P < 0.01; fourfold, P < 0.06, respectively), whereas IL-18 levels were unaffected (Fig. 2B and C). In line with activation of IL-1β transcription levels, both the intracellular content of pro-IL-1β and secretion of bioactive IL-1 were significantly elevated after exposure of adipocytes or intact adipose tissue to 25 mM of glucose (Fig. 2D and E). Because IL-1 production depends on caspase-1 activation, we determined caspase-1 activity levels in adipocytes treated with 5 or 25 mM of glucose. As shown in Fig. 2F, a significant increase in caspase-1 activity was observed in primary human adipocytes treated with 25 mM of glucose for 48 h, compared with adipocytes treated with 5 mM of glucose. In line with the enhanced caspase-1 activity, high glucose levels led to an increase in NLRP3 protein levels in primary human adipocytes (Fig. 2G). Although only a trend was observed (P = 0.07), our results imply that NLRP3 is one of the signaling molecules that translate high glucose levels into caspase-1 activation. Noticeably, similar results were obtained using human adipose tissue explants (data not shown). Overall, these results suggest that high glucose induces IL-1β transcription, activation of caspase-1, and secretion of IL-1 in human adipose tissue.
FIG. 2.
Hyperglycemia induces proinflammatory gene expression and results in an increased production of IL-1 by intact adipose tissue and adipocytes. A: IL-6, IL-8, and PPAR-γ gene expression levels in human intact adipose tissue (n = 3) after 6 or 48 h of glucose treatment. B: IL-1β gene expression levels in human adipose tissue (n = 3) treated with glucose for 6 or 48 h. C: IL-1β and IL-18 gene expression levels in human primary adipocytes (n = 3) treated with various concentrations of glucose. D and E: Intracellular pro-IL-1β levels measured in lysates from intact human adipose tissue (D) or human primary adipocytes (E) treated with 5 or 25 mM glucose for 48 h (n = 4) and IL-2 production from NOB-1 cells after exposure to medium from intact human subcutaneous adipose tissue of three different donors treated with 5 or 25 mM of glucose for 48 h. F: Caspase-1 activity assay in primary human adipocytes treated with 5 mM, 25 mM of glucose, or LPS (10 ng/mL) for 48 h. The left graph displays the results of one representative experiment. The right graph displays the average results of n = 6 experiments. G: NLRP3 protein expression levels in human primary adipocytes treated with 5 or 25 mM of glucose for 48 h. A representative Western blot and quantification of the results (n = 3) are shown. *P < 0.05; **P value < 0.01 using a one-way ANOVA test (A–C), Student t test (D and E), or a Wilcoxon rank test (F; right graph).
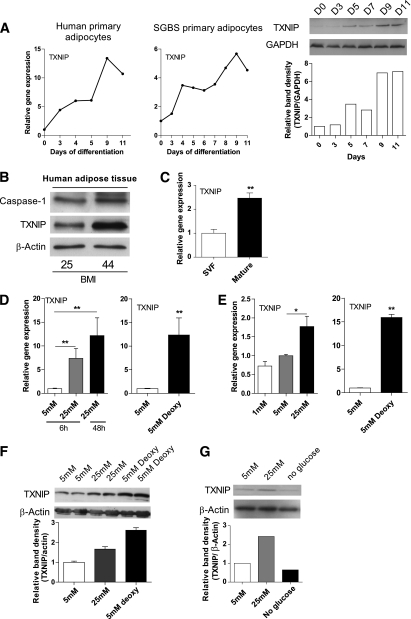
To assess whether high glucose induces TXNIP in human adipose tissue and adipocytes to a similar extent as observed in ob/ob animals, we first studied the regulation of TXNIP during the differentiation of human preadipocytes toward fully mature adipocytes. Whereas TXNIP is expressed in human preadipocytes, expression levels rapidly increased during differentiation toward mature adipocytes both at gene and protein expression levels (Fig. 3A).
FIG. 3.
TXNIP is present in human adipose tissue and adipocytes and is regulated by glucose. A: TXNIP gene and protein expression levels during adipocyte differentiation of human primary adipocytes or human SGBS adipocytes. B: Western blot images of caspase-1 and TXNIP protein levels in total human subcutaneous adipose tissue (n = 2). C: Relative gene expression levels of TXNIP in mature adipocytes or the SVF isolated from human adipose tissue (n = 3). D: Gene expression levels of TXNIP in intact adipose tissue treated with various concentrations of glucose (n = 3). E: Gene expression levels of TXNIP in human primary adipocytes treated with various concentrations of glucose (n = 3). F: Protein levels of TXNIP in human adipose tissue after 5 mM glucose, 25 mM glucose, or 5 mM deoxyglucose treatment for 48 h (n = 2). G: Protein levels of TXNIP after glucose starvation (no glucose), 5 mM glucose, and 25 mM glucose for 48 h in human primary adipocytes. *P < 0.05; **P < 0.01 using a one-way ANOVA test (D and E) or a Student t test (C).
In addition to caspase-1, TXNIP protein levels were detectable in total human subcutaneous adipose tissue and tended to increase in parallel with BMI (Fig. 3B). Because adipose tissue is composed of both adipocytes and nonadipocyte cells, including inflammatory cells, we determined gene expression levels in both fractions. Purity of the different fractions was confirmed with the markers adiponectin (adipocyte-specific) and F4/80+ (macrophage-specific) (Supplementary Fig. 2). Whereas TXNIP is expressed in the stromal vascular fraction (SVF), expression levels were 2.5 times higher in adipocytes (Fig. 3C). As shown in Fig. 3D and E, glucose was a potent regulator of TXNIP mRNA expression in both isolated adipocytes and adipose tissue explants. Furthermore, treatment of adipocytes with 5 mM 2-deoxyglucose leading to a chronic activation of the MondoA:Mlx, resulted in a more pronounced activation of TXNIP gene expression (Fig. 3D and E). In line with these transcriptional changes, protein levels of TXNIP varied in response to different glucose concentrations added to adipocytes or intact adipose tissue (Fig. 3F and G).
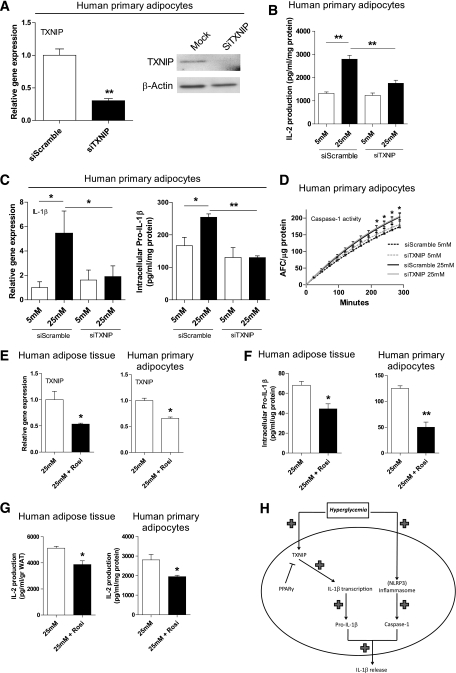
Because high glucose potently activates TXNIP expression in adipocytes (Fig. 3) and TXNIP has been shown to induce IL-1β secretion from mouse pancreatic islets cells in response to elevated glucose via activation of caspase-1 (13), we investigated whether TXNIP mediates high glucose-induced IL-1 production in human adipocytes using siRNA targeted against TXNIP. In adipocytes exposed to 25 mM of glucose, successful knockdown of TXNIP by siRNA (Fig. 4A) led to a significant reduction in bioactive IL-1 production toward basal IL-1 levels induced by 5 mM glucose (Fig. 4B). Additionally, IL-1β transcription and intracellular levels of pro-IL-1β were reduced upon siRNA-mediated depletion of TXNIP (Fig. 4C). Whereas high glucose treatment increased caspase-1 activity levels in adipocytes (Fig. 2F), siRNA-mediated knockdown of TXNIP had no effect on caspase-1 activation (Fig. 4D). Interestingly, reducing TXNIP gene expression with the PPAR-γ agonist Rosiglitazone in adipocytes and adipose tissue explants exposed to 25 mM glucose for 48 h (Fig. 4E) also led to a significant decrease in intracellular pro-IL-1β and bioactive IL-1 production (Fig. 4F and G). These results demonstrate that TXNIP contributes to high glucose-induced IL-1 release by modulating the transcription of IL-1β and the intracellular pool of pro-IL-1β.
FIG. 4.
TXNIP reduction results in a decline of high glucose-induced IL-1 production by modulating IL-1β gene expression. A: Gene and protein expression levels of TXNIP after siRNA treatment against TXNIP in human primary adipocytes (n = 6). B: IL-2 production from NOB-1 cells after exposure to medium from human primary adipocytes transfected with TXNIP siRNA and treated with 5 or 25 mM glucose for 48 h (n = 6). C: IL-1β mRNA expression and intracellular pro-IL-1β levels in TXNIP siRNA treated adipocytes exposed to 5 or 25 mM glucose for 48 h (n = 4). D: Caspase-1 activity assay in TXNIP siRNA treated human primary adipocytes exposed to 5 or 25 mM glucose for 48 h (n = 3). E: TXNIP gene expression levels in intact human adipose tissue and primary adipocytes exposed to 25 mM glucose for 48 h with or without Rosiglitazone (10 μM) treatment for 24 h (n = 3). F: Intracellular pro-IL-1β levels measured in lysates of intact human adipose tissue and primary adipocytes exposed to 25 mM glucose for 48 h with or without Rosiglitazone (10 μM) treatment for 10 h (n = 3). G: IL-2 production from NOB-1 cells after exposure to medium from intact adipose tissue and human primary adipocytes treated with 25 mM glucose for 48 h with or without Rosiglitazone (10 μM) stimulation for 10 h (n = 4). H: Role of TXNIP in hyperglycemia-induced release of IL-1β from adipose tissue. *P < 0.05; **P < 0.01 using a one-way ANOVA test (B and C) or a Student t test (D–G).
DISCUSSION
Several studies show that IL-1β deteriorates peripheral insulin sensitivity and inhibits insulin production by the pancreas (8,10). The notion that IL-1β is relevant in human (patho)physiology is supported by the finding that blocking of IL-1 signaling pathways in type 2 diabetes mellitus patients by treatment with IL-1 receptor antagonist Anakinra improves glycemic control (21). However, the precise mechanisms leading to an enhanced production of IL-1β in the inflamed adipose tissue over the course of development of insulin resistance have remained obscure. The importance of hyperglycemia-induced insulin resistance in type 2 diabetes mellitus patients is well accepted, although no current explanation is available for this process. The stimulatory role of hyperglycemia on IL-1β production has been, however, known for several years (7–9). The combination of this knowledge with the recent discovery of the important effect of IL-1β on the induction of insulin resistance (22) directed us to the hypothesis that hyperglycemia induces insulin resistance in adipose tissue through activation of caspase-1 and IL-1β secretion. Moreover, Zhou et al. have recently uncovered TXNIP as an essential mediator of hyperglycemia-induced caspase-1-dependent IL-1β production in the murine pancreatic β-cell (13), and we explored whether TXNIP could mediate a similar effect in human and murine adipocytes. Our data clearly show that adipose tissue-resident caspase-1 and TXNIP are activated in both hyperglycemic ob/ob animals and in human adipose tissue treated with 25 mM of glucose, resulting in an increased IL-1β production. Both the absence of caspase-1 and TXNIP (20) significantly improved insulin sensitivity and resulted in lower plasma glucose levels. Together, these findings strengthen the concept that aberrant IL-1β production via caspase-1 underlies the pathophysiology of type 2 diabetes mellitus in different cellular sources, linking this disease to auto-inflammatory syndromes.
The glucose-responsive TXNIP gene has been previously linked to diabetes and insulin resistance, because diabetic patients expressed consistently higher TXNIP mRNA gene levels in skeletal muscles (15), whereas animals lacking TXNIP displayed a hypoglycemic, hypoinsulinemic phenotype (23). Furthermore, it has been shown that PPAR-γ negatively regulates TXNIP expression in adipose tissue (24). In agreement, we showed that activation of PPAR-γ by its agonist Rosiglitazone led to reduced TXNIP levels together with a decline in IL-1β. The elucidation of the exact molecular mechanisms through which TXNIP contributes to enhanced IL-1β production by the adipose tissue will need further study. Whereas TXNIP has been shown to directly control caspase-1 activation in pancreatic β-cells (13), our results suggest that, at least in adipocytes, TXNIP mainly regulates IL-1β mRNA transcription levels and intracellular pro-IL-1β pools and does not directly affect caspase-1 activity levels. This discrepancy may largely be explained by cell-specific differences in adipocytes versus pancreatic β-cells. In line with our results, Masters et al. reported no changes in caspase-1 activation upon TXNIP depletion in macrophages. However, these authors also failed to detect differences in IL-1β production upon stimulation with ATP and uric crystals in wild-type versus TXNIP−/− macrophages (25). This could be explained by the constitutive large expression of TXNIP in macrophages that is not influenced by high levels of glucose. In contrast, TXNIP does play a role in adipocyte glucose homeostasis and elevated levels of glucose potently induce TXNIP expression in adipocytes (15).
Because excessive production of ROS has been found to increase IL-β activity and may contribute to the toxic effect of high glucose on β-cells (26), future studies will need to reveal a possible role of TXNIP-induced oxidative stress induced by high glucose in mediating the release of IL-1β by adipose tissue. In addition, it would be interesting to investigate whether other metabolic stress signals that are elevated during the development of insulin resistance bear the potential to induce caspase-1.
In conclusion, adipocyte-specific TXNIP upregulation induced by hyperglycemia may contribute to a sustained proinflammatory state and hyperglycemia-associated insulin resistance partly through inducing IL-1β transcription. Furthermore, elevated levels of glucose increased the activation of caspase-1 in adipocytes. Although more efforts are needed, the high glucose-induced increase in NLRP3 protein levels in primary human adipocytes suggests involvement of the NLRP3-inflammasome in mediating caspase-1 activation during hyperglycemic conditions. Therefore, we suggest that, at least in adipose tissue, both activation of TXNIP and caspase-1 during hyperglycemic conditions lead to an enhanced production of IL-1β (Fig. 4H). In this way, TXNIP links hyperglycemia to increased IL-1β production that may result in insulin resistance at the level of the adipose tissue. As a consequence, inhibition of TXNIP may represent a novel therapeutic target in the treatment of insulin resistance that will ameliorate adipose tissue functioning.
ACKNOWLEDGMENTS
M.G.N. was supported by a Vici grant of the Netherlands Organization for Scientific Research. R.S. was supported by a grant from the Dutch Diabetes Research Foundation.
No potential conflicts of interest relevant to this article were reported.
T.B.K. and R.S. researched data and wrote the article. L.J.v.T. wrote the article. J.d.G. and A.F.H.S. reviewed and wrote the article. L.A.B.J. planned the experiment and reviewed the article. C.J.T. and M.G.N. planned the experiment, contributed to discussion, and wrote the article.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0266/-/DC1.
REFERENCES
- 1.Alexandraki K, Piperi C, Kalofoutis C, Singh J, Alaveras A, Kalofoutis A. Inflammatory process in type 2 diabetes: the role of cytokines. Ann N Y Acad Sci 2006;1084:89–117 [DOI] [PubMed] [Google Scholar]
- 2.Katsuki A, Sumida Y, Murashima S, et al. Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1998;83:859–862 [DOI] [PubMed] [Google Scholar]
- 3.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 2008;118:2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007;132:2169–2180 [DOI] [PubMed] [Google Scholar]
- 5.Lin Y, Rajala MW, Berger JP, Moller DE, Barzilai N, Scherer PE. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J Biol Chem 2001;276:42077–42083 [DOI] [PubMed] [Google Scholar]
- 6.Lin Y, Berg AH, Iyengar P, et al. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem 2005;280:4617–4626 [DOI] [PubMed] [Google Scholar]
- 7.Asakawa H, Miyagawa J, Hanafusa T, Kuwajima M, Matsuzawa Y. High glucose and hyperosmolarity increase secretion of interleukin-1 beta in cultured human aortic endothelial cells. J Diabetes Complications 1997;11:176–179 [DOI] [PubMed] [Google Scholar]
- 8.Maedler K, Sergeev P, Ris F, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 2002;110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanmugam N, Reddy MA, Guha M, Natarajan R. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 2003;52:1256–1264 [DOI] [PubMed] [Google Scholar]
- 10.Maedler K, Størling J, Sturis J, et al. Glucose- and interleukin-1β-induced β-cell apoptosis requires Ca2+ influx and extracellular signal-regulated kinase (ERK) 1/2 activation and is prevented by a sulfonylurea receptor 1/inwardly rectifying K+ channel 6.2 (SUR/Kir6.2) selective potassium channel opener in human islets. Diabetes 2004;53:1706–1713 [DOI] [PubMed] [Google Scholar]
- 11.Wilson KP, Black JA, Thomson JA, et al. Structure and mechanism of interleukin-1 beta converting enzyme. Nature 1994;370:270–275 [DOI] [PubMed] [Google Scholar]
- 12.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009;10:241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 2010;11:136–140 [DOI] [PubMed] [Google Scholar]
- 14.Minn AH, Hafele C, Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology 2005;146:2397–2405 [DOI] [PubMed] [Google Scholar]
- 15.Parikh H, Carlsson E, Chutkow WA, et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 2007;4:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoltzman CA, Peterson CW, Breen KT, Muoio DM, Billin AN, Ayer DE. Glucose sensing by MondoA:Mlx complexes: a role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc Natl Acad Sci USA 2008;105:6912–6917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 1964;239:375–380 [PubMed] [Google Scholar]
- 18.Wabitsch M, Brenner RE, Melzner I, et al. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int J Obes Relat Metab Disord 2001;25:8–15 [DOI] [PubMed] [Google Scholar]
- 19.Netea MG, Kullberg BJ, Boerman OC, Verschueren I, Dinarello CA, Van der Meer JW. Soluble murine IL-1 receptor type I induces release of constitutive IL-1 alpha. J Immunol 1999;162:4876–4881 [PubMed] [Google Scholar]
- 20.Hui ST, Andres AM, Miller AK, et al. Txnip balances metabolic and growth signaling via PTEN disulfide reduction. Proc Natl Acad Sci USA 2008;105:3921–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–1526 [DOI] [PubMed] [Google Scholar]
- 22.Jager J, Grémeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 2007;148:241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chutkow WA, Patwari P, Yoshioka J, Lee RT. Thioredoxin-interacting protein (Txnip) is a critical regulator of hepatic glucose production. J Biol Chem 2008;283:2397–2406 [DOI] [PubMed] [Google Scholar]
- 24.Chutkow WA, Birkenfeld AL, Brown JD, et al. Deletion of the α-arrestin protein Txnip in mice promotes adiposity and adipogenesis while preserving insulin sensitivity. Diabetes 2010;59:1424–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masters SL, Dunne A, Subramanian SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol 2010;11:897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Saxena G, Mungrue IN, Lusis AJ, Shalev A. Thioredoxin-interacting protein: a critical link between glucose toxicity and β-cell apoptosis. Diabetes 2008;57:938–944 [DOI] [PMC free article] [PubMed] [Google Scholar]