Abstract
OBJECTIVE
During the past few decades, a rapidly increasing incidence of childhood type 1 diabetes (T1D) has been reported from many parts of the world. The change over time has been partly explained by changes in lifestyle causing rapid early growth and weight development. The current study models and analyzes the time trend by age, sex, and birth cohort in an exceptionally large study group.
RESEARCH DESIGN AND METHODS
The present analysis involved 14,721 incident cases of T1D with an onset of 0–14.9 years that were recorded in the nationwide Swedish Childhood Diabetes Registry from 1978 to 2007. Data were analyzed using generalized additive models.
RESULTS
Age- and sex-specific incidence rates varied from 21.6 (95% CI 19.4–23.9) during 1978–1980 to 43.9 (95% CI 40.7–47.3) during 2005–2007. Cumulative incidence by birth cohort shifted to a younger age at onset during the first 22 years, but from the birth year 2000 a statistically significant reversed trend (P < 0.01) was seen.
CONCLUSIONS
Childhood T1D increased dramatically and shifted to a younger age at onset the first 22 years of the study period. We report a reversed trend, starting in 2000, indicating a change in nongenetic risk factors affecting specifically young children.
A rapidly increasing incidence in childhood type 1 diabetes (T1D) has been reported from many countries during the last 20 years (1,2). Second to Finland, Sweden has the highest reported nationwide annual incidence of T1D in the world (1). From 1978 to 1997, the incidence of T1D among those aged 0–15 years was almost doubled in Sweden, with the largest increase among children aged 0–5 years (3). The European multicenter study (2) covering the years 1989–2003 showed a significant log linear increase in incidence in almost all the 20 EURODIAB centers representing 17 countries across the continent and predicted a doubling of new cases of T1D among children aged 0–5 years between 2005 and 2020.
Although short-term variations in incidence can be attributed to seasonality and burden of infectious diseases, western lifestyle factors, such as eating patterns in childhood, leading to accelerated growth and obesity have been suggested to account for the long-term increasing trend over time (4–6).
In the present 30-year follow-up (1978–2007) of the Swedish Childhood Diabetes Registry (SCDR), we describe the current time trend by age, sex, and birth cohort, and analyze the changes in incidence using statistical models.
RESEARCH DESIGN AND METHODS
The SCDR was approved by the research ethics committee at Karolinska Institutet and the Swedish data inspection board.
This study is based on 14,721 incident cases of childhood-onset T1D occurring from January 1, 1978, to December 31, 2007, and recorded in the SCDR. The SCDR has recorded incident cases of childhood-onset T1D (0–14.9 years) since July 1, 1977, with a high level of coverage (96–99% of cases) ascertained by internal revisions and matching to official population databases (7,8). Similar methods of data collection and verification have been used since the start of the register. During 2 years (1999 and 2000), three pediatric hospitals did not deliver data prospectively, but this has been adjusted afterward. Except for the yearly internal validation procedures as previously described (3) and the studies using external sources for validation, we have instituted a continuous validation with another source since 2003, i.e., the Swedish Quality Assessment Register, which covers age-groups 0–18 years. All children with newly diagnosed T1D in Sweden are initially treated at pediatric clinics in a hospital setting. The clinics report their T1D cases to the SCDR with date of diagnosis, birth date, and each patient’s unique personal identification number. Date of diagnosis is set to the date of the first insulin injection. Patients recorded July 1, 1977, to December 31, 1977, were excluded because it was a not a full year’s contribution of cases. We excluded three patients with diabetes onset after 15 years of age who were accidently registered. Age-standardized yearly incidence rates were extracted from the SCDR and relevant population data from Statistics Sweden (9). Mean annual incidence rates were calculated and described for the whole study population and stratified by sex and age-groups (0–4, 5–9, and 10–14 years).
A generalized additive model (GAM) for a Poisson response was used to investigate trends in incidence. GAMs are fitted for the Poisson family of distributions with the log link function. Smoothing terms are allowed in GAMs that permit flexible, nonlinear modeling of selected covariates. In the model, the impact of each calendar year at onset, age-group (0–4, 5–9, and 10–14 years), sex, and interaction terms were tested. A nonparametric smoothing function for the time trend (year) is used by a penalized regression spline approach, with automatic smoothness selection. Because the response variable is a rate rather than a count, as custom for the Poisson model, we include the population size in the respective age–sex group as an offset for each of the models.
To analyze possible trend shifts for the latest birth cohorts, we fit a linear regression curve with the cumulative incidence as a dependent variable and age at onset as a predictor. In the regression analysis, standard methods are used to test differences in slopes between the different birth cohorts using the cohort 2000 as the reference cohort.
The statistical software R was used for the data analysis. The analysis of the GAM was performed with the R functions gam. and anova.gam from the package mgcv.
RESULTS
Incidence rates by time period, age at onset, and sex.
A total of 14,721 Swedish children (7,769 boys and 6,952 girls) were registered with T1D before 15 years of age during the study period. When stratified by age at onset groups (0–4, 5–9, and 10–14 years), the mean age-specific annual incidence rates over the full observation period were 20.5, 33.9, and 37.6/100,000, respectively. Table 1 shows age- and sex-specific incidence in 3-year periods. The average increase in incidence rate for the entire cohort was highest between the periods 1999–2001 and 2002–2004, whereas the increase seems to level off during 2005–2007.
TABLE 1.
Age- and sex-specific rates of T1D in Sweden 1978–2007: incidence per 100,000 children per year in 3-year periods (95% CI)
| Time period | Sex | 0–4 years | 5–9 years | 10–14 years | 0–14 years |
|---|---|---|---|---|---|
| 1978–1980 |
M |
15.0 (10.2–19.7) |
21.0 (15.7–26.3) |
31.2 (24.9–37.5) |
22.8 (19.6–26.1) |
| F |
11.7 (8.0–15.3) |
23.3 (17.4–29.2) |
25.4 (20.0–30.7) |
20.4 (17.6–23.5) |
|
| TOT |
13.3 (10.1–16.6) |
22.1 (18.2–26.0) |
28.3 (24.1–32.7) |
21.6 (19.4–23.9) |
|
|
n |
198 |
369 |
503 |
1,070 |
|
| 1981–1983 |
M |
19.0 (13.4–24.4) |
29.1 (22.6–35.6) |
32.9 (26.2–39.5) |
27.3 (23.7–31.0) |
| F |
15.2 (10.6–19.8) |
28.2 (21.9–34.6) |
28.7 (23.0–34.4) |
24.4 (21.2–27.7) |
|
| TOT |
17.1 (13.4–20.8) |
28.7 (24.1–33.3) |
30.8 (26.2–35.4) |
25.8 (23.3–28.4) |
|
|
n |
244 |
449 |
518 |
1,211 |
|
| 1984–1986 |
M |
18.4 (13.0–23.8) |
26.2 (19.8–32.5) |
31.1 (24.6–37.6) |
25.6 (22.0–29.1) |
| F |
19.0 (13.5–24.5) |
28.3 (21.8–34.7) |
32.1 (25.7–38.6) |
26.8 (23.2–30.3) |
|
| TOT |
18.7 (14.8–22.6) |
27.2 (22.5–31.8) |
31.6 (26.9–36.2) |
26.2 (23.6–28.7) |
|
|
n |
267 |
369 |
522 |
1,185 |
|
| 1987–1989 |
M |
14.8 (10.2–19.5) |
29.3 (22.6–36.1) |
35.9 (28.6–43.2) |
27.2 (23.0–30.2) |
| F |
13.2 (8.5–17.9) |
26.3 (19.8–32.7) |
31.3 (24.8–37.8) |
24.0 (20.1–27.0) |
|
| TOT |
14.0 (10.8–17.3) |
27.8 (23.1–32.5) |
33.6 (28.6–38.7) |
25.6 (23.1–28.2) |
|
|
n |
219 |
402 |
517 |
1,138 |
|
| 1990–1992 |
M |
16.7 (12.1–21.3) |
27.5 (21.0–33.9) |
38.1 (30.5–45.7) |
28.0 (23.2–30.4) |
| F |
15.5 (10.7–20.3) |
28.8 (21.2–36.3) |
32.2 (24.9–39.4) |
25.9 (21.2–28.7) |
|
| TOT |
16.1 (12.9–19.4) |
28.1 (23.5–32.7) |
35.2 (30.0–40.5) |
26.9 (24.4–29.6) |
|
|
n |
284 |
420 |
521 |
1,225 |
|
| 1993–1995 |
M |
22.2 (16.9–27.5) |
30.6 (24.2–37.1) |
35.1 (27.9–42.4) |
29.6 (25.3–32.6) |
| F |
20.4 (15.6–21.2) |
36.7 (29.5–43.0) |
32.7 (25.4–40.1) |
30.2 (25.9–33.3) |
|
| TOT |
21.3 (17.6–25.0) |
33.7 (28.8–38.4) |
33.9 (28.9–39.1) |
29.9 (27.3–32.5) |
|
|
n |
383 |
562 |
508 |
1,453 |
|
| 1996–1998 |
M |
23.8 (18.0–29.7) |
32.8 (26.4–39.1) |
41.5 (33.8–49.2) |
33.2 (28.9–36.6) |
| F |
24.6 (18.9–30.3) |
35.3 (28.6–42.0) |
35.8 (28.9–42.7) |
32.2 (28.4–35.9) |
|
| TOT |
24.2 (20.0–28.5) |
34.0 (29.4–38.7) |
38.7 (33.4–44.1) |
32.7 (30.0–35.4) |
|
|
n |
380 |
622 |
610 |
1,612 |
|
| 1999–2001 |
M |
28.8 (22.0–35.6) |
39.1 (32.0–46.2) |
42.0 (34.7–49.4) |
37.0 (33.1–41.4) |
| F |
22.7 (17.0–28.5) |
39.6 (32.5–46.7) |
38.3 (31.1–45.5) |
33.9 (30.4–38.3) |
|
| TOT |
25.8 (21.2–30.5) |
39.3 (34.2–44.4) |
40.2 (35.1–45.4) |
35.4 (32.6–38.4) |
|
|
n |
357 |
687 |
708 |
1,752 |
|
| 2002–2004 |
M |
29.7 (22.8–36.6) |
47.4 (39.0–55.8) |
50.0 (42.3–57.8) |
42.9 (38.7–47.7) |
| F |
27.6 (21.6–33.7) |
54.3 (44.7–63.9) |
43.4 (35.9–50.7) |
42.1 (37.6–46.7) |
|
| TOT |
28.7 (23.9–33.5) |
50.9 (44.5–57.0) |
46.7 (41.5–52.2) |
42.5 (39.3–45.7) |
|
|
n |
408 |
765 |
873 |
2,046 |
|
| 2005–2007 |
M |
26.2 (20.0–32.4) |
46.1 (37.5–54.6) |
64.8 (55.6–74.0) |
46.7 (41.6–51.1) |
| F |
24.1 (17.7–30.6) |
49.7 (40.4–59.1) |
48.1 (39.5–56.7) |
41.2 (36.0–45.6) |
|
| TOT |
25.2 (20.8–29.6) |
47.9 (41.6–54.1) |
56.5 (50.5–62.9) |
43.9 (40.7–47.3) |
|
| n | 387 | 676 | 966 | 2,029 |
Generalized additive models.
Using linear models for time trends constitutes a great restriction; therefore, the more flexible GAMs were used. Table 2 shows that although calendar year and age at onset both significantly add to the model fit, sex alone gives a nonsignificant contribution. Addition of an age × year interaction term contributes significantly, indicating that the time trend differs by age at onset. Addition of an interaction term for year × sex did not contribute significantly to the model, but the interaction term for age at onset × sex significantly contributes, illustrating the known difference in age-specific incidence peak in the different sexes that coincides with puberty onset. The best fit model (model 7) explains 91% of the variation in incidence, i.e., the estimated and recorded cases are virtually the same.
TABLE 2.
Generalized additive models for the Poisson family with a log link
| Models | Residual deviance | Residual degrees of freedom | Change in deviance | Change in degrees of freedom | P value | R2 adjusted |
|---|---|---|---|---|---|---|
| (1) Null model |
1,923.98 |
179.00 |
— |
— |
||
| (2) Year |
1,174.77 |
172.68 |
749.21 |
6.32 |
<0.0001 |
0.49 |
| (3) Year + Age |
582.42 |
170.64 |
592.35 |
2.03 |
<0.0001 |
0.71 |
| (4) Year + Age + Sex |
579.44 |
169.64 |
2.98 |
1.00 |
0.08 |
0.71 |
| (5) Year + Age + Sex + Year × Age |
135.28 |
89.00 |
444.16 |
80.64 |
<0.0001 |
0.87 |
| (6) Year + Age + Sex + Year × Age + Year × Sex |
96.74 |
60.00 |
38.54 |
29.00 |
0.11 |
0.87 |
| (7) Year + Age + Sex + Year × Age + Age × Sex | 103.61 | 87.00 | 31.67 | 2.00 | <0.0001 | 0.91 |
The models are evaluated with the response variable being the incidence of T1D. A smooth term is estimated for the time trend and linear coefficients for age, sex, and interaction terms.
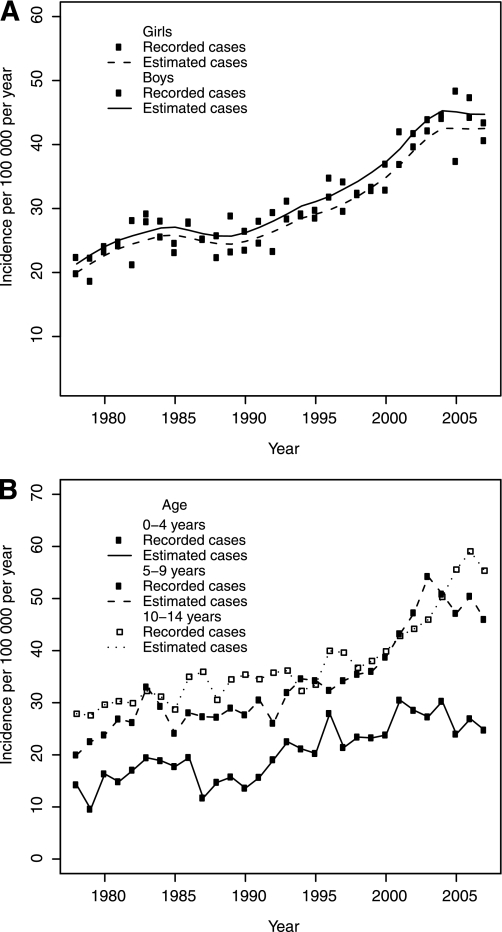
Figure 1A illustrates the observed and estimated (GAM model 4) incidence rates for the entire cohort and study period by sex. A modest increase by time is seen the first 6–7 years of registration followed by a transient decrease between 1985 and 1990. In the 1990s, a steep increase is seen, followed by a leveling off during the last 7 years of the study period.
FIG. 1.
A: Incidence of childhood diabetes by calendar year for boys and girls, respectively. Observed and estimated data, GAM; model 4. B: Incidence of childhood diabetes in three ages at onset groups by calendar year. Observed and estimated data, GAM; model 7.
The trend in incidence differed among the three age-groups over time and is illustrated in Table 1 and Fig. 1B. From the beginning to the end of the 1990s, the two youngest age-groups (0–4 and 5–9 years) had the highest relative increase. During the last decade, the 5–9- and 10–14-year age-groups had the highest rates of relative increase. The highest incidence is now seen in the 10–14-year age-group, as in the years before 2000, indicating birth cohort effects of risk exposures. Although the average incidence for the entire cohort has increased by every consecutive 3-year period, the youngest age-groups (0–4 and 5–9 years) have had a decreasing average incidence during the last 3-year period compared with the previous period.
Cohort analysis.
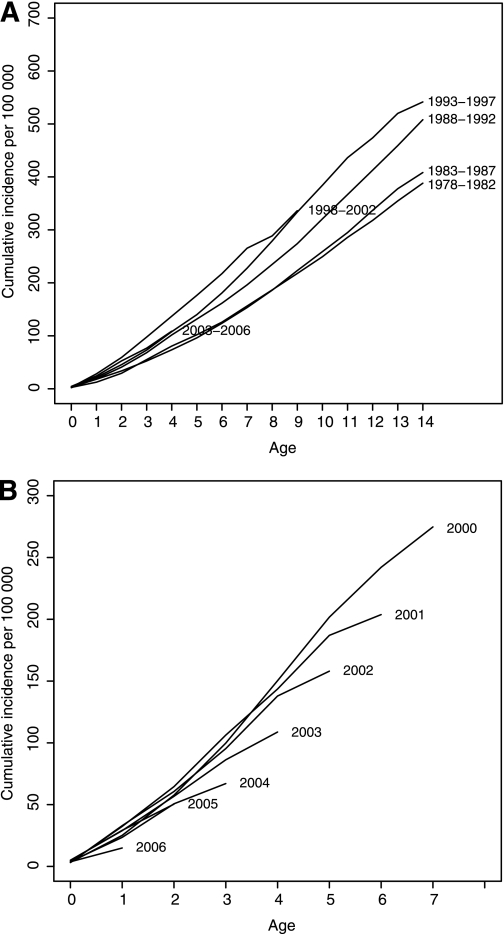
When studying the birth year cohorts for the entire study period in 5-year periods, there is a clear shift to younger age at onset in birth cohorts 1978–1999, but the birth years 1999–2002 and 2003–2006 display a change in this trend (Fig. 2A). This divergence is clearer in Fig. 2B, which shows the decline in cumulative incidence appearing in each consecutive birth year cohort for the years 2000–2006. This illustrates a possible trend break in incidence increase with older age at onset.
FIG. 2.
A: Cumulative incidence of childhood T1D in five calendar year groups. B: Cumulative incidence of childhood T1D for yearly birth cohorts 2000–2006.
To analyze the cumulative incidence trends in the cohorts of year 2000 and thereafter, we fit a linear regression model. In this analysis, all cohorts subsequent to year 2000 show a significant decrease in cumulative incidence at the 0.01 level. For the birth cohort of year 2006, the difference from the cohort of year 2000 is the largest. Here, the decrease in cumulative incidence is −30.12 for each year increase in age at onset for the 2006 cohort compared with the 2000 cohort (P = 0.007). A similar analysis of the birth cohorts 1978–1987 showed no clear trend that could explain the transient decrease in mean incidence seen between 1985 and 1990.
DISCUSSION
During the past few decades, a large number of studies from single centers, nationwide registers, and multicenter studies covering many parts of the world have shown a dramatic increase in the incidence of childhood-onset T1D (1–3,10,11). These studies clearly indicate the strong impact of lifestyle-related risk factors in childhood-onset T1D. In most reports, the incidence increase is steepest among the youngest children, and taken together with the notions of a stable or even decreasing incidence rate in young adults (12–15), these findings indicate an accelerated preclinical autoimmune destruction of the β-cell to younger age at onset suggested to cause at least part of the increase over time (6,16,17). The current study indicates for the first time a reversed pattern with a shift to older age at onset by birth cohort among children born after the year 2000. The change in incidence by time may be transient as reported from Norway (11) and seen in the Swedish data 1985–1990, but the shift to older age at onset by birth cohort in the last 7-year period is of clear interest because it indicates a more specific change in a risk exposure that affects the youngest children.
A major strength of the SCDR is that we were able to follow prospectively, with a high level of ascertainment of cases, nationwide incidence rate over a long time. Thus, this study allows us to consider shifts in time trends in large datasets. Our rich data combined with precise statistical methods, i.e., using a flexible model approach (GAM), increase the accuracy of the statistical analysis. A weakness in analyzing cumulative incidences in children born after 1993 in our study is obviously that the birth year cohorts are not followed for the full age span. Thus, an unknown number of potential new cases may change the future picture, highlighting the need to continue monitoring incidence trends, performing analysis by birth cohort, and including incidence data from older age-groups (12).
Population-based case-control studies confirm that high calorie intake (18,19) and rapid growth and weight development are risk markers for childhood-onset diabetes (20–22).
During the past decade, efforts have been made to decrease child obesity through preventive health care programs at child health care centers and schools through guidelines from the Swedish National Institute of Public Health (23). Recent studies after the implementation of these guidelines have shown a declining prevalence of overweight and obese 4-year-old Swedish children (24). Certainly other environmental risk exposures that specifically affect young children may contribute to the pattern of change in trend now seen in Sweden, but the ecological relationship with a change in overweight in Sweden is striking.
Some studies have tried to predict future incidence and prevalence of childhood diabetes using different models of time trend data (2,10). Obviously, such predictions are dependent on the stability of trends and a large enough dataset. A prediction based on our own 30-year data and GAM models would imply a grave picture, suggesting an increase of new T1D cases by >60% in the coming decade (data not shown). However, such a forecast may be an overestimate in case the trend break now seen is not transient.
In conclusion, our data point at a break in the worrying trend of increase in childhood-onset T1D in Sweden, indicating that prevention by changes in lifestyle habits is possible. Still, adequate health care resources must be allocated to meet children’s needs in both promoting better lifestyle habits for potential prevention of T1D and type 2 diabetes and taking care of the many children worldwide in whom diabetes will develop.
ACKNOWLEDGMENTS
The study was supported by grants from the Swedish Research Council project number 0735 and the Västerbotten County Council. The founding sources had no role in data collection or analysis, interpretation of results, trial design, patient recruitment, or any other aspect of the study.
No potential conflicts of interest relevant to this article were reported.
Y.B. wrote the first draft of the article and contributed to all discussion. I.W. contributed to discussion, advised on and undertook the statistical analyses, and commented on subsequent versions of the article. T.L. contributed to discussion and analysis of data and commented on subsequent versions of the article. A.M. contributed to the planning of the project and commented on subsequent versions of the article. G.D. initiated this study and contributed to the planning of the analyses, discussions, and writing of the article.
Results of this study were reported at the EASD Annual Meeting, Stockholm, Sweden, 20–24 September 2010 and ISPAD Annual Meeting, Buenos Aires, Argentina, 27–30 October 2010.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0813/-/DC1.
*The Swedish Childhood Diabetes Study Group: Steering committee: G. Dahlquist (chair), T. Lind (deputy chair), L. Mustonen (secretary), M. Eliasson, A. Möllsten, S. Schön, L. Nyström, K. Steen-Carlsson, I. Waernbaum, and the local contact people (see Supplementary Data) who diligently provide individual patient data and contribute to validation procedures.
REFERENCES
- 1.DIAMOND Project Group Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabet Med 2006;23:857–866 [DOI] [PubMed] [Google Scholar]
- 2.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, EURODIAB Study Group Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009;373:2027–2033 [DOI] [PubMed] [Google Scholar]
- 3.Dahlquist G, Mustonen L, Swedish Childhood Diabetes Study Group Analysis of 20 years of prospective registration of childhood onset diabetes time trends and birth cohort effects. Acta Paediatr 2000;89:1231–1237 [DOI] [PubMed] [Google Scholar]
- 4.Patterson CC, Dahlquist G, Soltész G, Green A, EURODIAB ACE Study Group. Europe and Diabetes Is childhood-onset type I diabetes a wealth-related disease? An ecological analysis of European incidence rates. Diabetologia 2001;44(Suppl. 3):B9–B16 [DOI] [PubMed] [Google Scholar]
- 5.Substudy EURODIAB, EURODIAB Substudy 2 Study Group Rapid early growth is associated with increased risk of childhood type 1 diabetes in various European populations. Diabetes Care 2002;25:1755–1760 [DOI] [PubMed] [Google Scholar]
- 6.Dahlquist G. Can we slow the rising incidence of childhood-onset autoimmune diabetes? The overload hypothesis. Diabetologia 2006;49:20–24 [DOI] [PubMed] [Google Scholar]
- 7.Dahlquist G, Mustonen L. Childhood onset diabetes—time trends and climatological factors. Int J Epidemiol 1994;23:1234–1241 [DOI] [PubMed] [Google Scholar]
- 8.Nyström L, Dahlquist G, Rewers M, Wall S. The Swedish childhood diabetes study. An analysis of the temporal variation in diabetes incidence 1978-1987. Int J Epidemiol 1990;19:141–146 [DOI] [PubMed] [Google Scholar]
- 9.SCB. STATISTICS SWEDEN: Swedish Population (in one-year groups) 1860-2008 [article online]. Available from http://www.scb.se/Statistik/BE/BE0101/2008A01/Be01010Folkm%c3%a4ngd1860-2008eng.xls. Accessed 2 June 2010
- 10.Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 2008;371:1777–1782 [DOI] [PubMed] [Google Scholar]
- 11.Aamodt G, Stene LC, Njostad PR, Sovik O, Joner G, Norwegian Childhood Diabetes Study Group Spatiotemporal trends and age-period-cohort modeling of the incidence of type 1 diabetes among children <15 years in Norway 1973-1982 and 1989-2003. Diabetes Care 2007;30:884–889 [DOI] [PubMed] [Google Scholar]
- 12.Pundziute-Lyckå ADG, Dahlquist G, Nyström L, et al. Swedish Childhood Diabetes Study Group The incidence of type I diabetes has not increased but shifted to a younger age at diagnosis in the 0-34 years group in Sweden 1983-1998. Diabetologia 2002;45:783–791 [DOI] [PubMed] [Google Scholar]
- 13.Ostman J, Lönnberg G, Arnqvist HJ, et al. Gender differences and temporal variation in the incidence of type 1 diabetes: results of 8012 cases in the nationwide Diabetes Incidence Study in Sweden 1983-2002. J Intern Med 2008;263:386–394 [DOI] [PubMed] [Google Scholar]
- 14.Weets I, De Leeuw IH, Du Caju MV, et al. Belgian Diabetes Registry The incidence of type 1 diabetes in the age group 0-39 years has not increased in Antwerp (Belgium) between 1989 and 2000: evidence for earlier disease manifestation. Diabetes Care 2002;25:840–846 [DOI] [PubMed] [Google Scholar]
- 15.Feltbower RG, McKinney PA, Parslow RC, Stephenson CR, Bodansky HJ. Type 1 diabetes in Yorkshire, UK: time trends in 0-14 and 15-29-year-olds, age at onset and age-period-cohort modelling. Diabet Med 2003;20:437–441 [DOI] [PubMed] [Google Scholar]
- 16.Wilkin TJ. The accelerator hypothesis: weight gain as the missing link between Type I and Type II diabetes. Diabetologia 2001;44:914–922 [DOI] [PubMed] [Google Scholar]
- 17.Gale EA. Spring harvest? Reflections on the rise of type 1 diabetes. Diabetologia 2005;48:2445–2450 [DOI] [PubMed] [Google Scholar]
- 18.Dahlquist GG, Blom LG, Persson LA, Sandström AI, Wall SG. Dietary factors and the risk of developing insulin dependent diabetes in childhood. BMJ 1990;300:1302–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pundziute-Lyckå A, Persson LA, Cedermark G, et al. Diet, growth, and the risk for type 1 diabetes in childhood: a matched case-referent study. Diabetes Care 2004;27:2784–2789 [DOI] [PubMed] [Google Scholar]
- 20.Blom L, Persson LA, Dahlquist G. A high linear growth is associated with an increased risk of childhood diabetes mellitus. Diabetologia 1992;35:528–533 [DOI] [PubMed] [Google Scholar]
- 21.Hyppönen E, Virtanen SM, Kenward MG, Knip M, Akerblom HK, Childhood Diabetes in Finland Study Group Obesity, increased linear growth, and risk of type 1 diabetes in children. Diabetes Care 2000;23:1755–1760 [DOI] [PubMed] [Google Scholar]
- 22.Patterson CDG, EURODIAB Substudy 2 Study Group Rapid early growth is associated with increased risk of childhood type 1 diabetes in various European populations. Diabetes Care 2002;25:1755–1760 [DOI] [PubMed] [Google Scholar]
- 23.The Swedish National Institute of Public Health. Eating habits and foods [article online]. Available from http://www.fhi.se/en/About-FHI/Public-health-policy/10-Eating-habits-and-foods/ Accessed 2 September 2010
- 24.Bergström E, Blomquist HK. Is the prevalence of overweight and obesity declining among 4-year-old Swedish children? Acta Paediatr 2009;98:1956–1958 [DOI] [PubMed] [Google Scholar]