Abstract
OBJECTIVE
Glucose-dependent insulinotropic polypeptide (GIP) is a member of a structurally related group of hormones that also includes glucagon, glucagon-like peptides, and secretin. GIP is an incretin, known to modulate glucose-induced insulin secretion. Recent studies have shown that glucagon is necessary for early insulin-positive differentiation, and a similar role for incretins in regulating embryonic insulin-positive differentiation seems probable. Here we studied the role of GIP signaling in insulin-positive differentiation in the embryonic mouse pancreas.
RESEARCH DESIGN AND METHODS
The ontogeny of the GIP ligand and GIP receptor in the embryonic pancreas was investigated by immunohistochemistry and RT-PCR. GIP signaling was inhibited in cultured embryonic pancreata using morpholine-ring antisense against GIP ligand and receptor, or small interfering RNA (siRNA) for GIP ligand and receptor. Markers of endocrine cells and their progenitors were studied by immunohistochemistry and RT-PCR.
RESULTS
GIP and GIP receptor mRNA were both detected in the embryonic pancreas by embryonic day 9.5 and then persisted throughout gestation. GIP was generally coexpressed with glucagon by immunostaining. The GIP receptor was typically coexpressed with insulin. Morpholine-ring antisense or siRNA against either GIP ligand or GIP receptor both inhibited the differentiation of insulin-positive cells. Inhibition of GIP or its receptor also led to a decrease in the number of Pdx-1–positive and sox9-positive cells in the cultured embryonic pancreas. The number of Pax6- and Nkx2.2-positive cells, representative of developing pancreatic endocrine cells and β-cells, respectively, was also decreased.
CONCLUSIONS
GIP signaling may play a role in early embryonic pancreas differentiation to form insulin-positive cells or β-cells.
Glucose-dependent insulinotropic polypeptide (GIP) is an incretin. Incretins are hormones released from the gut in response to nutrient ingestion that potentiate glucose-stimulated insulin secretion (1). GIP and glucagon-like peptide 1 (GLP-1) are the two main incretins, identified as mediators of the process by which administration of oral glucose provokes a greater stimulation of insulin release than does an intravenous glucose challenge (2). This connection between gut and the pancreatic islets has been termed the “enteroinsular axis” (3) and appears to be responsible for 50% of postprandial insulin release (2,4). GIP is released from enteroendocrine K-cells in the duodenum, primarily in response to the ingestion of glucose or fat, and potentiates insulin secretion in a glucose-dependent manner (5). A recent study now reports the presence of a bioactive form of GIP in the α-cells that promotes insulin secretion in the adult islet β-cells (6). GLP-1 is a proglucagon-derived peptide hormone that is synthesized and secreted by the enteroendocrine L-cells in the distal ileum and the colon. Preproglucagon can be alternatively processed to produce glicentin or oxyntomodulin and GLP-1 (7).
The incretin GLP-1 can enhance β-cell growth and differentiation. GLP-1 receptor null mice, however, do not show a loss of β-cell development. Interestingly, these mice were found to have upregulated GIP release and GIP-induced insulin release (8). On the other hand, the GIP receptor (GIPR) null mice, which also did not show any obvious β-cell defect, showed enhanced sensitivity to GLP-1, suggesting that there may be important cooperation between these two incretin signaling pathways (9).
We and others previously demonstrated that glucagon signaling to the glucagon receptor is necessary for early insulin-positive differentiation (10,11). This glucagon dependency also was suggested by the observation that Pax6 or prohormone convertase 2 null mice, which both lack glucagon-positive cells, also lack early insulin-positive differentiation (12,13). Although it was reported that GIP is produced in enteroendocrine K-cells in the duodenum of the adult, GIP was also found to be produced in the human fetal pancreas by 18 weeks’ gestation (14). The function of this GIP in the developing pancreas remains to be elucidated. Here we show that GIP is located in the embryonic mouse pancreas, and loss-of-function studies in vitro suggest that insulin differentiation in the embryonic pancreas depends on GIP signaling.
RESEARCH DESIGN AND METHODS
Isolation of embryonic pancreas
All animal experiments were performed in accordance with the guidelines for animal experimentation of the Children’s Hospital of Pittsburgh, Pennsylvania. Male and female CD-1 mice were mated overnight. The presence of a vaginal plug the next morning was indicative of pregnancy, and noon of that day was designated as embryonic day 0.5 (E0.5). Embryonic pancreata were harvested on various embryonic days from E9.5 to E18.5 using microdissection techniques as described previously (15).
Pancreas organ culture conditions
E11.5 pancreata with surrounding mesenchyme were grown using the hanging drop method by placing them in a standard 35-mm dish with Dulbecco’s modified Eagle’s medium F-12 (Gibco BRL, Grand Island, NY) containing 10% FBS and 1% antibiotic/antimycotic solution (10,000 units/mL penicillin G, 10,000 units/mL streptomycin sulfate, and 200 μg/mL amphotericin B [Gibco BRL]). Each drop contains 40 μl media. The media was changed every day. Tissue cultures were maintained in 5% CO2 at 37°C for 6 days.
Morpholine-ring antisense
The media containing 20 or 40 μmol/L Morpholino (Gene Tools, Philomath, OR) scrambled missense or antisense for GIP or GIPR were used with the hanging drop cultures (10). Antisense sequences to both GIP and GIPR were designed using a 20-bp sequence complimentary to a sequence spanning the GIP, GIPR, or preproglucagon adenine, thymine, guanine (ATG) start sequence (5- GGTCTTCATTTTTTATTCTGCCTTG-3′) or (5′-TTTATCACTGTTTGTCTTCTGCTTG-3′) (5′-GGTCT TCATTTTTTATTCTGCCTTG-3′), respectively. A 20-bp control oligo sequence for target genes was designed using randomized nucleotides. Control or antisense treatment was continued for 6 days, where media was replaced every day with fresh control or antisense-containing media. Tissues were cultured in 5% CO2 at 37°C for 6 days.
Small interfering RNA
RNA interference was performed with small interfering RNA (siRNA) (16). The siRNA oligonucleotides were synthesized and purified by Ambion, which also provided the scrambled negative control. E11.5 pancreata were transfected with 100 nmol/L siRNA in 1% FBS culture medium by using the SiPort reagent (Ambion, Austin, TX). After incubation for 24 h, the culture media were replaced with regular siRNA-free culture medium. Media were replaced every day, and pancreata were harvested, fixed, and processed for histology and immunohistochemistry after 6 days.
Bromodeoxyuridine proliferation assay
Day 11 embryonic pancreata were treated with control, GIP ligand, or GIPR antisense as described above; however, after 24 h, 10−5 mol/L bromodeoxyuridine (BrdU) was added to the culture. The harvested pancreata were fixed in 4% paraformaldehyde overnight at 4°C, embedded in OCT, and sectioned. The sections were treated with 2 N HCl for 15 min and then with 0.5% Pepsin in 0.1 N HCl for another 15 min at room temperature. To determine the proportion of proliferating cells, sections were stained with a BrdU-specific antibody that will bind to denatured and acid-degraded BrdU-labeled DNA (Dako, Carpentaria, CA). Cells positive for BrdU were counted in at least 10 sections obtained from a minimum of five pancreata in each group.
Immunohistochemistry
Tissues were fixed in 4% paraformaldehyde in PBS solution for 6 h. They were then transferred to 30% sucrose in PBS overnight and then embedded in Tissue Tek OCT compound (Sakura Finetek, Torrance, CA), frozen in liquid nitrogen, and cut into 6-μm sections using a Microm HM 550 cryostat (Richard-Allan Scientific, Kalamazoo, MI). Primary antibodies to the following antigens were used at the indicated dilution: insulin (guinea pig antiswine; Dako), 1:400; glucagon (rabbit antihuman; Dako), 1:400; α-amylase (rabbit antihuman; Sigma-Aldrich, St. Louis, MO), 1:400; Pdx-1 (Abcam, Cambridge, U.K.) 1:2,000; GIP (rabbit antihuman; catalog #T4110; Bachem, Bubendorf, Switzerland), 1:200; GIPR (rabbit antihuman; Novus Biologicals, Littleton, CO); Nkx2.2 (mouse antichick; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), 1:5; Pax6 (rabbit antimouse; Covance, Richmond, CA), 1:300; sox9 (Millipore, Billerica, MA), 1:1,000; and Caspase (Cell Signaling Technology, Danvers, MA), 1:200. Primary antibodies were incubated at room temperature for 60 min for insulin/glucagon/Pdx-1/α-amylase and overnight at 4°C for GIP and GIPR. Secondary antibodies, including fluorescein isothiocyanate–conjugated, AMCA-conjugated, and CY3-conjugated goat/donkey antirabbit/antiguinea pig/antimouse (Jackson Immunoresearch Laboratory, West Grove, PA), were used. Some of the sections were counterstained with 4'6'-diamidino-2-phenylindole dihydrochloride (DAPI) nuclear stain. Images were taken using a fluorescent microscope (Axiostar, Carl Zeiss, GmbH, Jena, Göttingen, Germany).
Image quantification serial sections of pancreas were mounted on slides. On average, 15–20 sections per pancreas were analyzed for each expressed gene that was quantified. Each group had a minimum of three to five pancreata. Fluorescent images of sections were scanned at the same magnification and exposure conditions. Quantification was done with ImageJ software (National Institutes of Health, Bethesda, MD). First, total area of the pancreas was measured and then specific areas were determined on the same section. The percentage of insulin-specific area was then determined. Total cell number of DAPI nuclear staining and percentage of Pdx-1/Pax6/Nkx2.2-positive cells was quantified manually. Statistical analysis was done by Student t test or ANOVA. Data are presented as mean ± SEM. A P value <0.05 was considered statistically significant.
Isolation of RNA and RT-PCR
Total RNA was isolated from whole pancreas using the RNeasy kit (Qiagen, Valencia, CA). Isolated RNA was treated with Promega RQ1 DNase (Promega, Madison, WI) for 1 h at 37°C to remove any contaminating genomic DNA. Oligo(dt)-primed reverse transcription of RNA was done for 2 h at 37°C using the Sensiscript Reverse Transcriptase kit (Qiagen). First-strand cDNA was used as a template for PCR. Optimum reaction conditions for PCR were obtained with 5 μL 10× PCR buffer (200 mmol/L Tris-HCl [pH 8.4] and 500 mmol/L KCl), 3 mmol/L MgCl2, 0.2 mmol/L dNTP, 0.025 units AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA), and 2 μmol/L primers. Amplifications were performed starting with a 10-min denaturation step at 95°C, followed by 40 cycles of denaturation at 95°C for 30 s, 58°C for 40 s for primer annealing, and 72°C for 45 s for extension.
Semiquantitative PCR
Real-time semiquantitative PCR was carried out with SyberGreen PCR Master Mix in an iCycler iQ apparatus (Bio-Rad, Hercules, CA). The amplification conditions were as follows: 94°C for 5 min, followed by 40 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s. A mouse β-tubulin primer set was used to normalize the amount of total cDNA for each gene expression experiment. Serial concentrations of cDNA (107, 106, 105, 104, 103, and 102 molecules/μL) were used for the calibration of a standard curve for the given primer. The PCR products were separated and visualized on the agarose gel. At least five different samples were analyzed at each sequential age to quantify the target gene. Primer sequences used are listed as forward then reverse, 5′ to 3′: β-tubulin, 5′-CCTTTTGGCCAGATCTTCAG-3′ and 5′-AACCAACTCAGCTCCCTCTG-3′, which amplify a product of 102 bp; GIP, 5′-GAGTTCCGATCCCATGCTAA-3′ and 5′-TGTGCCTCTTTGTCCTCCTT-3′, which amplify a product of 239 bp; and GIPR, 5′-CTCATCTTCATCCGCATCCT-3′ and 5′-GGAAACCCTGGAAGGAACTT-3′, which amplify a product of 226 bp.
RESULTS
GIP and GIPR ontogeny in the developing pancreas
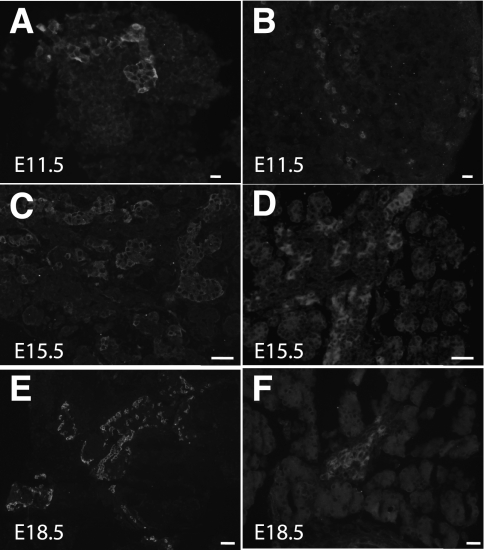
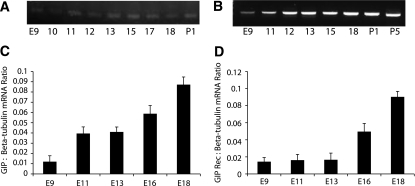
The ontogeny of GIP ligand and GIPR in the developing pancreas was investigated by immunohistochemistry at serial gestational days. GIP-positive staining could be identified as early as E9.5 and continued through E18.5 (Fig. 1A, C, and E; Supplementary Fig. 1A, E, I, and M). Pdx-1–positive cells (Supplementary Fig. 1B, F, J, and N) were present in pancreatic epithelium at early stages and in β-cells at later stages. Neither of these two Pdx-1–positive cell populations stained positive for GIP (Supplementary Fig. 1D, H, L, and P). Glucagon-positive staining and merged images (Supplementary Fig. 1C, G, K, O, and D, H, L, P) revealed that GIP ligand appeared to colocalize with glucagon, but not with Pdx-1 or insulin (insulin data not shown). RT-PCR for GIP ligand showed that mRNA expression of GIP also was present as early as E9.5 and continued through later gestational stages and into the postnatal period (Fig. 2A).
FIG. 1.
Immunohistochemistry showing the ontogeny of GIP ligand (A, C, and E) and GIPR (B, D, and F). GIP ligand is present in the early embryonic pancreas, and the expression increases during late gestational ages. GIPR expression was weak at early ages, but strong expression was found at later gestational ages (magnification size bars: A and B = 20 μmol/L; C and D = 50 μmol/L; E and F = 100 μmol/L).
FIG. 2.
RT-PCR for GIP ligand showed that mRNA expression of GIP was present as early as E9.5 and continued through later gestational stages and into postnatal stages (A). Semiquantitative RT-PCR showed increasing GIP expression after E9.5 (C). B: RT-PCR of GIPR (GIP Rec) showed that there was consistent expression of GIPR in early and late developmental and postnatal stages. D: Semiquantitative RT-PCR expression pattern for GIPR showed low relative values at days 9–13, followed by a progressive increase in expression over increasing gestational age. Error bars represent SEM.
Semiquantitative RT-PCR showed a gradually increasing GIP mRNA level beginning at E9.5 (Fig. 2C). In situ hybridization was unable to detect GIP ligand, perhaps because of a low expression level (data not shown). GIP is a member of a family of structurally related hormones that includes glucagon and glucagon-like peptides. Thus, to check for immunostaining cross-reactivity, we preincubated GIP, GLP-1, or glucagon peptide (100 μg/mL) with GIP antibody or glucagon antibody before immunohistochemistry of embryonic mouse pancreas. GIP immunostaining was not influenced by preincubation with glucagon peptide or GLP-1 peptide, but was appropriately blocked by GIP peptide (Supplementary Fig. 2). Similarly, glucagon immunostaining was not influenced by preincubation with GIP peptide or GLP-1 peptide, but was blocked appropriately by glucagon peptide (Supplementary Fig. 2). Thus, our GIP antibody does not appear to have any significant cross-reactivity with GLP-1 peptide or glucagon peptide. However, Fujita et al. (6) used a similar antibody against GIP and found cross-reactivity between GIP and GLP-1 in adult islet α-cells. The discrepancy between their results and ours may be due to the use of a different anti-GIP antibody in the two studies.
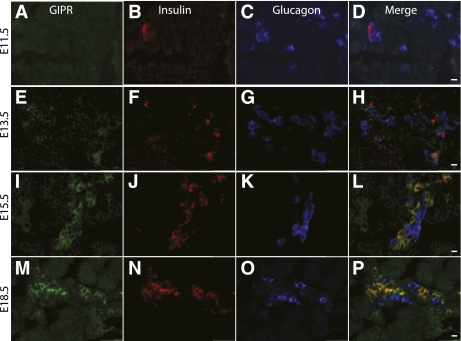
Significant GIPR staining was not seen until E13.5 and then continued through E18.5 (Fig. 1D and F). Immunostaining for insulin (Fig. 3B, F, J, and N), glucagon (Fig. 3C, G, K, and O), and GIPR (Fig. 3A, E, I, and M) showed that GIPR expression overlapped primarily with insulin expression. At E13.5 (Fig. 3H) and E14.5 (data not shown), many GIPR-positive cells were also positive for either glucagon or insulin, but by E15.5, the glucagon/GIPR double-positive cells were not detected (Fig. 3L and P), and only insulin/GIPR double-positive cells were seen. RT-PCR for the GIPR showed expression of GIPR mRNA in early and late developmental and postnatal stages (Fig. 2B). Semiquantitative RT-PCR for GIPR mRNA showed lower values at days 9–13, followed by a progressive increase in expression over increasing gestational age (Fig. 2D), corresponding roughly to the second wave of β-cell production, since later-gestation GIPR expression was only seen in insulin-positive cells.
FIG. 3.
Immunohistochemical staining for GIPR at serial gestational ages E11.5 (A–D), E13.5 (E–H), E15.5 (I–L), and E18.5 (M–P). Definite GIPR-positive staining was first identified at E13.5 and continued through E18.5 (A, E, I, and M). Insulin (B, F, J, and N), glucagon (C, G, K, and O), and merged images (D, H, L, and P) are shown. GIPR expression overlapped primarily with insulin. At E13.5, a few glucagon cells also expressed GIPR (compare 3D and 3H) in addition to the insulin-positive cells that expressed GIPR (magnification size bars: D and H = 20 μmol/L; L and P = 50 μmol/L). (A high-quality digital representation of this figure is available in the online issue.)
Inhibition of GIP signaling in the embryonic pancreas led to decreased insulin-positive differentiation
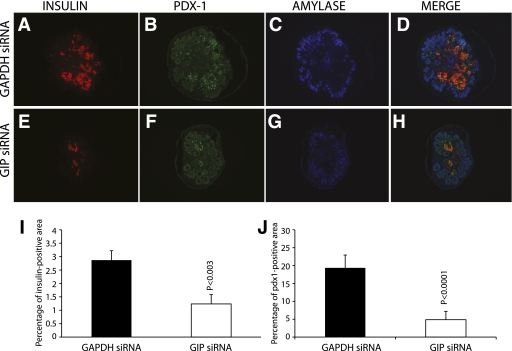
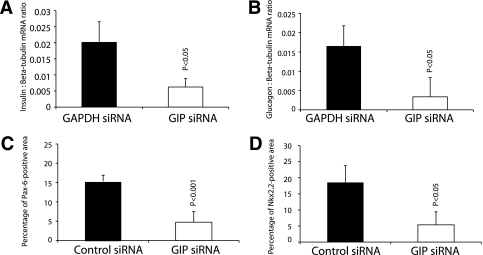
We blocked GIP expression in day 11 embryonic pancreas using different strategies. First, we cultured E11 whole developing pancreas for 6 days in the presence of Cy3-conjugated control (GAPDH) siRNA or GIP siRNA (not fluorochrome-conjugated) at 100 nmol/L. Cy3-conjugated GAPDH siRNA was successfully incorporated into cultured pancreata (Supplementary Fig. 3A). There was no obvious gross difference between pancreata treated with GAPDH siRNA or with GIP siRNA, as assessed under a dissecting microscope (Supplementary Fig. 3B and D). Semiquantitative RT-PCR of GIP mRNA from GIP siRNA-treated E11 embryonic pancreas for 6 days in culture showed inhibition of GIP mRNA expression (Supplementary Fig. 3E). GIP immunostaining of GIP siRNA-treated pancreas confirmed downregulation of GIP ligand (Supplementary Fig. 3F and G). The total GIP-positive staining area was calculated and was found to be significantly decreased with GIP siRNA treatment (Supplementary Fig. 3H). The area of insulin-positive staining was calculated by using image analysis software, and the proportion of the entire pancreas that was insulin positive was calculated. The total and percentage of insulin staining area of GIP siRNA-treated cultured pancreas was significantly less than GAPDH siRNA-treated pancreas (Fig. 4A, E, and I). Pdx-1–positive staining (Fig. 4B and F) was similarly decreased. The proportion of Pdx-1–positive cells in the pancreas was also significantly decreased (Fig. 4J). There was no change in the area of amylase-positive staining (Fig. 4C and G, quantitation not shown). Semiquantitative RT-PCR was performed for insulin and preproglucagon mRNA (Fig. 5A and B), which showed significant decreases in these mRNAs after GIP siRNA treatment. Pancreatic endocrine precursor cell markers such as Pax6 and Nkx2.2 were also investigated. Semiquantitative RT-PCR analysis showed that both Pax6 and Nkx2.2 mRNA were significantly reduced (Fig. 5C and D). To confirm, immunohistochemistry for Pax6 and Nkx2.2 was performed on GIP siRNA-treated pancreas, showing few Pax6- (Supplementary Fig. 4A and B) and Nkx2.2-positive cells (Supplementary Fig. 4C and D).
FIG. 4.
Immunohistochemistry of GIP siRNA-treated embryonic pancreas showed decreased insulin and Pdx-1–positive staining (A, B, E, and F). There was no apparent change in amylase staining (C and G). Area of insulin staining was calculated by using image analysis software, and the positive proportion of the entire pancreas was then calculated. The insulin-staining area of GIP siRNA-treated cultured pancreas was significantly less than the GAPDH siRNA-treated pancreas (n = 3, P = 0.003) (I). Pdx-1–positive cells were also significantly decreased in GIP siRNA-treated pancreas (J). (A high-quality digital representation of this figure is available in the online issue.)
FIG. 5.
Semiquantitative RT-PCR was performed for insulin and glucagon (A and B, respectively) and showed significant decreases of mRNA with GIP siRNA. Similarly, the Pax6- and Nkx2.2-positive staining areas were decreased significantly in GIP siRNA-treated pancreas (C and D). Error bars represent SEM.
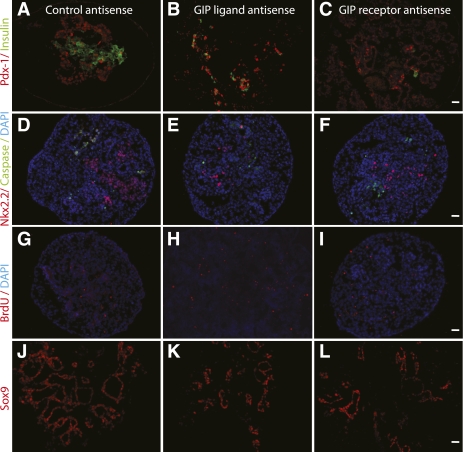
Next we inhibited GIP signaling by morpholine-ring antisense as an alternative to siRNA. Pancreases cultured for 6 days in the presence of 40 μmol/L morpholine-ring antisense against GIP showed greatly suppressed GIP expression when compared with Morpholino control antisense-treated tissues (Supplementary Figs. 5A and B and 6A). GIP Morpholino antisense at 40 μmol/L significantly (P < 0.05) suppressed insulin-positive differentiation (Fig. 6A and B; Supplementary Fig. 6B). Similar to siRNA inhibition, GIP Morpholino antisense treatment also resulted in a significant decrease in Pdx-1 (Fig. 6A and B) and Nkx2.2 expression (Fig. 6D and E). sox9-positive cells were also decreased in GIP ligand antisense-treated samples (Fig. 6J and K). Interestingly, we did not see any increase in cleaved caspase staining in GIP ligand antisense-treated samples, suggesting cells were not undergoing apoptosis (Fig. 6D and E). We did not detect any significant change in the number of BrdU-positive cells between control and GIP antisense-treated samples (Fig. 6G and H).
FIG. 6.
E11 pancreas cultured for 6 days in the presence of morpholine-ring antisense against GIP, GIPR, or control. Immunohistochemistry was performed for insulin and Pdx-1 (A, B, and C), Nkx2.2 and caspase (D, E, and F), BrdU (G, H, and I), and sox9 (J, K, and L). Both ligand and receptor antisense treatment significantly affected endocrine differentiation, as shown by decreased insulin and Nkx2.2 staining (B, C, E, and F). We also saw a decrease in an undifferentiated progenitor cell population suggested by decreased Pdx-1 and sox9 staining. However, the antisense treatment did not induce apoptosis (D, E, and F) or change the proliferation rate (G, H, and I) (magnification size bars: 20 μmol/L). (A high-quality digital representation of this figure is available in the online issue.)
Blocking of GIPR expression in explant cultures in vitro led to decreased insulin-positive differentiation
A significant decrease in insulin-positive differentiation was found in embryonic pancreas treated with GIPR morpholine-ring antisense (Fig. 6C; Supplementary Fig. 5D and E). The insulin-positive area was significantly decreased, compared with control-treated pancreas (Supplementary Fig. 7A). Semiquantitative RT-PCR for insulin and glucagon mRNA also showed a significant reduction after 40 μmol/L GIPR morpholine-ring antisense treatment (Supplementary Fig. 7B and C). Pdx-1 and sox9 (markers of progenitor cells) were also reduced in GIP-receptor antisense-treated pancreas (Fig. 6C and L). Similar to GIP ligand antisense, here again after blocking GIP the receptor, we did not see any significant change in apoptosis (Fig. 6F) or cell proliferation (Fig. 6I).
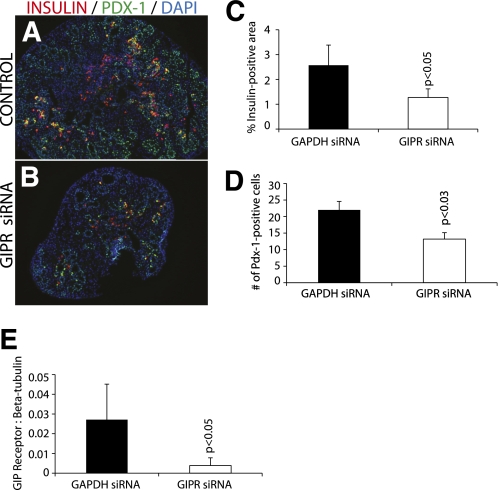
Similarly, immunohistochemistry after GIPR siRNA treatment (instead of Morpholino treatment) of cultured pancreas showed reduced insulin (Fig. 7A–C) and Pdx-1 (Fig. 7A, B, and D). Semiquantitative RT-PCR for GIPR mRNA showed a significant reduction of GIPR mRNA after GIPR siRNA treatment (Fig. 7E), confirming effective knockdown of expression by the siRNA. Immunohistochemistry of GIPR siRNA-treated pancreas also showed a significant decrease in Pax6- (P = 0.001) and Nkx2.2-positive (P = 0.0008) cell numbers (Supplementary Fig. 8).
FIG. 7.
GIPR siRNA. Immunohistochemistry of GIPR siRNA-treated cultured pancreas for insulin and Pdx-1 (A and B) is shown. In GIPR siRNA-treated cultured pancreas, there was a significant decrease in the number of insulin-positive staining (C). Pdx-1–positive cells were counted, and there was a significant decrease in GIPR siRNA-treated tissues (D) (magnification size bars: 100 μmol/L). Semiquantitative RT-PCR for GIPR was performed to confirm effective gene knockdown (E). (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
GIP and GIPR ontogeny
In mature animals, GIP is synthesized and secreted from intestinal K-cells located mainly in the duodenum and proximal jejunum. GIP is secreted in response to nutrient ingestion, especially glucose or fat (17). GIPR is a member of the seven transmembrane–spanning, heterotrimeric G-protein–coupled receptor superfamily. In the adult pancreas, GIPR expression can be seen in the islets (18). However, relatively little is known about the ontogeny of GIP and GIPR. In our study, GIP mRNA and protein was found in the developing embryonic pancreas. The specificity of the antibody used in this study was confirmed by peptide preincubation with exogenous peptides GLP-1, GIP, or glucagon before immunohistochemistry. Our experiments confirmed that GIP is localized to the developing pancreas, especially in glucagon-positive cells. Interestingly, beginning at E13.5, GIPR was localized to insulin-positive cells, consistent with its known presence in β-cells in the adult (18). There was then a transition from early GIPR expression before E15.5, when most GIPR-positive cells were glucagon positive/insulin negative, to later in gestation, when nearly all GIPR-positive cells were insulin positive/glucagon negative. These results suggest a possible paracrine-signaling pathway from glucagon-positive cells to insulin progenitor cells. Such signaling from glucagon cells to induce insulin cell formation is supported by studies in which removal of glucagon or glucagon receptor attenuates early insulin cell differentiation (10,11,13). Tseng et al. (19) were unable to detect GIP mRNA in the pancreas of late-gestation fetal rats. Late gestational pancreas is rife with RNases, and their lack of detection of what presumably is a low mRNA copy number for GIP may explain this discrepancy.
GIP signal inhibition in early pancreatic development
GIPR knockout mice (20,21) did not appear to have pancreatic abnormalities that would suggest a developmental defect. We specifically studied the embryonic pancreas of GIPR null mutant mice (a gift from Dr. S. Seino, Osaka, Japan), which also appeared to be normal (data not shown). However, these mice are known to exhibit mild glucose intolerance in response to an oral glucose challenge. It is possible that this glucose intolerance phenotype, as well as a possible embryonic phenotype in GIPR null mutant mice, is dampened because of compensation from other signaling pathways, such as GLP-1 signaling. GIPR null mutant mice have been shown to have enhanced GLP-1 receptor sensitivity (21). However, GIPR/GLP1-R double null mutant mice are also born normal, so further alternative pathways may be in play in the double mutant mice. The glucose intolerance in adult GIPR null mutant mice appeared to be due to reduced levels of glucose-stimulated insulin secretion. Interestingly, GIPR knockout mice had a normal glucose curve after an intraperitoneal glucose challenge, suggesting that enteral glucose, with stimulation of intestinal (K-cell) GIP release, was bypassed when the glucose was given intraperitoneally.
In our study, siRNA and morpholine-ring antisense against GIP or GIPR were used to inhibit GIP signaling in the developing pancreas in vitro. Inhibition of GIP by these methods resulted in a decrease in insulin-positive differentiation, as well as a decrease in Pdx-1–, Pax6-, and Nkx2.2-positive cells. Interestingly, we did not see an increase in sox9-positive cells, as we saw in a previous study where inhibition of ngn-3–mediated endocrine differentiation resulted in a significant increase in sox9- and DBA-positive progenitor cells (22). In addition, we did not see any obvious change in proliferation or cell death after GIP or GIPR antisense treatment. Thus, the lack of endocrine differentiation in these antisense-treated cultures could represent a failure to differentiate. We have described previously that inhibition of ngn-3 expression in the embryonic pancreas led to a significant increase in DBA-positive ductal cells, with several of those cells coexpressing sox-9, suggesting that they may represent expansion of an pancreatic epithelial progenitor pool (22). Similarly, we also saw an increase in duct cells in the embryonic pancreas when we inhibited glucagon signaling using Morpholino antisense (K.P., C.S., P.G., G.K.G., unpublished data). Similar effects on an epithelial progenitor cell population may occur after GIP signaling inhibition.
The discrepancy between our in vitro results and findings from genetically altered mice may be due to the sudden inhibition of GIP signaling that occurs with our in vitro systems, without the chance for an adaptive response to compensate for the loss of GIP signaling that may occur in null mutant mice. This possibility could be tested with conditional incretin receptor mutants. Counter-intuitively, GIPR knockout mice have been shown to develop an increase in β-cell area in the adult, perhaps in response to the oral glucose intolerance mentioned above.
Paracrine GIP signaling from α-cells to β-cells
We and others previously demonstrated that glucagon is necessary for the early development of insulin-positive cells in the pancreas because of paracrine actions of glucagon (10,11). Similarly, our results imply that GIP signaling to the GIPR is also necessary for early β-cell development. Such a role for GIP ligand signaling to its receptor is also suggested by the embryonic localization of GIP and GIPR in adjacent glucagon- and insulin-positive cells, respectively. In the adult, GIP from intestinal K-cells works to increase insulin secretion and inhibits β-cell apoptosis (4). There, GIP works via a humoral pathway wherein GIP is rapidly metabolized by the ubiquitous enzyme, dipeptidyl-peptidase IV (DPPIV) (23), and then the metabolite GIP 3-42 can work as an antagonist of the GIPR (24). DPPIV can exist as a soluble form in plasma, or can be attached to the endothelial cell surfaces (25). One possible developmental pathway that may allow GIP to act in the embryonic pancreas is that GIP secreted locally from glucagon-positive cells in the pancreas may avoid degradation of DPPIV because of close paracrine signaling to nearby cells. Interestingly, our inhibition of GIP signaling led to a decrease in glucagon-positive cells, both in GIP and GIPR inhibition studies (Fig. 5B; Supplementary Fig. 5B andE). This loss of glucagon cells suggests that developing insulin-positive cells, which are GIPR positive, may normally signal back to the glucagon-positive cells to maintain these glucagon-positive cells. Also, immunohistochemistry of the GIPR in early developing pancreas showed that GIPR was transiently present in glucagon-positive cells, but then disappeared by midgestation (Fig. 3H). This expression pattern suggests that GIP could also work in an autocrine manner in α-cell development early in pancreatic development.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health (066671/100), Juvenile Diabetes Research Foundation (066564/340), Tobacco Fund (066584/180), and Children's Hospital of the University of Pittsburgh funds (80016) (to G.K.G.).
No potential conflicts of interest relevant to this article were reported.
K.P. and M.K. researched data. K.P. wrote the manuscript. S.T., C.S., N.L., J.P., P.G., and Y.E.-G. contributed to discussion and review. G.K.G. contributed to discussion and reviewed and edited the manuscript. M.M. and S.S. contributed to discussion and review.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db09-0035/-/DC1.
REFERENCES
- 1.Creutzfeldt W. The incretin concept today. Diabetologia 1979;16:75–85 [DOI] [PubMed] [Google Scholar]
- 2.Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest 1967;46:1954–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unger RH, Eisentraut AM. Entero-insular axis. Arch Intern Med 1969;123:261–266 [PubMed] [Google Scholar]
- 4.Efendic S, Portwood N. Overview of incretin hormones. Horm Metab Res 2004;36:742–746 [DOI] [PubMed] [Google Scholar]
- 5.Yip RG, Wolfe MM. GIP biology and fat metabolism. Life Sci 2000;66:91–103 [DOI] [PubMed] [Google Scholar]
- 6.Fujita Y, Wideman RD, Asadi A, et al. Glucose-dependent insulinotropic polypeptide is expressed in pancreatic islet alpha-cells and promotes insulin secretion. Gastroenterology 2010;138:1966–1975 [DOI] [PubMed] [Google Scholar]
- 7.Drucker DJ. Minireview: the glucagon-like peptides. Endocrinology 2001;142:521–527 [DOI] [PubMed] [Google Scholar]
- 8.Pederson RA, Satkunarajah M, McIntosh CH, et al. Enhanced glucose-dependent insulinotropic polypeptide secretion and insulinotropic action in glucagon-like peptide 1 receptor –/– mice. Diabetes 1998;47:1046–1052 [DOI] [PubMed] [Google Scholar]
- 9.Pamir N, Lynn FC, Buchan AM, et al. Glucose-dependent insulinotropic polypeptide receptor null mice exhibit compensatory changes in the enteroinsular axis. Am J Physiol Endocrinol Metab 2003;284:E931–E939 [DOI] [PubMed] [Google Scholar]
- 10.Prasadan K, Daume E, Preuett B, et al. Glucagon is required for early insulin-positive differentiation in the developing mouse pancreas. Diabetes 2002;51:3229–3236 [DOI] [PubMed] [Google Scholar]
- 11.Vuguin PM, Kedees MH, Cui L, et al. Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology 2006;147:3995–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dohrmann C, Gruss P, Lemaire L. Pax genes and the differentiation of hormone-producing endocrine cells in the pancreas. Mech Dev 2000;92:47–54 [DOI] [PubMed] [Google Scholar]
- 13.Vincent M, Guz Y, Rozenberg M, et al. Abrogation of protein convertase 2 activity results in delayed islet cell differentiation and maturation, increased alpha-cell proliferation, and islet neogenesis. Endocrinology 2003;144:4061–4069 [DOI] [PubMed] [Google Scholar]
- 14.Portela-Gomes GM, Johansson H, Olding L, Grimelius L. Co-localization of neuroendocrine hormones in the human fetal pancreas. Eur J Endocrinol 1999;141:526–533 [DOI] [PubMed] [Google Scholar]
- 15.Gittes GK, Galante PE, Hanahan D, Rutter WJ, Debase HT. Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development 1996;122:439–447 [DOI] [PubMed] [Google Scholar]
- 16.Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature 2003;423:876–881 [DOI] [PubMed] [Google Scholar]
- 17.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 [DOI] [PubMed] [Google Scholar]
- 18.Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 1993;133:2861–2870 [DOI] [PubMed] [Google Scholar]
- 19.Tseng CC, Boylan MO, Jarboe LA, Williams EK, Sunday ME, Wolfe MM. Glucose-dependent insulinotropic peptide (GIP) gene expression in the rat salivary gland. Mol Cell Endocrinol 1995;115:13–19 [DOI] [PubMed] [Google Scholar]
- 20.Miyawaki K, Yamada Y, Ban N, et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 2002;8:738–742 [DOI] [PubMed] [Google Scholar]
- 21.Miyawaki K, Yamada Y, Yano H, et al. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci U S A 1999;96:14843–14847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prasadan K, Tulachan S, Guo P, Shiota C, Shah S, Gittes G. Endocrine-committed progenitor cells retain their differentiation potential in the absence of neurogenin-3 expression. Biochem Biophys Res Commun 2010;396:1036–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995;136:3585–3596 [DOI] [PubMed] [Google Scholar]
- 24.Gault VA, Parker JC, Harriott P, Flatt PR, O’Harte FP. Evidence that the major degradation product of glucose-dependent insulinotropic polypeptide, GIP(3-42), is a GIP receptor antagonist in vivo. J Endocrinol 2002;175:525–533 [DOI] [PubMed] [Google Scholar]
- 25.Mentlein R. Dipeptidyl-peptidase IV (CD26): role in the inactivation of regulatory peptides. Regul Pept 1999;85:9–24 [DOI] [PubMed] [Google Scholar]