Abstract
OBJECTIVE
High cholesterol levels in circulating immune complexes (IC), surrogate markers of modified LDL, are associated with increased carotid intima-media thickness (IMT) and cardiovascular events in type 1 diabetes. Different modifications of LDL are involved in IC formation, but which of these are predictive of vascular events is not known. Therefore, we measured oxidized LDL (oxLDL), advanced glycation end products–modified LDL (AGE-LDL), and malondialdehyde-modified LDL (MDA-LDL) in IC and determined their relationship with increased carotid IMT and compared the strength of the association with that observed with conventional risk factors.
RESEARCH DESIGN AND METHODS
Levels of oxLDL, AGE-LDL, and MDA-LDL were measured in circulating IC isolated from sera of 479 patients of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort, collected at baseline. Internal and common carotid IMT were measured 8 and 14 years later by DCCT/EDIC.
RESULTS
OxLDL, AGE-LDL, and MDA-LDL levels in circulating IC were significantly correlated with diabetes duration, BMI, and lipid and blood pressure, but not with age. Multivariate logistic regression models indicated that individuals in the highest versus lowest quartile of oxLDL and AGE-LDL in IC had a 6.11-fold [confidence interval (CI) 2.51–14.8] and a 6.4-fold (CI 2.53–16.2) increase in the odds of having high carotid IMT, respectively, after adjusting for conventional risk factors. Parallel analyses resulted in odds ratios of 2.62 (CI 1.24, 5.55) for LDL-C, 1.45 (CI 0.69, 3.03) for diastolic blood pressure, and 2.33 (CI 1.09, 4.99) for A1C.
CONCLUSIONS
OxLDL and AGE-LDL in circulating IC were significantly associated with progression and increased levels of carotid IMT in type 1 diabetes.
Chronic inflammation has an established role in the pathogenesis of atherosclerosis and acute vascular events (1–3). Therefore, identification of factors that may trigger inflammation and acute vascular events remains a high priority. Modified forms of LDL have emerged as major factors in the pathogenesis of atherosclerosis, and oxidized LDL (oxLDL) has been shown to trigger proinflammatory events (4–8) through the activation of pathways associated with innate immunity (4,6,9,10).
However, oxLDL is also involved in triggering adaptive immunity pathways involved in the pathogenesis of atherosclerosis (11,12). T cell activation appears to be linked to LDL modification since peptides derived from oxLDL have been shown to be recognized by T cells (13). However, the strongest link between modified LDL and adaptive immunity involves the activation of the humoral immune system. Most forms of modified LDL are immunogenic and induce the formation of autoantibodies in humans (14,15). A direct consequence of autoantibody synthesis against modified LDL is the formation of immune complexes (IC). These IC are detectable both in serum (11) and in the atheromatous plaque, where both oxLDL and oxLDL antibodies have been found (16,17).
The antibodies present in circulating IC isolated from subjects with type 1 diabetes are predominantly IgG of the proinflammatory subclasses 1 and 3 (18,19) and react with oxLDL, malondialdehyde-modified LDL (MDA-LDL), and advanced glycation end products–modified LDL (AGE-LDL) (15). In vitro studies have demonstrated that oxLDL-IC have proatherogenic and proinflammatory properties that greatly exceed those of oxLDL (20).
In patients with type 1 diabetes, more than 90% of modified LDL circulate as soluble IC and only traces of modified lipoproteins circulating free are captured in the serum after removal of IC (21). Therefore, to assess the relationship of modified LDL with atherosclerosis, the modified lipoprotein in IC needs to be measured, because the formation of soluble IC interferes with the accuracy of the assays, both of modified LDL antibodies and of modified LDL (14,21). In contrast, the measurement of modified LDL levels obtained from isolated IC appears to yield reproducible results, and in the case of MDA-LDL, we showed that the capture assay and a biochemical assay for MDA had an excellent correlation (r = 0.706) (21).
In a small prospective study of 98 subjects with type 1 diabetes we have previously examined the role of modified LDL-IC in the development of cardiovascular disease (CVD) using the concentrations of cholesterol in IC (22,23), as a surrogate marker for antibody-associated modified LDL. The concentrations of cholesterol in the IC correlated with the development of coronary artery disease over a period of 7 years (24,25). By use of the same approach, we measured cholesterol in IC from a cohort of 1,050 individuals from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. We found that cholesterol was present in significantly higher concentrations in the IC from patients who showed progression of the internal carotid intima-media thickness (IMT) and that the cholesterol content of IC was a significant positive predictor of internal carotid IMT progression (26).
Subsequently, in our laboratory, we developed capture immunoassays that allow us to quantify specific modified forms of LDL once they are dissociated from their corresponding antibodies (21). We used these capture immunoassays to measure oxLDL, AGE- LDL, and MDA-LDL in isolated and fractionated modified LDL-IC obtained from stored serum samples collected from the DCCT/EDIC cohort at baseline and examined the relationship between the concentration of oxLDL, AGE-LDL, and MDA-LDL in LDL-IC and the carotid IMT measurements performed in the DCCT/EDIC cohort ∼8 and 14 years after the samples were collected. This allowed us to address the hypothesis that the levels of specific modified forms of LDL in circulating IC play a role in the development of vascular disease in type 1 diabetes and to compare their predictive value with that of conventional predictors of CVD in type 1 diabetes.
RESEARCH DESIGN AND METHODS
This study was performed on a subgroup of 479 subjects from DCCT/EDIC cohort. The DCCT cohort included 1,441 patients who were 13–39 years of age and had type 1 diabetes for 1–15 years at study entry (27). None of the patients at entry into the study (1983–89) had hypertension or dyslipidemia and therefore were not on lipid-lowering or antihypertensive therapy.
The DCCT cohort was randomly assigned to intensive or conventional diabetes therapy and followed for an average of 6.5 years. In 1994, after intensive therapy had been demonstrated to have major beneficial effects on microvascular complications, the interventional phase of the study was stopped and the observational phase was initiated (28). During the ongoing EDIC observational phase, the patients have been under the care of their personal physicians and encouraged to practice intensive diabetes therapy.
Of the 1,441 DCCT participants, 90–95% were followed during EDIC and 905 of these individuals had blood collected longitudinally as part of a substudy. From these 905 subjects, 518 patients were selected for a case-control study of LDL-IC and albuminuria. Patients with at least two measurements of albumin excretion rate (AER) above 60 mg/24 h were selected as cases, and 3 to 4 patients without albuminuria per patient with albuminuria were selected as controls. From the 518 subjects with LDL-IC measured, 479 had IMT measured during EDIC (29).
Serum samples were obtained after an overnight fast at entry into the DCCT study and assayed at the time or stored at −80°C. The DCCT and EDIC were approved by the Institutional Review Board of all participating DCCT/EDIC centers, and all participants provided written informed consent.
Assessment of carotid IMT
Carotid ultrasonography was first performed 1 to 2 years after initiation of EDIC (5–13 years after DCCT baseline) and repeated 5 years later. The measurement of IMT in the DCCT/EDIC cohort has been described in detail (30,31). In brief, a single longitudinal lateral view of the distal 10 mm of the right and left common carotid arteries (CCA) and three longitudinal views in different imaging planes of each internal carotid artery (ICA) were obtained by certified technicians at the clinical centers, recorded on S-VHS tapes and read in a central unit (Tufts Medical Center, Boston, MA) by a single reader, masked to participant characteristics. The maximum IMT (in mm) of the CCA was defined as the mean of the maximal IMT for near and far walls on both right and left sides. The maximum IMT of the ICA was defined in the same way, and the results of the three scans (i.e., anterior, lateral, and posterior views of both sides) were averaged.
Measurement of AGE-LDL, oxLDL, and MDA-LDL in human circulating IC
We measured oxLDL, MDA-LDL, and AGE-LDL by first precipitating circulating IC from serum and then fractionating these IC by protein G affinity chromatography, separating the predominant IgG antibody from modified LDL, as described previously (19,26). The reactivity of modified LDL separated from LDL-IC with antibodies specific for different LDL modifications (oxLDL, MDA-LDL, and AGE-LDL) was then assayed with capture assays developed in our laboratory (21). The characteristics of the antibodies used in the assay and the specificity and reproducibility of the capture assays have been reported previously (15,21). Coefficients of variation for 50 samples measured in two separate assays were 5.2% for oxLDL, 0.5% for MDA-LDL, and 8.3% for AGE-LDL. The development of standards for calibration of the oxLDL, MDA-LDL, and AGE-LDL assays, as well as sensitivity, reproducibility, and recovery data for the capture assays, has been reported elsewhere (21). The levels of the different LDL modifications in human circulating IC were expressed in function of the amount of apolipoprotein B contained in the IC, and the final values were given as the concentration per milliliter of serum.
Other procedures
At baseline DCCT, each participant completed a standardized medical history, physical examination, electrocardiogram, and laboratory testing including hemoglobin A1c (28,32), fasting lipid profiles, and 4-h urine collections for measurement of AER and creatinine clearance. Covariates for the current analyses were obtained from DCCT baseline history, physical examination, and laboratory data (fasting lipids and renal function). The methodologies to measure conventional CVD risk factors have been described elsewhere (28,32). Retinopathy status at baseline was assessed with stereo fundus photography (33).
Statistical analysis
Prospective analyses were carried out in which the levels of oxLDL, AGE-LDL, and MDA-LDL in LDL-IC, measured at baseline DCCT, functioned as a biomarker for individual levels of LDL, degree of oxidative stress, and immune response. Internal and common IMT levels 8–14 years later (EDIC years 1 and 6) were the outcomes of interest. All modified LDL values were standardized to milligrams of apolipoprotein B per liters of serum and are expressed as milligrams per liters; in addition, modified LDL values were log transformed because of their skewed, nonnormal distribution. Spearman correlations were determined for modified LDL levels and baseline DCCT variables of interest. Means and proportions adjusted for treatment group, retinopathy status, age, and sex were determined for participant DCCT baseline characteristics stratified by modified LDL quartile using linear and logistic regression as appropriate. Linear regression was used to determine estimates of the β-coefficient and semipartial R2 for the relationship between the level of each type of modified LDL measured in LDL-IC and internal and common IMT (ICA and CCA IMT) measurements at EDIC years 1 and 6 and to calculate least square means for EDIC years 1 and 6 ICA/CCA IMT across quartiles of each modified LDL. Trends in both internal and common IMT across quartiles of each modified LDL were tested using a F-statistic obtained from a generalized linear model adjusted for treatment group, retinopathy cohort, age, sex, diabetes duration, hemoglobin A1c, logarithm of AER, and ultrasonography equipment.
Logistic regression was used to model two outcomes: 1) the odds ratio associated with being in the upper versus lower measurements of ICA IMT (i.e., upper quintile versus lower four quartiles) at EDIC year 6 and 2) the odds ratio associated with high progression of ICA IMT from EDIC year 1 to EDIC year 6 (i.e., high progression being defined as being in the upper quintile of ICA IMT change). For logistic regression analysis each IC was categorized into quartiles. The association between modified LDL quartiles and being in the upper quintile of ICA IMT was assessed separately for modified LDL after controlling for the covariates included in the linear regression models with the addition of LDL and HDL cholesterol, systolic and diastolic blood pressure, and current smoking status. Additionally, for each IC studied appropriate interaction terms were used to determine whether covariates modified the relationship between each type of modified LDL and having high ICA IMT at EDIC year 6. Finally, the c-statistic or area under the receiver operating curve (ROC AUC) was used to compare the discriminatory power of various multivariate models. Parallel analyses were completed for the outcome high progression of ICA IMT from EDIC year 1 to 6. Reported P values are two-sided with a type I error rate for significance of α = 0.05. All analysis were performed using the SAS v. 9.2 system (SAS Institute, Cary, NC).
RESULTS
At DCCT baseline, the mean age of the study population was 27.1 ± 7.0 years, the mean duration of diabetes was 6.0 ± 4.2 years, and 247 (51.6%) of the 479 subjects studied were men and 45.7% were assigned to the DCCT intensive treatment group. A comparison of DCCT baseline characteristics of the 479 subjects with the remaining DCCT cohort showed that duration of diabetes was longer, they were more likely to have retinopathy at baseline, and BMI and AER were higher. Blood pressure, lipid, and hemoglobin A1c as well as age, sex, drinking, and smoking status were similar in those included and excluded in this study’s subcohort.
At DCCT baseline, the levels of oxLDL, AGE-LDL, and MDA-LDL in isolated LDL-IC were significantly correlated with diabetes duration, BMI, and lipid and blood pressure levels, but not with age. Correlations with LDL cholesterol, although statistically significant, were of moderate magnitude (r = 0.15 to 0.23, P < 0.0007 to P < 0.0001). The levels of MDA-LDL, AGE-LDL, and oxLDL in LDL-IC were all highly intercorrelated (r = 0.71 to 0.83, P < 0.0001).
The percentage of men increased with increasing quartiles of oxLDL in LDL-IC (Table 1). After treatment group, retinopathy status, age, and sex were adjusted, the duration of diabetes remained similar while BMI, hemoglobin A1c, LDL cholesterol, and triglyceride levels increased across quartiles of oxLDL in LDL-IC. HDL cholesterol levels decreased across increasing quartiles of oxLDL in LDL-IC. Systolic blood pressure, diastolic blood pressure, AER, and creatinine clearance did not increase or decrease across increasing oxLDL quartiles. Finally, neither current smoking status nor alcohol consumption appeared to be associated with oxLDL quartiles.
TABLE 1.
DCCT baseline characteristics (means or proportions and 95% confidence intervals) of the study population stratified by quartile of oxLDL in LDL-IC (n = 479) adjusted for treatment group, retinopathy cohort, age, and sex
| oxLDL in LDL-IC quartiles (cut points, mg/L) |
Trend |
||||
|---|---|---|---|---|---|
| 1st (5–89) | 2nd (90–162) | 3rd (163–305) | 4th (306–1382) | P | |
| Age (years)* | 27.0 (25.7, 28.2) | 27.1 (25.9, 28.4) | 27.3 (26.0, 28.5) | 27.1 (25.9, 28.4) | 0.8235 |
| Men (%)* | 42.0 (33.5, 51.0) | 47.5 (38.7, 56.4) | 59.2 (50.2, 67.6) | 57.5 (48.5, 66.0) | 0.0047 |
| Intensive treatment group (%)* | 52.1 (43.2, 60.9) | 48.3 (39.5, 57.2) | 46.7 (37.9, 55.6) | 35.8 (27.8, 44.8) | 0.0135 |
| Primary retinopathy cohort (%)* | 54.6 (45.6, 63.3) | 44.2 (35.6, 53.1) | 51.7 (42.8, 60.5) | 70.8 (62.1, 78.3) | 0.0058 |
| Diabetes duration (years) | 5.5 (5.0, 6.0) | 6.5 (6.0, 7.0) | 6.1 (5.6, 6.6) | 5.9 (5.4, 6.4) | 0.4827 |
| Hemoglobin A1c (%) | 8.7 (8.4, 9.0) | 8.6 (8.3, 8.8) | 8.9 (8.6, 9.2) | 9.3 (9.0, 9.6) | 0.0014 |
| Body mass index (kg/m2) | 23.1 (22.6, 23.6) | 23.3 (22.8, 23.8) | 23.4 (22.9, 23.9) | 24.1 (23.6, 24.6) | 0.0062 |
| Blood pressure (mmHg) | |||||
| Systolic | 113 (111, 115) | 115 (113, 117) | 115 (113, 117) | 115 (113, 117) | 0.2216 |
| Diastolic | 73 (71, 74) | 73 (71, 74) | 73 (71, 74) | 74 (73, 76) | 0.2266 |
| Cholesterol (mg/dL) | |||||
| HDL | 53 (51, 55) | 51 (49, 53) | 50 (48, 52) | 49 (47, 51) | 0.0058 |
| LDL | 103 (98, 108) | 102 (97, 108) | 113 (108, 118) | 118 (113, 123) | <0.0001 |
| Triglycerides (mg/dL)† | 66 (62, 71) | 68 (63, 73) | 76 (70, 81) | 82 (77, 88) | <0.0001 |
| AER (mg/24 h)† | 10.7 (9.4, 12.3) | 11.6 (10.2, 13.3) | 12.5 (10.9, 14.3) | 12.6 (11.0, 14.4) | 0.0754 |
| Creatinine clearance (ml/min) | 125 (120, 129) | 126 (121, 131) | 131 (126, 136) | 125 (121, 130) | 0.4882 |
| Current smoker (%) | 13.5 (8.4, 20.9) | 25.6 (18.4, 34.4) | 17.0 (11.3, 24.8) | 23.3 (16.3, 32.2) | 0.2083 |
| Current drinker (%) | 26.9 (19.3, 36.0) | 14.0 (8.8, 21.5) | 16.7 (11.1, 24.5) | 16.5 (10.8, 24.3) | 0.0961 |
*Unadjusted.
†Because of nonnormal distributions, geometric means are presented.
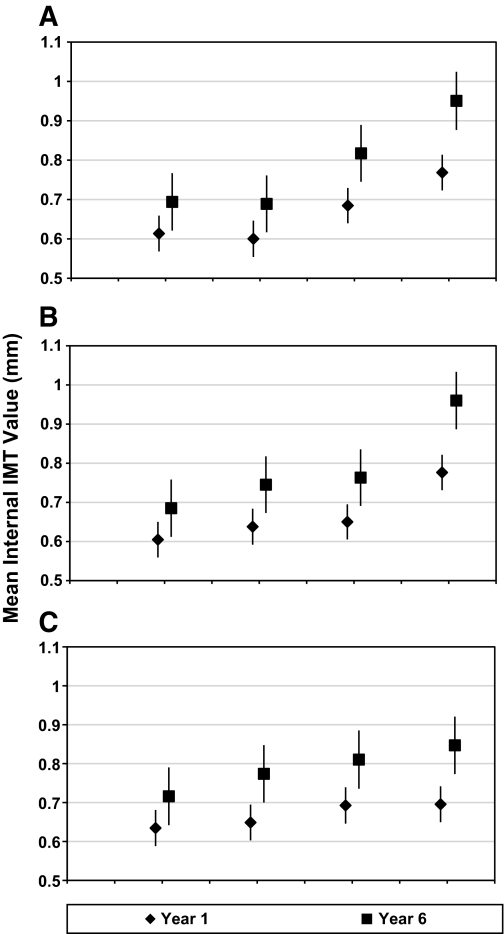
After covariates were adjusted, the concentrations of modified LDL in isolated IC were each significantly associated with EDIC year 1 and EDIC year 6 ICA IMT with higher modified LDL levels predicting higher ICA IMT (Table 2). For common carotid artery IMT, associations were slightly weaker with log MDA-LDL failing to predict higher CCA IMT at EDIC year 1 or EDIC year 6. Additionally, log oxLDL and log AGE–LDL IC were associated with progression of ICA and CCA IMT from EDIC year 1 to EDIC year 6. With focus on mean ICA IMT levels as the outcome and stratification by oxLDL quartiles, ICA IMT levels increased across oxLDL quartiles at EDIC year 1 (Linear Trend Test; P < 0.001) and EDIC year 6 (Linear Trend Test; P < 0.001) after adjusting for treatment group, retinopathy cohort, age, sex, diabetes duration, hemoglobin A1c, logarithm of AER, and ultrasonography equipment (Fig. 1A). Similar findings were observed across AGE-LDL quartiles (Fig. 1B), whereas slightly weaker but still statistically significant findings were observed across MDA-LDL quartiles (Fig. 1C).
TABLE 2.
Adjusted linear regression models for modified LDL in LDL-IC* predicting internal and common carotid IMT
| β-Coefficient estimate | P | Semipartial R2 (%) | |
|---|---|---|---|
| Internal IMT (mm)–EDIC year 1 | |||
| Ln oxLDL in IC† | 0.05965 | <0.0001 | 4.28 |
| Ln AGE-LDL in IC† | 0.04684 | <0.0001 | 4.25 |
| Ln MDA-LDL in IC† | 0.02369 | 0.0306 | 1.03 |
| Common IMT (mm)–EDIC year 1 | |||
| Ln oxLDL in IC† | 0.00855 | 0.0460 | 0.85 |
| Ln AGE-LDL in IC† | 0.00645 | 0.0562 | 0.78 |
| Ln MDA-LDL in IC† | 0.00282 | 0.4181 | 0.14 |
| Internal IMT (mm)–EDIC year 6 | |||
| Ln oxLDL in IC† | 0.11000 | <0.0001 | 5.52 |
| Ln AGE-LDL in IC† | 0.08942 | <0.0001 | 5.89 |
| Ln MDA-LDL in IC† | 0.04208 | 0.0172 | 1.24 |
| Common IMT (mm)–EDIC year 6 | |||
| Ln oxLDL in IC† | 0.01908 | 0.0035 | 1.82 |
| Ln AGE-LDL in IC† | 0.01475 | 0.0043 | 1.76 |
| Ln MDA-LDL in IC† | 0.00781 | 0.1420 | 0.47 |
| Internal IMT progression (mm)–year 6 adjusted for year 1 | |||
| Ln oxLDL in IC‡ | 0.05904 | 0.0015 | 2.24 |
| Ln AGE-LDL in IC‡ | 0.05036 | 0.0006 | 2.63 |
| Ln MDA-LDL in IC‡ | 0.02178 | 0.1437 | 0.48 |
| Common IMT progression (mm)–year 6 adjusted for year 1 | |||
| Ln oxLDL in IC‡ | 0.01318 | 0.0225 | 1.12 |
| Ln AGE-LDL in IC‡ | 0.01014 | 0.0262 | 1.07 |
| Ln MDA-LDL in IC‡ | 0.00603 | 0.1976 | 0.36 |
*β-Coefficient estimates are per unit increase in natural log transformed levels of modified LDL forms in isolated LDL-IC (in mg/L).
†Adjusted for DCCT treatment group; DCCT retinopathy cohort; and baseline DCCT age, sex, diabetes duration, hemoglobin A1c (%), logarithm of AER, and ultrasonography equipment.
‡Additionally, adjusted for EDIC year 1 IMT level; EDIC year 6 adjusted for EDIC year 1 is equivalent to progression from year 1 to year 6 adjusted for year 1.
FIG. 1.
Internal IMT means (in millimeters) for years 1 and 6 adjusted for age, sex, study group, retinopathy status, duration of diabetes at study entry, percent hemoglobin A1c, logarithm of AER, and ultrasonography equipment. A: The quartiles of oxLDL in isolated LDL-IC are as follows: 1, 5–89 (mg/L); 2, 90–162; 3, 163–305; and 4, 306–1382. Linear Trend Test: year 1 (F = 27.21; P < 0.001); year 6 (F = 27.91; P < 0.001). B: The quartiles of AGE in isolated LDL-IC are as follows: 1, 0.15–2.64 (mg/L); 2, 2.65–6.42; 3, 6.43–12.03; and 4, 12.17–305.34. Linear Trend Test: year 1 (F = 25.28; P < 0.001); year 6 (F = 24.85; P < 0.001). C: The quartiles of MDA in isolated LDL-IC are as follows: 1, 3–43 (mg/L); 2, 44–108; 3, 109–202; and 4, 203–1296. Linear Trend Test: year 1 (F = 4.59; P = 0.033); year 6 (F = 6.39; P = 0. 012).
Multivariate logistic regression models were used to further examine the ability of the concentrations of oxLDL and AGE-LDL in isolated LDL-IC to predict ICA IMT (Table 3). The outcome, high ICA IMT, was defined as being in the upper quintile as compared with the lower four quintiles of ICA IMT at EDIC year 6 (high IMT defined as >0.845 mm). Individuals in the highest quartile of oxLDL in isolated LDL-IC had a sevenfold increased odds [7.72 (95% CI 3.27, 18.3)] of having high versus normal ICA IMT relative to those in the lowest quartile of oxLDL, after controlling for treatment group, retinopathy cohort, age, sex, diabetes duration, hemoglobin A1c, logarithm of AER, and ultrasonography equipment. Additionally, adjusting for LDL cholesterol, HDL cholesterol, diastolic blood pressure, and smoking status attenuated the odds ratios somewhat to 6.11 (95% CI 2.51, 14.8). Parallel analyses for AGE-LDL resulted in odds ratios of 7.82 (95% CI 3.17, 19.3) and 6.40 (95% CI 2.53, 16.2), respectively. Parallel analyses for MDA-LDL, not shown in Table 3, resulted in odds ratios of 2.74 (95% CI 1.27, 5.92) and 2.39 (95% CI 1.06, 5.38), respectively. None of the covariates examined were found to modify associations between oxLDL or AGE-LDL levels in isolated LDL-IC and having high ICA IMT at EDIC year 6.
TABLE 3.
Adjusted* odds ratios (and 95% confidence intervals) from multivariate logistic regression models for a given difference in risk factor level for being in the upper quintile versus the lower four quintiles† of ICA IMT at EDIC year 6
| IC | oxLDL in LDL-IC |
AGE-LDL in LDL-IC |
||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Quartile | ||||
| Lowest | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 1.98 (0.78, 5.02) | 1.77 (0.68, 4.60) | 3.65 (1.44, 9.26) | 3.66 (1.40, 9.56) |
| 3 | 3.27 (1.35, 7.91) | 2.88 (1.16, 7.15) | 2.71 (1.06, 6.93) | 2.75 (1.05, 7.21) |
| 4 | 7.72 (3.27, 18.3) | 6.11 (2.51, 14.8) | 7.82 (3.17, 19.3) | 6.40 (2.53, 16.2) |
| Age (1-year increase) | 1.12 (1.08, 1.17) | 1.10 (1.06, 1.15) | 1.12 (1.07, 1.16) | 1.10 (1.05, 1.15) |
| Sex (men vs. women) | 1.97 (1.16, 3.35) | 1.27 (0.69, 2.33) | 2.10 (1.24, 3.57) | 1.30 (0.71, 2.38) |
| Study group (intensive vs. conventional) | 0.72 (0.42, 1.22) | 0.67 (0.39, 1.16) | 0.68 (0.40, 1.15) | 0.64 (0.37, 1.11) |
| Retinopathy cohort (second vs. primary) | 0.58 (0.26, 1.28) | 0.51 (0.22, 1.18) | 0.69 (0.32, 1.48) | 0.60 (0.27, 1.35) |
| Duration (1-year increase) | 1.07 (0.98, 1.17) | 1.08 (0.98, 1.18) | 1.09 (0.99, 1.19) | 1.09 (1.00, 1.20) |
| Hemoglobin A1c (1-unit increase, %) | 1.08 (0.91, 1.28) | 1.06 (0.89, 1.26) | 1.11 (0.94, 1.31) | 1.08 (0.91, 1.29) |
| Ln of AER (1-unit increase, mg/24 h) | 1.14 (0.78, 1.66) | 0.95 (0.64, 1.41) | 1.11 (0.77, 1.62) | 0.93 (0.62, 1.37) |
| Cholesterol (10-unit increase, mg/dL) | ||||
| LDL | —- | 1.10 (1.00, 1.21) | —- | 1.11 (1.02, 1.22) |
| HDL | —- | 0.69 (0.53, 0.91) | —- | 0.67 (0.51, 0.88) |
| Diastolic blood pressure‡ (10-unit increase, mmHg) | —- | 1.39 (0.99, 1.95) | —- | 1.52 (1.07, 2.15) |
| Current smoking (yes vs. no) | —- | 2.31 (1.25, 4.26) | —- | 2.23 (1.19, 4.15) |
| ROC AUC | 0.794 | 0.818 | 0.790 | 0.817 |
*All models are additionally adjusted for ultrasonography equipment.
†The numerical cut point for high IMT at EDIC year 6 was greater than 0.845 mm.
‡Diastolic rather than systolic blood pressure was included because although not significantly associated with high ICA IMT, it was a stronger predictor than systolic blood pressure in our study population.
Similar analyses were completed to examine the ability of oxLDL and AGE-LDL in isolated LDL-IC to predict progression of ICA IMT (Table 4). The outcome, high progression of ICA IMT, was defined as being in the upper quintile as compared with the lower four quintiles of change in ICA IMT from EDIC year 1 to 6 (high IMT change defined as ICA IMT year 6 − year 1 > 0.179 mm). Adjusted odds ratios for ICA IMT progression were 4.08 (95% CI 1.80–9.23) and 2.62 (95% CI 1.18–5.79), respectively, for oxLDL and AGE-LDL in isolated LDL-IC comparing the highest with the lowest LDL-IC quartile.
TABLE 4.
Adjusted* odds ratios (and 95% confidence intervals) from multivariate logistic regression models for a given difference in risk factor level for being in the upper quintile versus the lower four quintiles† of progression of ICA IMT from EDIC year 1 to EDIC year 6
| IC | oxLDL in LDL-IC Model 1 | AGE-LDL in LDL-IC Model 2 |
|---|---|---|
| Quartile | ||
| Lowest | 1.00 | 1.00 |
| 2 | 1.33 (0.53–3.29) | 1.66 (0.73–3.79) |
| 3 | 2.29 (0.98–5.35) | 1.65 (0.73–3.72) |
| 4 | 4.08 (1.80–9.23) | 2.62 (1.18–5.79) |
| Age (1-year increase) | 1.05 (1.01–1.10) | 1.05 (1.01–1.09) |
| Sex (men vs. women) | 1.07 (0.60–1.93) | 1.09 (0.61–1.94) |
| Study group (intensive vs. conventional) | 0.88 (0.51–1.50) | 0.84 (0.50–1.42) |
| Retinopathy cohort (second vs. primary) | 0.55 (0.25–1.23) | 0.60 (0.28–1.36) |
| Duration (1-year increase) | 1.08 (0.99–1.18) | 1.09 (0.99–1.19) |
| Hemoglobin A1c (1-unit increase, %) | 1.05 (0.88–1.24) | 1.08 (0.91–1.27) |
| Ln of AER (1-unit increase, mg/24 h) | 0.98 (0.67–1.43) | 0.95 (0.65–1.37) |
| Cholesterol (10 unit increase, mg/dL) | ||
| LDL | 1.06 (0.97–1.16) | 1.07 (0.98–1.17) |
| HDL | 0.80 (0.62–1.04) | 0.77 (0.60–0.99) |
| Diastolic blood pressure‡ (10-unit increase, mmHg) | 1.47 (1.05–2.06) | 1.55 (1.11–2.17) |
| Current smoking (yes vs. no) | 2.25 (1.22–4.15) | 2.25 (1.23–4.14) |
| Year 1 IMT | 1.96 (0.78–4.88) | 2.24 (0.89–5.64) |
| ROC AUC | 0.779 | 0.764 |
*Both models are additionally adjusted for ultrasonography equipment.
†The numerical cut point for high IMT progression from EDIC year 1 to EDIC year 6 was greater than 0.179 mm.
‡Diastolic rather than systolic blood pressure was included because although not significantly associated with high ICA IMT, it was a stronger predictor than systolic blood pressure in our study population.
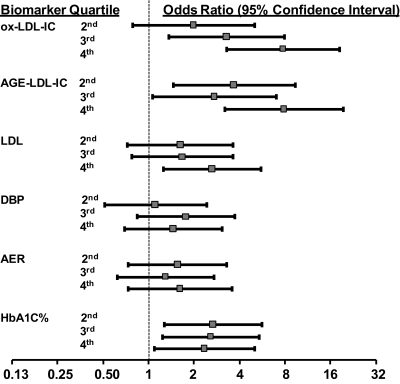
Finally, a comparison of the discriminatory power of oxLDL and AGE-LDL concentrations in isolated LDL-IC with that of LDL cholesterol, diastolic blood pressure, AER, and hemoglobin A1c shows the adjusted odds ratios for having high versus normal ICA IMT, comparing those in the highest versus lowest quartile of LDL cholesterol, diastolic blood pressure, AER, and hemoglobin A1c, were 2.62 (95% CI 1.24, 5.55), 1.45 (95% CI 0.69, 3.03), 1.61 (95% CI 0.73, 3.56), and 2.33 (95% CI 1.09, 4.99), respectively (Fig. 2). Furthermore, ROC AUC, used as mentioned in methods to compare the discriminatory power of the various multivariate models, were 0.750, 0.740, and 0.739, respectively, for LDL cholesterol, diastolic blood pressure, and AER models, and 0.747 for the hemoglobin A1c model. In comparison, the ROC AUC for the analogous models for oxLDL and AGE-LDL in LDL-IC were 0.794 and 0.790, respectively.
FIG. 2.
Adjusted* odds ratios with 95% confidence intervals (calculated from multivariate logistic regression models) for given levels of oxLDL in isolated LDL-IC, AGE-LDL in isolated LDL-IC, LDL cholesterol, diastolic blood pressure (DBP), albumin excretion rate (AER), and hemoglobin A1c (levels in the 2nd, 3rd, and 4th quartiles relative to quartile 1) to predict internal carotid artery IMT at EDIC year 6. OxLDL-IC categories are 5–89, 90–162, 163–305, and 306–1382 mg/L; AGE-LDL-IC categories are 0.15–2.64, 2.65–6.42, 6.43–12.03, and 12.17–305.34 mg/L; LDL categories are 29–89, 90–105, 106–126, and 127–219 mg/dL; DBP categories are 40–66, 68–73, 74–79, and 80–98 mmHg; AER categories are 1.4–6.0, 7.0–10, 11–19, and 20–151 mg/24 h; hemoglobin A1c categories are 5.9–7.7, 7.7–8.5, 8.5–9.9, and 9.9–14.4%. High carotid artery IMT was defined as being in the upper quintile as compared with the lower 4 quintiles of internal carotid artery IMT at EDIC year 6. The numerical cut point for high IMT at EDIC year 6 was greater than 0.845 mm. *Adjusted for age, sex, study group, retinopathy status, duration of diabetes at study entry, logarithm of AER (except for when AER is the categorical variable), percent hemoglobin A1c (except for when hemoglobin A1c is the categorical variable), and ultrasonography equipment.
DISCUSSION
Our study has shown that high levels of oxLDL and AGE-LDL in circulating IC, even when measured at a very young age and when the patient is completely free of macrovascular disease, are strongly predictive of increased IMT and thickening over a period of 8–14 years. The associations of the modified lipoprotein levels in circulating IC with ICA IMT were generally larger than those of classical predictive factors such as albumin excretion rate, LDL cholesterol, hemoglobin A1c, or blood pressure.
The intercorrelation between the levels of MDA-LDL, AGE-LDL, and oxLDL in circulating IC was to be expected, since the precipitated IC are not necessarily made of LDL molecules with single modifications but rather of LDL molecules with multiple epitopes formed by different mechanisms, recognized by antibodies of different specificities. Furthermore, some of the epitopes, such as carboxymethyllysine (CML), are shared by AGE-LDL and oxLDL (15). Therefore, our measurements reflect the relative distribution of epitopes related to copper oxidation, MDA, and AGE modifications in the population of modified LDL molecules involved in IC formation. Interestingly, the levels of MDA-LDL were not as predictive of ICA IMT. We have shown that copper-oxidized LDL contains non-MDA epitopes recognized by human antibodies (15). This observation suggests that although MDA epitopes are present in modified LDL from isolated IC, the antibodies involved in IC formation react predominantly with non-MDA epitopes, and this could explain why the measurement of oxLDL in isolated LDL-IC is a better predictor of elevated carotid artery IMT.
The proinflammatory properties of LDL-IC have been well characterized (34). Their pathogenic potential results from the fact that human modified LDL antibodies are predominantly of the IgG isotype, which can diffuse easily across the endothelial barrier. In addition, modified LDL antibodies are predominantly of the IgG1 and IgG3 isotypes (14,19,20,35,36), able to activate the complement system by the classical pathway (37) and to interact with Fcγ-receptors in phagocytic cells (38), specifically macrophages in the vessel wall, and therefore promote cell activation and inflammation. Given that both circulating and complexed human autoantibodies to both oxLDL and AGE-LDL are predominantly IgG of the IgG1 an IgG3 isotypes (14,19,20,35,36), it would be fully expected that they could play a pathogenic role in chronic inflammatory processes, such as atherosclerosis.
A limitation of our study is that the measurement of modified lipoproteins in IC isolated from peripheral blood is only a surrogate marker for the formation of extravascular IC; however, it is very likely that these peripheral IC levels are reasonable surrogate markers since both modified LDL and the corresponding antibodies have been identified in atheromatous lesions (16,17). A second limitation of the study is that the 479 participants with IC measurements available, because selected for a case-control study of albuminuria, were not a random sample of the entire DCCT/EDIC study population. To overcome this selection bias we have controlled for DCCT retinopathy cohort, AER, diabetes duration, and hemoglobin A1c, and we determined that covariates, including DCCT treatment group, were not acting as effect modifiers of associations of interest, indicating that the predictive ability of modified LDL-IC was similar across different levels of these variables. However, there may still be some residual confounding, which we were unable to account for in our analysis.
The measurement of LDL modifications in isolated IC reflects three important steps in the development of the arteriosclerotic process: increased levels of LDL cholesterol, clearly associated with the development of atherosclerosis; increased oxidation and glycoxidative modification of LDL in diabetes; and the impact of the humoral immune response in the inflammatory process associated with atherosclerosis.
Although AGE-LDL modification is more accentuated in hyperglycemic patients, LDL oxidation seems to be a universal event, affecting the general population. The same predominance of IgG1 and IgG3 oxLDL antibodies also has been found in nondiabetic patients and healthy controls (18). Therefore, the pathogenic role of modified LDL in circulating IC should not be limited to diabetic patients. However, it is possible that patients with type 1 diabetes not only generate higher levels of modified LDL through glyco-oxidative processes but, given the complex constellation of genetic factors associated with their autoimmune disease, they could have an enhanced and more potent autoimmune response to modified lipoproteins. It is therefore quite important to investigate whether the same high predictive value of modified LDL in circulating IC for CVD events is also present in type 2 diabetes and in the general population.
Regardless of the results of such studies, it is, however, quite clear that high levels of oxLDL and AGE-LDL in circulating IC have a major impact on the progression of carotid IMT in type 1 diabetes and they may help to identify patients at high risk for CVD events.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a program project funded by the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grant PO1-HL55782; by NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants R01-DK081352 and R01-DK088778; and by Juvenile Diabetes Research Foundation Grant 2006-49. This work was also supported by the Research Service of the Ralph H. Johnson Department of Veterans Affairs Medical Center.
The DCCT/EDIC was sponsored through research contracts from the Division of Diabetes, Endocrinology and Metabolic Diseases (NIDDK) of the NIH. Additional support was provided by the National Center for Research Resources through the GCRC program and by Genentech through a Cooperative Research and Development Agreement with the NIDDK. The DCCT/EDIC group provided the samples to be analyzed and the clinical data used in data analysis. No other potential conflicts of interest relevant to this article were reported.
M.F.L.-V. wrote the article and provided the researched data. K.J.H. and N.L.B. wrote the article and were primarily responsible for the statistical analysis of the researched data. J.L. and D.M.N. revised and edited the article and provided consultation with respect to data analysis and data presentation in the article. G.V. wrote the article and provided the researched data.
Footnotes
This article contains supplementary data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0915/-/DC1.
*A complete list of Epidemiology of Diabetes Interventions and Complications Research Group participants is available in the supplementary data online.
REFERENCES
- 1.Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–874 [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999;340:115–126 [DOI] [PubMed] [Google Scholar]
- 3.Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat Med 2002;8:1211–1217 [DOI] [PubMed] [Google Scholar]
- 4.Drake TA, Hannani K, Fei H, Lavi S, Berliner JA. Minimally oxidized low-density lipo-protein induces tissue factor expression in cultured human endothelial cells. Am J Pathol 1991;138:601–607 [PMC free article] [PubMed] [Google Scholar]
- 5.Kusuhara M, Chait A, Cader A, Berk BC. Oxidized LDL stimulates mitogen-activated protein kinases in smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol 1997;17:141–148 [DOI] [PubMed] [Google Scholar]
- 6.Liao F, Berliner JA, Mehrabian M, et al. Minimally modified low density lipoprotein is biologically active in vivo in mice. J Clin Invest 1991;87:2253–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quinn MT, Parthasarathy S, Fong LG, Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci USA 1987;84:2995–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ylä-Herttuala S. Biochemistry of the arterial wall in developing atherosclerosis. Ann N Y Acad Sci 1991;623:40–59 [DOI] [PubMed] [Google Scholar]
- 9.Terkeltaub R, Banka CL, Solan J, Santoro D, Brand K, Curtiss LK. Oxidized LDL induces monocytic cell expression of interleukin-8, a chemokine with T-lymphocyte chemotactic activity. Arterioscler Thromb 1994;14:47–53 [DOI] [PubMed] [Google Scholar]
- 10.Hazen SL. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J Biol Chem 2008;283:15527–15531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virella G, Lopes-Virella MF. Atherogenesis and the humoral immune response to modified lipoproteins. Atherosclerosis 2008;200:239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol 2010;134:33–46 [DOI] [PubMed] [Google Scholar]
- 13.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA 1995;92:3893–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virella G, Lopes-Virella MF. Lipoprotein autoantibodies: measurement and significance. Clin Diagn Lab Immunol 2003;10:499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Virella G, Thorpe SR, Alderson NL, et al. Definition of the immunogenic forms of modified human LDL recognized by human autoantibodies and by rabbit hyperimmune antibodies. J Lipid Res 2004;45:1859–1867 [DOI] [PubMed] [Google Scholar]
- 16.Ylä-Herttuala S, Palinski W, Rosenfeld ME, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest 1989;84:1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ylä-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb 1994;14:32–40 [DOI] [PubMed] [Google Scholar]
- 18.Mironova M, Virella G, Lopes-Virella MF. Isolation and characterization of human antioxidized LDL autoantibodies. Arterioscler Thromb Vasc Biol 1996;16:222–229 [DOI] [PubMed] [Google Scholar]
- 19.Virella G, Carter RE, Saad A, Crosswell EG, Game BA, Lopes-Virella MF, DCCT/EDIC Study Group Distribution of IgM and IgG antibodies to oxidized LDL in immune complexes isolated from patients with type 1 diabetes and its relationship with nephropathy. Clin Immunol 2008;127:394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saad AF, Virella G, Chassereau C, Boackle RJ, Lopes-Virella MF. OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J Lipid Res 2006;47:1975–1983 [DOI] [PubMed] [Google Scholar]
- 21.Virella G, Derrick MB, Pate V, Chassereau C, Thorpe SR, Lopes-Virella MF. Development of capture assays for different modifications of human low-density lipoprotein. Clin Diagn Lab Immunol 2005;12:68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mironova M, Virella G, Virella-Lowell I, Lopes-Virella MF. Anti-modified LDL antibodies and LDL-containing immune complexes in IDDM patients and healthy controls. Clin Immunol Immunopathol 1997;85:73–82 [DOI] [PubMed] [Google Scholar]
- 23.Mironova MA, Klein RL, Virella GT, Lopes-Virella MF. Anti-modified LDL antibodies, LDL-containing immune complexes, and susceptibility of LDL to in vitro oxidation in patients with type 2 diabetes. Diabetes 2000;49:1033–1041 [DOI] [PubMed] [Google Scholar]
- 24.Lopes-Virella MF, Virella G, Orchard TJ, et al. Antibodies to oxidized LDL and LDL-containing immune complexes as risk factors for coronary artery disease in diabetes mellitus. Clin Immunol 1999;90:165–172 [DOI] [PubMed] [Google Scholar]
- 25.Orchard TJ, Virella G, Forrest KY, Evans RW, Becker DJ, Lopes-Virella MF. Antibodies to oxidized LDL predict coronary artery disease in type 1 diabetes: A nested case-control study from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes 1999;48:1454–1458 [DOI] [PubMed] [Google Scholar]
- 26.Lopes-Virella MF, McHenry MB, Lipsitz S, et al. DCCT/EDIC Research Group Immune complexes containing modified lipoproteins are related to the progression of internal carotid intima-media thickness in patients with type 1 diabetes. Atherosclerosis 2007;190:359–369 [DOI] [PubMed] [Google Scholar]
- 27.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 28.Epidemiology of Diabetes Interventions and Complications (EDIC) Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med 1998;128:262–269 [DOI] [PubMed] [Google Scholar]
- 30.Nathan DM, Lachin J, Cleary P, et al. Diabetes Control and Complications Trial. Epidemiology of Diabetes Interventions and Complications Research Group Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003;348:2294–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group Effect of intensive diabetes treatment on carotid artery wall thickness in the epidemiology of diabetes interventions and complications. Diabetes 1999;48:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The DCCT Research Group Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter study. Clin Chem 1987;33:2267–2271 [PubMed] [Google Scholar]
- 33.Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98(Suppl. 5):823–833 [PubMed] [Google Scholar]
- 34.Virella G, Tsokos G. Immune complex diseases. In Medical Immunology. Virella G, Ed. London, NY, Informa, 2007, p. 323–334 [Google Scholar]
- 35.Virella G, Koskinen S, Krings G, Onorato JM, Thorpe SR, Lopes-Virella M. Immunochemical characterization of purified human oxidized low-density lipoprotein antibodies. Clin Immunol 2000;95:135–144 [DOI] [PubMed] [Google Scholar]
- 36.Virella G, Thorpe SR, Alderson NL, et al. Autoimmune response to advanced glycosylation end-products of human LDL. J Lipid Res 2003;443:487–493 [DOI] [PubMed] [Google Scholar]
- 37.Michaelsen TE, Sandlie I, Bratlie DB, Sandin RH, Ihle O. Structural difference in the complement activation site of human IgG1 and IgG3. Scand J Immunol 2009;70:553–564 [DOI] [PubMed] [Google Scholar]
- 38.Burton DR, Woof JM. Human antibody effector function. Adv Immunol 1992;51:1–84 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.