Abstract
OBJECTIVE
Although Smad3 has been considered as a downstream mediator of transforming growth factor-β (TGF-β) signaling in diabetes complications, the role of Smad7 in diabetes remains largely unclear. The current study tests the hypothesis that Smad7 may play a protective role and has therapeutic potential for diabetic kidney disease.
RESEARCH DESIGN AND METHODS
Protective role of Smad7 in diabetic kidney disease was examined in streptozotocin-induced diabetic mice that have Smad7 gene knockout (KO) and in diabetic rats given Smad7 gene transfer using an ultrasound-microbubble-mediated technique.
RESULTS
We found that mice deficient for Smad7 developed more severe diabetic kidney injury than wild-type mice as evidenced by a significant increase in microalbuminuria, renal fibrosis (collagen I, IV, and fibronectin), and renal inflammation (interleukin-1β [IL-1β], tumor necrosis factor-α [TNF-α], monocyte chemoattractant protein-1 [MCP-1], intracellular adhesion molecule-1 [ICAM-1], and macrophages). Further studies revealed that enhanced renal fibrosis and inflammation in Smad7 KO mice with diabetes were associated with increased activation of both TGF-β/Smad2/3 and nuclear factor-κB (NF-κB) signaling pathways. To develop a therapeutic potential for diabetic kidney disease, Smad7 gene was transferred into the kidney in diabetic rats by an ultrasound-microbubble-mediated technique. Although overexpression of renal Smad7 had no effect on levels of blood glucose, it significantly attenuated the development of microalbuminuria, TGF-β/Smad3-mediated renal fibrosis such as collagen I and IV and fibronectin accumulation and NF-κB/p65-driven renal inflammation including IL-1β, TNF-α, MCP-1, and ICAM-1 expression and macrophage infiltration in diabetic rats.
CONCLUSIONS
Smad7 plays a protective role in diabetic renal injury. Overexpression of Smad7 may represent a novel therapy for the diabetic kidney complication.
Diabetic nephropathy is a major complication of diabetes (1–4). Approximately 20–40% of patients with type 1 or type 2 diabetes mellitus (DM) develop diabetic nephropathy (5,6). Diabetic nephropathy is characterized by excessive deposition of extracellular matrix (ECM) proteins in the mesangium and tubulointerstitium, thickness of basement membrane of the glomeruli, and loss of podocytes with the development of microalbuminuria and a decline of renal function (2,3,7). Both in vivo and in vitro studies have demonstrated that renal fibrosis and inflammation play an important role in the pathogenesis of diabetic kidney disease (2–4,7–10). Transforming growth factor-β (TGF-β) family is a crucial mediator in the development of diabetic nephropathy (11–15).
It is now clear that after binding to its receptors, TGF-β signals through two critical downstream mediators, Smad2 and Smad3, to exert its biological activities such as ECM production. In addition, TGF-β1 also induces an inhibitory Smad called Smad7, which negatively regulates activation of Smad2/3 by TGF-β receptor competition and degradation via the ubiquitin-proteasome degradation mechanism (16,17). Recent studies have demonstrated that overexpression of Smad7 is capable of inhibiting renal fibrosis and inflammation by blocking the activation of both TGF-β/Smad and nuclear factor-κB (NF-κB) signaling pathway (18–22). In contrast, deletion of Smad7 promotes renal fibrosis and inflammation (23), suggesting that Smad7 may be a key regulator and a therapeutic agent for renal fibrosis and inflammation (24). Although it has been reported that Smad3 is pathogenic in fibrosis including diabetic kidney disease (25,26), the role of Smad7 in diabetes complications remains unexplored, and it is unknown whether blockade of the TGF-β signaling pathway by Smad7 has therapeutic potential for diabetes complications. Thus, in the current study, we uncovered the role of Smad7 in diabetic kidney disease induced in Smad7 knockout (KO) mice and developed new therapeutic strategy for diabetic kidney complication by targeting the TGF-β/Smad pathway with ultrasound-microbubble-mediated Smad7 gene therapy.
RESEARCH DESIGN AND METHODS
Animal models.
Diabetes was induced in genetically identical littermate Smad7 KO and wild-type (WT) mice (CD-1 background mice, male, aged 12–14 weeks, 32.91 ± 0.80 vs. 43.68 ± 1.06 g) by a daily intraperitoneally injection of 50 mg/kg streptozotocin (STZ) for 5 consecutive days as recommended by Animal Models of Diabetic Complications Consortium (27). Smad7 KO mice were generated by functionally deleting exon I in the Smad7 gene as previously described (28), and the genotype was confirmed by PCR with primers as shown in Supplementary Fig. 1A. Nonfasting blood glucose was monitored weekly for the first 2 weeks after the last STZ injection and then every 4 weeks by blood glucose meter (Optium Xceed systems, Victoria, Australia). Urinary samples (16 h) were collected in metabolic cages for microalbuminuria assay. Groups of eight mice were killed at 24 weeks for examination. In addition, groups of six normal Smad7 KO and WT mice that received sodium citrate buffer, instead of STZ, were used as normal age-matched control.
To develop a therapeutic strategy for diabetic kidney disease with ultrasound-microbubble-mediated Smad7 gene transfer, a diabetic rat model was used because when compared with mice, rats are more sensitive to STZ-induced diabetic renal injury and more technically feasible for gene delivery via the left renal artery for 4 weeks as previously described (19). Briefly, experimental diabetes was induced in Sprague-Dawley rats (male, aged 6 weeks, 220–240 g) by a single injection of STZ (60 mg/kg i.p.). Groups of six diabetic rats were randomized to three groups: 1) diabetes group (DM), 2) diabetes treated with empty vector control, and 3) diabetes treated with Smad7 (Smad7) using the ultrasound-microbubble gene transfer technique as described below. Blood glucose and 24-h microalbuminuria were examined as described above. All animals were killed at week 5 after STZ injection. A group of six rats treated with sodium citrate buffer was used as normal age-matched control. The experimental protocols for both diabetic models induced in mice or rats were approved by the Institutional Animal Experimentation Ethics Committee.
Ultrasound-mediated gene transfer of inducible Smad7 gene-bearing microbubbles into the kidney.
An established gene transfer technique using the ultrasound-mediated inducible Smad7 gene-bearing microbubbles was used in this study as described previously (18,19). Briefly, after 1 day STZ injection, the left rat kidney was transfected with a mixture of pTRE–Flag-M2Smad7 and pEFpurop-Tet-on with Optison (echo contrast agents, Mallinckrodt, St. Louis, MO) in 1:1 vol/vol ratio, containing 25 µg of the designated plasmids in 0.5 mL saline, via the left renal artery with temporarily clipping off the renal blood supply (<5 min). The ultrasound transducer (Ultax UX-301; Celcom Medico, Japan) was directly applied onto the left kidney with a continuous-wave output of 1-MHz ultrasound at 5% power output, for a total of 60 s with 30-s intervals. Immediately after ultrasound, all animals were received with doxycycline in the drinking water (200 μg/mL) to induce Smad7 expression. Control animals had the same protocol but received the pTRE/Tet-on empty vectors without Smad7.
Smad7 gene transfection rate and transgene expression within the kidney was determined by immunohistochemistry with monoclonal antibody to Flag-M2, and levels of Smad7 expression were determined by quantitative real-time PCR, Western blot, and immunohistochemistry with anti-Smad7 antibody as previously described (18).
Real-time PCR.
Renal cortex was collected by carefully removing the renal pelvis and medullar tissues and was frozen at −80°C freezer for analysis of the gene of interest by quantitative real-time PCR as previously described (23). The primers used for detection of both mouse and rat mRNA expression including interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), intracellular adhesion molecule-1 (ICAM-1), monocyte chemoattractant protein-1 (MCP-1), TGF-β1, Smad7, collagen I, and GAPDH were described previously (19,23), whereas the primers for collagen IV and fibronectin were described below: mouse collagen IV: forward 5′-GCAACGGTACAAAGGGAGAGAG-3′, reverse 5′-CTTCATTCCTGGTAACCCTGGTG-3′; mouse fibronectin: forward 5′-TACCAAGGTCAATCCACACCCC-3′, reverse 5′-CAGATGGCAAAAGAAAGCAGAGG-3′; rat fibronectin: forward 5′-TCTATCCAGGGAGGGCAGTGT-3′, reverse 5-GTTTGCCAAGGGCTCATCTC-3′. House keep gene GAPDH was used as an internal standard. The ratio for the mRNA was examined against GAPDH and was expressed as mean ± SE.
Western blot analysis.
Renal cortical tissue samples were lysed with radioimmunoprecipitation assay (RIPA) buffer, and proteins were extracted for Western blot analysis as described previously (22). Briefly, after protein was transferred onto a nitrocellulose membrane, the membrane was incubated overnight with primary antibodies against phospho-IκBα (ser32), phospho-p65 (ser276), phospho-Smad2, phospho-Smad3 (Cell Signaling Technology), fibronectin (Dako, Glostrup, Denmark), IκBα, p65, Smad7 (Santa Cruz Biotechnology), and collagen I and collagen IV (Southern Biotech, Birmingham, AL), followed by the LI-COR IRDye 800-labeled secondary antibodies (Rockland Immunochemicals, Gilbertsville, PA). The signals were detected with Odyssey Infrared Imaging System (Li-COR Biosciences, Lincoln, NE) and quantitated with ImageJ program (National Institutes of Health). The ratio for the protein examined was normalized against GAPDH and expressed as mean ± SE.
Microalbumin urine and urinary creatinine analysis.
Urinary microalbumin was detected by a competitive ELISA according to the manufacturer's protocol. Mouse urinary creatinine was measured by a colorimetric method (QuantiChrom Creatinine Assay Kit, Hayward, CA). Although microalbuminuria in rats was expressed as milligrams per 24 h, results of mouse microalbuminuria were expressed as protein/urine creatinine (in μg/mg).
Histology and immunohistochemistry.
Changes in renal morphology were examined in methyl Carnoy’s fixed, paraffin-embedded tissue sections (4 μm) stained with the Periodic Acid–Schiff (PAS) method. Immnunostaining was performed on paraffin sections using a microwave-based antigen retrieval technique (29). The antibodies used in the study were as followed: fibronectin (Dako), collagen I and IV (Southern Tech), ICAM-1, MCP-1, IL-1β, and TNF-α (Santa Cruz Biotechnology). After being immunostained with the secondary antibodies, sections were developed with diaminobenzidine to produce a brown color and counterstained with hematoxylin.
Quantitation of immunostaining was carried on coded slides as previously described (18,22,23). Expression of collagen IV, fibronectin, IL-1β, TNF-α, MCP-1, and ICAM-1 in the entire cortical tubulointerstitium (a cross-section of the kidney) was determined using the quantitative Image Analysis System (AxioVision 4, Carl Zeiss, Germany) as previously described (22,23), whereas expression in glomeruli was determined as described below. Briefly, 20 glomeruli were randomly selected from each section and positive signals within the selected glomerulus were highlighted, measured, and expressed as percent positive area of the entire glomerulus. The number of phopsho-p65, phospho-Smad2/3, F4/80+, ED1+ cells was counted in 20 consecutive glomeruli and expressed as cells/glomerular cross-section, whereas positive cells in the tubulointerstitium were counted under high-power fields (×40) by means of a 0.0625-mm2 graticule fitted in the eyepiece of the microscope and expressed as cells per millimeters squared.
Statistical analysis.
Data from studies were expressed as mean ± SE. Statistical analyses were performed using one-way analysis of variance (ANOVA) from the Prism Program (Prism 5.0 GraphPad Software, San Diego, CA) for collagen matrix and inflammatory parameters in the rat model of diabetes, whereas a two-way ANOVA was used for disease parameters obtained from Smad7 WT and KO mice. In addition, a repeated ANOVA analysis was used for albumin excretion analysis over the entire disease course in both mouse and rat models.
RESULTS
Smad7 KO mice have aggravated diabetic renal injury.
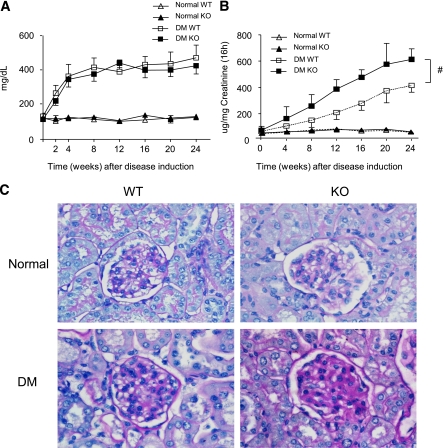
After multiple low doses of STZ injection for 5 consequent days, both Smad7 KO and WT mice developed equal levels of hyperglycemia (blood glucose 300–400 mg/dL) and body weight (38.99 ± 1.60 vs. 41.60 ± 1.42 g) over the 24-week periods (Fig. 1A), indicating a comparable damage with pancreatic islets of Smad7 WT and KO mice. However, Smad7 KO mice developed more severe microalbuminuria than the WT mice over the 24-week disease course (Fig. 1B). Pathologically, an increase in mesangial matrix and thickness of glomerular basement membrane was apparent in WT mice at week 24 of diabetes, which was further enhanced in diabetic Smad7 KO mice (Fig. 1C).
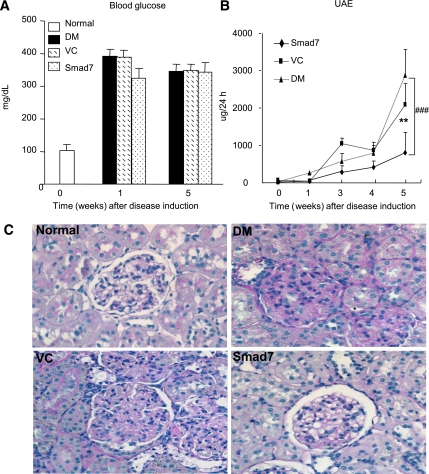
FIG. 1.
Diabetic kidney injury is enhanced in Smad7 KO mice. A: Blood glucose. Levels of blood glucose are significantly increased at week 2 after STZ injection and maintained at equal higher levels over the 24-week period in both Smad7 KO and WT mice. B: Urinary albumin exertion (UAE). Smad7 KO mice had more severe microalbuminuria than the WT mice. C: Histology (PAS-stained sections). Data are expressed as mean ± SE for group of eight mice. #P < 0.05 vs. WT DM group by 2-way ANOVA. Magnification ×400. (A high-quality color representation of this figure is available in the online issue.)
Renal fibrosis is enhanced in diabetic Smad7 KO mice.
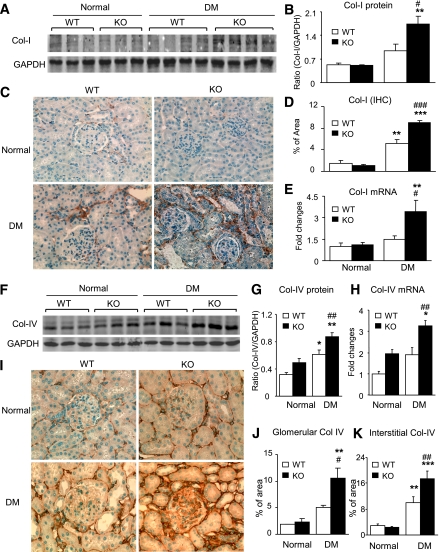
As shown in Fig. 2, analysis with immunohistochemistry, Western blot, and real-time PCR showed that deletion of Smad7 largely increased collagen I (Fig. 2A–E), collagen IV (Fig. 2F–K), and fibronectin (Supplementary Fig. 2A–E) expression and deposition in the diabetic kidney in both glomeruli and tubulointerstitium when compared with the diseased WT mice. Immunohistochemistry revealed that although increased collagen I deposition was largely confined to the area of tubulointerstitium (Fig. 2C), an abundant collagen IV and fibronectin accumulation was noted in both glomerular and tubulointerstitial areas and was largely increased in Smad7 KO mice as shown in Fig. 2I–K and in Supplementary Fig. 2C and D.
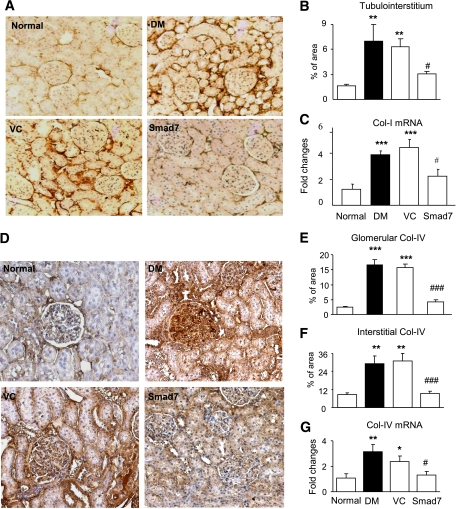
FIG. 2.
Renal fibrosis is enhanced in diabetic Smad7 KO mice. A–E: Collagen I (Col I) expression. F–K: Collagen IV (Col IV) expression. Results show that when compared with the WT mice, Smad7 KO mice significantly enhance renal collagen I and IV expression as demonstrated by Western blot analysis (A, B, F, and G), immunohistochemistry (IHC; C and I) and quantitative analysis in glomeruli (J) and tubulointerstitium (D and K), and real-time PCR at the mRNA level (E and H). Data represent mean ± SE for groups of eight animals. *P < 0.05, **P < 0.01, ***P < 0.001 vs. normal; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. WT DM mice. Magnification ×400 (A and F). (A high-quality color representation of this figure is available in the online issue.)
Renal inflammation was exacerbated in diabetic Smad7 KO mice.
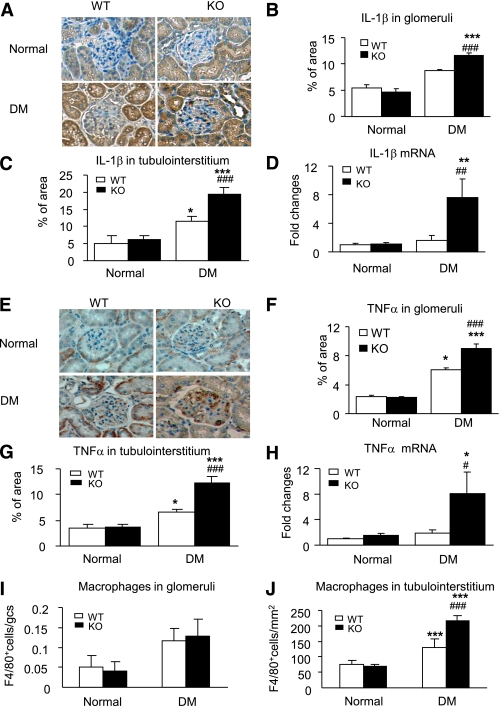
Because inflammation is a critical process in the development of diabetes complication (30,31), we examined whether disruption of Smad7 gene influences renal inflammation under diabetic conditions. As shown in Fig. 3 and in Supplementary Fig. 3, immnunohistochemistry and real-time PCR revealed that when compared with Smad7 WT mice, mice deficient for Smad7 exhibited a substantial increase in renal inflammation in both glomeruli and tubulointerstitium, resulting in a four- to sixfold upregulation of proinflammatory cytokines IL-1β (Fig. 3A–D) and TNF-α (Fig. 3E–H) and a two- to threefold increase in adhesion molecule ICAM-1 (Supplementary Fig. 3A–D) and chemokine MCP-1 (Supplementary Fig. 3E–H). Enhanced expression of renal IL-1β, TNF-α, ICAM-1, and MCP-1 in Smad7 KO mice was associated with a significant increase in macrophage infiltration (Fig. 3I and J).
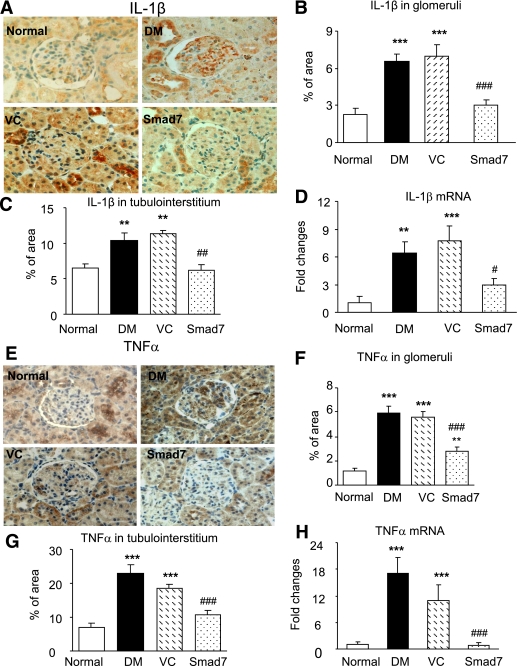
FIG. 3.
Renal inflammation is enhanced in diabetic Smad7 KO mice. A–D: IL-1β expression. E–H: TNF-α expression. I and J: Macrophages infiltrating the glomerulus and the tubulointerstitium. Results show that when compared with the WT mice, Smad7 KO mice develop more severe renal inflammation by enhancing renal IL-1β and TNF-α expression in glomeruli (A, B, E, and F) and in renal cortex (C and G) as demonstrated by immunohistochemistry and mRNA expression real-time PCR (D and H). Data represent mean ± SE for groups of eight animals. *P < 0.05, **P < 0.01, ***P < 0.001 vs. normal; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. WT DM mice. Magnification ×400 (A and E). (A high-quality color representation of this figure is available in the online issue.)
Enhanced activation of TGF-β/Smad3 and NF-κB signaling is a key mechanism by which deletion of Smad7 promotes diabetic renal injury.
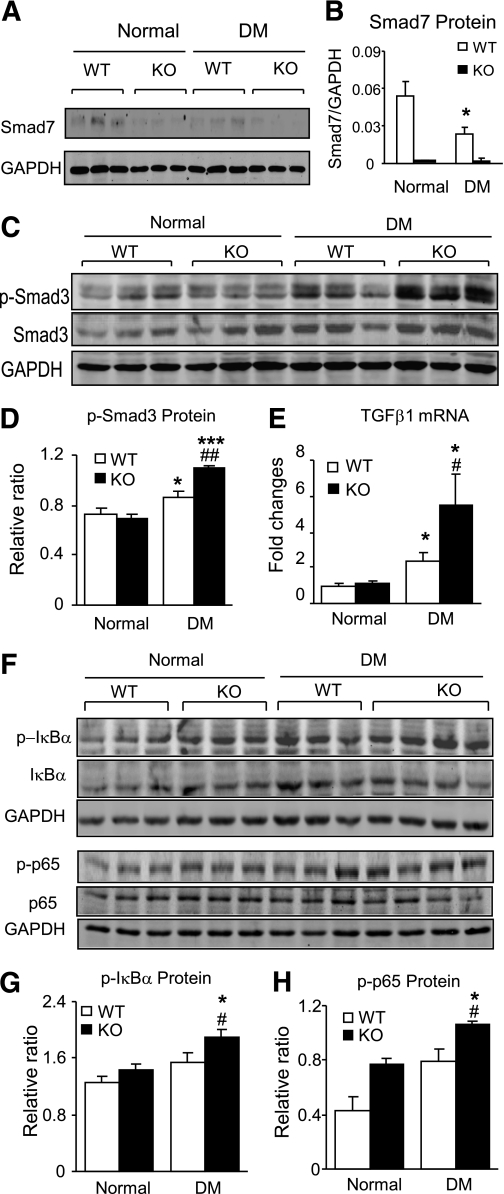
We next investigated the mechanisms by which Smad7 KO mice promoted diabetic renal fibrosis and inflammation by studying the TGF-β/Smad and NF-κB signaling pathways. We first examined Smad7 expression in normal and diabetic mice. Western blot showed that whereas renal Smad7 was not detectable in both normal and diabetic Smad7 KO mice, it was significantly decreased in WT mice with diabetes (Fig. 4A and B). Inhibition of renal Smad7 in diabetic WT mice resulted in a notable increase in phosphorylation of Smad2/3, which was further enhanced in the diabetic kidney of Smad7 KO mice (Fig. 4C and D). Further study by real-time PCR revealed that enhanced activation of Smad3 signaling in Smad7 KO mice was associated with a marked increase in renal TGF-β1 mRNA expression (Fig. 4E), demonstrating that deletion of Smad7 substantially enhanced TGF-β/Smad3 signaling in the diabetic kidney disease.
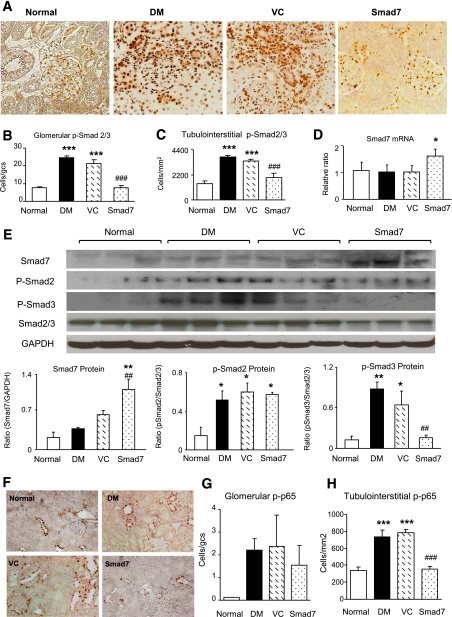
FIG. 4.
Enhanced TGF-β/Smad and NF-κB signaling pathways in diabetic Smad7 KO mice. Western blot analysis (A and B) shows that when compared with normal WT mice, expression of Smad7 is significantly decreased in diabetic WT mice but undetectable in both normal and diabetic KO mice. C and D: Phosphorylation of Smad3. Western blot analysis shows an enhanced TGF-β/Smad3 signaling (p-Smad3) in diabetic Smad7 mice compared with diabetic WT mice. E: Real-time PCR detects an increased renal TGF-β1 mRNA expression in diabetic Smad7 KO mice. F: Activation of NF-κB. Western blot analysis reveals that an increased phosphorylation of IκBα in Smad7 KO mice is associated with enhanced phosphorylation of NF-κB/p65 (p-p65). Note that phosphorylation causes degradation of IκBα and NF-κB/p65 protein, respectively. J: Quantitation of phosphorylated IκBα (p-IκBα). H: Quantitation of phosphorylated p65 (p-p65). Data represent mean ± SE for groups of eight animals. *P < 0.05, ***P < 0.001 vs. normal; #P < 0.05, ##P < 0.01 vs. WT DM mice.
Similarly, enhanced renal inflammation in Smad7 KO mice was associated with a further increase in NF-κB activation in the diabetic kidney when compared with the diabetic Smad7 WT mice (Fig. 4F–H). Indeed, when compared with diabetic WT mice, deletion of Smad7 significantly enhanced NF-κB activation as demonstrated by higher levels of phosphorylated IκBα and NF-κB/p65 (Fig. 4F–H).
Overexpression of renal Smad7 attenuates diabetic kidney injury in a rat model of diabetes.
To further confirm the protective role of Smad7 and develop a novel therapy for diabetic kidney disease with Smad7, we delivered an inducible Smad7 gene into the left diabetic rat kidney via the left renal artery using an ultrasound-microbubble-mediated technique as previously described (18). We first determined the Smad7 gene transfection rate and Smad7 transgene expression mediated by ultrasound-microbubble technique. As shown in Supplementary Fig. 4, immunohistochemistry detected that endogenous Smad7 was high in the normal rat kidney in both glomerular and tubulointerstitial cells (Supplementary Fig. 4B) but was reduced in the diabetic kidney (Supplementary Fig. 4D). In contrast, ultrasound-mediated Smad7 gene transfer into the left kidney resulted in extremely higher levels of Smad7 transgene expression as demonstrated by the finding that almost all glomerular cells and tubulointerstitial cells were strongly positive for Flag-M2 and Smad7 protein (Supplementary Fig. 4E and F). Interestingly, a few Flag-M2 positive cells with low to moderate levels of Smad7 expression were also detected in both glomeruli and tubulointerstitium in the right kidney (Supplementary Fig. 4G and H), although no ultrasound energy was directly applied onto the right kidney.
We then examined the therapeutic effect of Smad7 on diabetic kidney injury. As shown in Fig. 5A and B, gene transfer of Smad7 attenuated microalbuminuria, although levels of blood glucose remained high (>300 mg/dL). Histopathologically, diabetic kidney treated with Smad7 also exhibited an improved ECM deposition in glomeruli and tubulointerstitium when compared with the diabetic rats treated with or without empty vector control (Fig. 5C).
FIG. 5.
Smad7 gene therapy attenuates diabetic kidney injury in rats. A: Blood glucose. B: Microalbuminuria. C: Histology. When compared with the diabetic rats (DM) and empty vector control DM rats (VC), Smad7 gene therapy attenuates microalbuminuria and ECM deposition within the glomerulus and tubulointerstitium. Data represent mean ± SE for groups of six animals. **P < 0.01 vs. normal; ###P < 0.001 vs. DM and VC. (A high-quality color representation of this figure is available in the online issue.)
Overexpression of renal Smad7 inhibits renal fibrosis and inflammation in a rat model of diabetes.
Immunohistochemistry and real-time PCR analysis showed that when compared with normal rat kidney, moderate renal fibrosis was developed in the diabetic kidney treated with or without control empty vectors. This was demonstrated by a significant upregulation and accumulation of collagen I (Fig. 6A–C), collagen IV (Fig. 6D–G), collagen III (Supplementary Fig. 5A–C), and fibronectin (Supplementary Fig. 5D–F) in both glomeruli and tubulointerstitium. In contrast, these fibrotic changes were largely attenuated in the diabetic kidney treated with Smad7 gene transfer (Fig. 6 and Supplementary Fig. 5).
FIG. 6.
Overexpression of renal Smad7 inhibits renal fibrosis in diabetic rats. A–C: Collagen I expression. D–G: Collagen IV expression. Results show that when compared with diabetic rats (DM) and diabetic rats with control vector treatment (VC), Smad7 gene transfer significantly inhibits renal collagen I and IV expression as demonstrated by immunohistochemistry (A, B, and D–F) and real-time PCR at the mRNA levels (C and G). Data represent mean ± SE for groups of six animals. *P < 0.05, **P < 0.01, ***P < 0.001 vs. normal; #P < 0.05, ###P < 0.001 vs. DM and VC mice. Magnification ×200 (A and D). (A high-quality color representation of this figure is available in the online issue.)
Because deletion of Smad7 enhanced renal inflammation in diabetic mice (Fig. 3 and Supplementary Fig. 3), we examined whether overexpression of Smad7 reduces renal inflammation in diabetic rats. Immunohistochemistry and real-time PCR detected that significant renal inflammation was developed in diabetic rats treated with or without empty control vectors, including upregulation of IL-1β (Fig. 7A–D), TNF-α (Fig. 5E–H), ICAM-1 (Supplementary Fig. 6A–D), MCP-1 (Supplementary Fig. 6E–H), and macrophage infiltration (Supplementary Fig. 7) in both glomeruli and tubulointerstitum. In contrast, overexpression of Smad7 largely attenuated these inflammatory responses (Fig. 7 and Supplementary Fig. 6) and blocked macrophage accumulation in glomeruli and tubulointerstitium in the diabetic kidney (Supplementary Fig. 7).
FIG. 7.
Overexpression of renal Smad7 inhibits renal inflammation in diabetic rats. A–D: IL-1β expression. E–H: TNF-α expression. Immunohistochemistry and real-time PCR demonstrate that when compared with diabetic rats (DM) and diabetic rats treated with control vector (VC), Smad7 gene transfer significantly inhibits renal inflammation including IL-1β and TNF-α protein expression in glomeruli (A, B, E, and F) and tubulointerstitium (C and G) and their mRNA expression (D and H). Data represent mean ± SE for groups of six animals. *P < 0.05, **P < 0.01, ***P < 0.001 vs. normal; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. DM and VC mice. Magnification ×400 (A and E). (A high-quality color representation of this figure is available in the online issue.)
Blockade of activation of TGF-β/Smad3 and NF-κB signaling is a key mechanism by which overexpression of Smad7 inhibits diabetic renal injury.
As shown in Fig. 8, immunohistochemistry revealed that a significant increase in activation of TGF-β/Smad signaling (Fig. 8A–C) and NF-κB/p65 signaling (Fig. 8F–H) was apparent in the diabetic rat kidney in both glomeruli and tubulointerstitium treated with or without control plasmids. In contrast, overexpression of Smad7 in the rat kidney of diabetes substantially blocked activation of both TGF-β/Smad and NF-κB/p65 signaling (Fig. 8A–C and F–H). Further studies by real-time and Western blot analysis detected that gene transfer of Smad7 resulted in higher levels of Smad7 mRNA and protein expression in the diabetic rat kidney, thereby inhibiting Smad3, but not Smad2, phosphorylation (Fig. 8D and E).
FIG. 8.
Overexpression of renal Smad7 blocks activation of the TGF-β/Smad and NF-κB signaling pathways in diabetic rats. A: Phosphorylated Smad2/3 (p-Smad2/3) nuclear location. B and C: Quantitative analysis of nucleated p-Smad2/3 in glomeruli and tubulointerstitium. D: Renal Smad7 mRNA expression by real-time PCR. E: Western blot analysis of renal Smad7 and phosphorylation of Smad2 (p-Smad2) and Smad3 (p-Smad3). Note that Smad7 gene transfer upregulates renal Smad7, thereby inhibiting phosphorylation of Smad3, but not Smad2. F: Phosphorylated NF-κB/p65 nuclear location. G and H: Quantitative analysis of phosphorylated NF-κB/p65 in the glomerulus and tubulointerstitium. Data represent mean ± SE for groups of six animals. *P < 0.05, **P < 0.01, ***P < 0.001 vs. normal; ##P < 0.05, ###P < 0.001 vs. DM and VC mice. Magnification ×400 (A and F). (A high-quality color representation of this figure is available in the online issue.)
DISCUSSION
Increasing evidence has shown a critical role for the TGF-β signaling in the development of diabetes complication (32). The current study added new information for a protective role of Smad7, a negative inhibitor of TGF-β/Smad signaling, in diabetic kidney disease and provided new evidence for prevention or treatment of diabetic kidney complication by targeting the TGF-β and NF-κB signaling pathways with Smad7.
A significant finding from the current study is that loss of renal Smad7 may be a mechanism for the development of diabetic kidney complication. This was supported by the finding that renal Smad7 was significantly decreased in 24-week diabetic mice, which is consistent with other studies in 17-week diabetic rats (33), in human mesangial cells under high glucose conditions (34), in obstructive kidney disease (18), and in remnant kidney disease (19). Because Smad7 is known as an inhibitory of TGF-β/Smad signaling and is capable of inducing a NF-κB inhibitor IκBα to block NF-κB activation (14,16), therefore, loss of renal Smad7 in the diabetic kidney may promote TGF-β/Smad-mediated renal fibrosis and NF-κB-driven inflammation. The findings of enhanced diabetic kidney injury such as microalbuminuria, renal fibrosis, and renal inflammation in Smad7 KO mice further supported this notion.
The current study revealed that antifibrosis may be a mechanism by which Smad7 protects kidneys from diabetic injury. Indeed, activation of TGF-β/Smad signaling has been shown in the experimental and human diabetic kidneys (35,36) and is responsible for ECM production in vitro under high glucose and advanced glycation end products conditions (36,37). The functional importance for TGF-β signaling in diabetic kidney complication was demonstrated in a mouse model of diabetes where deletion of Smad3, a key downstream mediator of TGF-β signaling, inhibited diabetic renal injury (25,26). The current study added new evidence for a role of Smad7 in negatively regulating TGF-β/Smad-mediated diabetic kidney damage as evidenced by the finding that loss of Smad7 sustained, but overexpression of Smad7 blocked, TGF-β/Smad2/3-mediated renal injury in diabetes in terms of microalbuminuria and collagen matrix (collagen I and IV and fibronectin) expression in the glumerulus and tubulointerstitium. The protective role of Smad7 in diabetic kidney fibrosis is also consistent with previous findings from other disease models of obstructive and remnant kidney that deletion of Smad7 promotes renal fibrosis, which is inhibited by overexpression of renal Smad7 (18,19,23).
Inhibition of renal inflammation may be another mechanism for a protective role of Smad7 in diabetic renal injury. It is now clear that renal inflammation may also play a role in diabetic kidney disease (30). A number of recent studies have shown that many inflammatory mediators such as IL-1β, TNF-α, MCP-1, ICAM-1, and macrophage infiltration are increased in diabetes complications and mice lacking these mediators are protected against diabetic renal injury (9,38–42). In line with these findings, we also found that enhanced diabetic renal injury in Smad7 KO mice was associated with a significant increase in renal inflammation as evidenced by a further upregulation of IL-1β, TNF-α, MCP-1, ICAM-1, and macrophage infiltration when compared with the diabetic WT mice. In contrast, overexpression of Smad7 in the kidney blocked the development of renal inflammation. These observations strongly suggest a critical role for Smad7 in controlling renal inflammation in diabetes.
NF-κB activation may be a critical mechanism in the development of diabetic kidney disease (43). Indeed, NF-κB is a critical signaling pathway of the inflammatory cascade. The phosphorylation of the NF-κB inhibitor will release the NF-κBp50/p65 subunits, resulting in nuclear accumulation and transcriptional regulation of the target genes (44). We have previously shown that Smad7 controls inflammation by negatively regulating NF-κB signaling via induction of IκBα, an inhibitor of NF-κB, thereby blocking IκBα from degradation and preventing the activation of NF-κB signaling in vivo and in vitro (14,20,23). In the current study, we found that disruption of Smad7 enhanced the phosphorylation of IκBα, thereby resulting in increased activation of NF-κB/p65 signaling in the diabetic kidney. In contrast, overexpression of renal Smad7 inhibited NF-κB activation in the kidney with diabetes. All these findings suggest that Smad7 may negatively regulate the NF-κB signaling pathway to control renal inflammation in diabetic kidney complication. Thus, disruption of Smad7 enhanced, but overexpression of renal Smad7 inhibited, renal inflammation under diabetic conditions.
Taken together, results from the current study revealed that Smad7 may have therapeutic potential for diabetic kidney disease. The findings that ultrasound-mediated Smad7 gene transfer into the left kidney produced a marked inhibition of diabetic kidney injury were very impressive. However, it should be pointed out that because of the use of pan-ultrasound, the energy acted directly onto the left kidney may also reach the right kidney in some degree to influence the uptake of circulating Smad7 as evidenced by a small number of Flag-M2 positive cells, resulting in mild to moderate Smad7 expression in the right kidney. It should also be made aware that outcomes from the current study in both mouse and rat models of diabetes may not directly apply to the diabetic conditions in humans. Nevertheless, the findings from this study may provide new evidence for a better understanding of the biological activities of Smad7 in diabetic kidney disease in terms of protection against renal inflammation and fibrosis. In conclusion, Smad7 may play a protective role in diabetic kidney complication. Blockade of TGF-β/Smad3-mediated renal fibrosis and NF-κB-driven renal inflammation may be mechanisms by which Smad7 protects kidney from diabetic injury. In addition, the ability of overexpression of renal Smad7 to inhibit diabetic kidney disease indicates that Smad7 may be a therapeutic agent for diabetic kidney complication.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from Research Grant Council of Hong Kong SAR (GRF 768207 and 767508 and CUHK5/CRF/09) and Genzyme Innovations Program. No other potential conflicts of interest relevant to this article were reported.
H.Y.C. conceived mouse experiments and analyzed data and drafted the article. X.R.H. generated Smad7 KO mice and conceived experiments of both animal models. W.W. and J.H.L. carried out a rat model of diabetes and analyzed data. R.L.H. made Smad7 KO mice and reviewed and edited the article. A.C.K.C. conceived data analysis and discussion. H.Y.L. designed, supervised, and wrote the article.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0403/-/DC1.
REFERENCES
- 1.Caramori ML, Kim Y, Huang C, et al. Cellular basis of diabetic nephropathy: 1. Study design and renal structural-functional relationships in patients with long-standing type 1 diabetes. Diabetes 2002;51:506–513 [DOI] [PubMed] [Google Scholar]
- 2.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest 1984;74:1143–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 1997;99:342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White KE, Bilous RW. Type 2 diabetic patients with nephropathy show structural-functional relationships that are similar to type 1 disease. J Am Soc Nephrol 2000;11:1667–1673 [DOI] [PubMed] [Google Scholar]
- 5.Finne P, Reunanen A, Stenman S, Groop PH, Grönhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 2005;294:1782–1787 [DOI] [PubMed] [Google Scholar]
- 6.Remuzzi G, Schieppati A, Ruggenenti P. Clinical practice. Nephropathy in patients with type 2 diabetes. N Engl J Med 2002;346:1145–1151 [DOI] [PubMed] [Google Scholar]
- 7.Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol 2003;14:1358–1373 [DOI] [PubMed] [Google Scholar]
- 8.Banba N, Nakamura T, Matsumura M, Kuroda H, Hattori Y, Kasai K. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int 2000;58:684–690 [DOI] [PubMed] [Google Scholar]
- 9.Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int 2004;65:116–128 [DOI] [PubMed] [Google Scholar]
- 10.Ziyadeh FN, Hoffman BB, Han DC, et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA 2000;97:8015–8020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filippi CM, Juedes AE, Oldham JE, et al. Transforming growth factor-β suppresses the activation of CD8+ T-cells when naive but promotes their survival and function once antigen experienced: a two-faced impact on autoimmunity. Diabetes 2008;57:2684–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeves WB, Andreoli TE. Transforming growth factor beta contributes to progressive diabetic nephropathy. Proc Natl Acad Sci USA 2000;97:7667–7669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Kim Y, Caramori ML, et al. Cellular basis of diabetic nephropathy: II. The transforming growth factor-β system and diabetic nephropathy lesions in type 1 diabetes. Diabetes 2002;51:3577–3581 [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Huang XR, Li AG, et al. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol 2005;16:1371–1383 [DOI] [PubMed] [Google Scholar]
- 15.Belghith M, Bluestone JA, Barriot S, Mégret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 2003;9:1202–1208 [DOI] [PubMed] [Google Scholar]
- 16.Hayashi H, Abdollah S, Qiu Y, et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell 1997;89:1165–1173 [DOI] [PubMed] [Google Scholar]
- 17.Kavsak P, Rasmussen RK, Causing CG, et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell 2000;6:1365–1375 [DOI] [PubMed] [Google Scholar]
- 18.Lan HY, Mu W, Tomita N, et al. Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol 2003;14:1535–1548 [DOI] [PubMed] [Google Scholar]
- 19.Hou CC, Wang W, Huang XR, et al. Ultrasound-microbubble-mediated gene transfer of inducible Smad7 blocks transforming growth factor-beta signaling and fibrosis in rat remnant kidney. Am J Pathol 2005;166:761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng YY, Hou CC, Wang W, Huang XR, Lan HY. Blockade of NFkappaB activation and renal inflammation by ultrasound-mediated gene transfer of Smad7 in rat remnant kidney. Kidney Int Suppl 2005; 94:S83–S91 [DOI] [PubMed] [Google Scholar]
- 21.Ka SM, Huang XR, Lan HY, et al. Smad7 gene therapy ameliorates an autoimmune crescentic glomerulonephritis in mice. J Am Soc Nephrol 2007;18:1777–1788 [DOI] [PubMed] [Google Scholar]
- 22.Huang XR, Chung AC, Zhou L, Wang XJ, Lan HY. Latent TGF-beta1 protects against crescentic glomerulonephritis. J Am Soc Nephrol 2008;19:233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung AC, Huang XR, Zhou L, Heuchel R, Lai KN, Lan HY. Disruption of the Smad7 gene promotes renal fibrosis and inflammation in unilateral ureteral obstruction (UUO) in mice. Nephrol Dial Transplant 2009;24:1443–1454 [DOI] [PubMed] [Google Scholar]
- 24.Lan HY. Smad7 as a therapeutic agent for chronic kidney diseases. Front Biosci 2008;13:4984–4992 [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto M, Maezawa Y, Yokote K, et al. Mice lacking Smad3 are protected against streptozotocin-induced diabetic glomerulopathy. Biochem Biophys Res Commun 2003;305:1002–1007 [DOI] [PubMed] [Google Scholar]
- 26.Wang A, Ziyadeh FN, Lee EY, et al. Interference with TGF-beta signaling by Smad3-knockout in mice limits diabetic glomerulosclerosis without affecting albuminuria. Am J Physiol Renal Physiol 2007;293:F1657–F1665 [DOI] [PubMed] [Google Scholar]
- 27.Breyer MD, Böttinger E, Brosius FC, 3rd, et al. AMDCC Mouse models of diabetic nephropathy. J Am Soc Nephrol 2005;16:27–45 [DOI] [PubMed] [Google Scholar]
- 28.Li R, Rosendahl A, Brodin G, et al. Deletion of exon I of SMAD7 in mice results in altered B cell responses. J Immunol 2006;176:6777–6784 [DOI] [PubMed] [Google Scholar]
- 29.Lan HY, Mu W, Nikolic-Paterson DJ, Atkins RC. A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens. J Histochem Cytochem 1995;43:97–102 [DOI] [PubMed] [Google Scholar]
- 30.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 2008;19:433–442 [DOI] [PubMed] [Google Scholar]
- 31.Williams MD, Nadler JL. Inflammatory mechanisms of diabetic complications. Curr Diab Rep 2007;7:242–248 [DOI] [PubMed] [Google Scholar]
- 32.Ziyadeh FN. Different roles for TGF-beta and VEGF in the pathogenesis of the cardinal features of diabetic nephropathy. Diabetes Res Clin Pract 2008;82(Suppl. 1):S38–S41 [DOI] [PubMed] [Google Scholar]
- 33.Dixon A, Maric C. 17Beta-estradiol attenuates diabetic kidney disease by regulating extracellular matrix and transforming growth factor-beta protein expression and signaling. Am J Physiol Renal Physiol 2007;293:F1678–F1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Kang SW, Kim JL, Sung HY, Kwun IS, Kang YH. Isoliquiritigenin entails blockade of TGF-beta1-SMAD signaling for retarding high glucose-induced mesangial matrix accumulation. J Agric Food Chem 2010;58:3205–3212 [DOI] [PubMed] [Google Scholar]
- 35.Hong SW, Isono M, Chen S, Iglesias-De La Cruz MC, Han DC, Ziyadeh FN. Increased glomerular and tubular expression of transforming growth factor-beta1, its type II receptor, and activation of the Smad signaling pathway in the db/db mouse. Am J Pathol 2001;158:1653–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li JH, Huang XR, Zhu HJ, et al. Advanced glycation end products activate Smad signaling via TGF-beta-dependent and independent mechanisms: implications for diabetic renal and vascular disease. FASEB J 2004;18:176–178 [DOI] [PubMed] [Google Scholar]
- 37.Li JH, Huang XR, Zhu HJ, Johnson R, Lan HY. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int 2003;63:2010–2019 [DOI] [PubMed] [Google Scholar]
- 38.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int 2006;69:73–80 [DOI] [PubMed] [Google Scholar]
- 39.Okada S, Shikata K, Matsuda M, et al. Intercellular adhesion molecule-1-deficient mice are resistant against renal injury after induction of diabetes. Diabetes 2003;52:2586–2593 [DOI] [PubMed] [Google Scholar]
- 40.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Tesch GH. Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J Am Soc Nephrol 2005;16:1711–1722 [DOI] [PubMed] [Google Scholar]
- 41.Thomas HE, Irawaty W, Darwiche R, et al. IL-1 receptor deficiency slows progression to diabetes in the NOD mouse. Diabetes 2004;53:113–121 [DOI] [PubMed] [Google Scholar]
- 42.Ehses JA, Lacraz G, Giroix MH, et al. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci USA 2009;106:13998–14003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Starkey JM, Haidacher SJ, LeJeune WS, et al. Diabetes-induced activation of canonical and noncanonical nuclear factor-κB pathways in renal cortex. Diabetes 2006;55:1252–1259 [DOI] [PubMed] [Google Scholar]
- 44.Hatakeyama S, Kitagawa M, Nakayama K, et al. Ubiquitin-dependent degradation of IkappaBalpha is mediated by a ubiquitin ligase Skp1/Cul 1/F-box protein FWD1. Proc Natl Acad Sci USA 1999;96:3859–3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.