Abstract
OBJECTIVE
Arachidonic acid is metabolized by 12-lipoxygenase (12-LOX) to 12-hydroxyeicosatetraenoic acid (12-HETE) and has an important role in the regulation of angiogenesis and endothelial cell proliferation and migration. The goal of this study was to investigate whether 12-LOX plays a role in retinal neovascularization (NV).
RESEARCH DESIGN AND METHODS
Experiments were performed using retinas from a murine model of oxygen-induced ischemic retinopathy (OIR) that was treated with and without the LOX pathway inhibitor, baicalein, or lacking 12-LOX. We also analyzed vitreous samples from patients with and without proliferative diabetic retinopathy (PDR). Western blotting and RT-PCR were used to assess the expression of 12-LOX, vascular endothelial growth factor (VEGF), and pigment epithelium–derived factor (PEDF). Liquid chromatography–mass spectrometry was used to assess the amounts of HETEs in the murine retina and human vitreous samples. The effects of 12-HETE on VEGF and PEDF expression were evaluated in Müller cells (rMCs), primary mouse retinal pigment epithelial cells, and astrocytes.
RESULTS
Retinal NV during OIR was associated with increased 12-LOX expression and 12-, 15-, and 5-HETE production. The amounts of HETEs also were significantly higher in the vitreous of diabetic patients with PDR. Retinal NV was markedly abrogated in mice treated with baicalein or mice lacking 12-LOX. This was associated with decreased VEGF expression and restoration of PEDF levels. PEDF expression was reduced in 12-HETE–treated rMCs, astrocytes, and the retinal pigment epithelium. Only rMCs and astrocytes showed increased VEGF expression by 12-HETE.
CONCLUSIONS
12-LOX and its product HETE are important regulators of retinal NV through modulation of VEGF and PEDF expression and could provide a new therapeutic target to prevent and treat ischemic retinopathy.
Retinal neovascularization (NV) is a vision-threatening complication of ischemic retinopathy that develops in various retinal disorders, including diabetic retinopathy and retinopathy of prematurity (ROP). Retinal NV is controlled by a balanced production of pro- and antiangiogenic factors, including vascular endothelial growth factor (VEGF) and pigment epithelium–derived factor (PEDF), respectively (1). However, under some pathological conditions, including diabetic retinopathy and ROP, this balance is disrupted by enhanced production of proangiogenic and/or downregulation of antiangiogenic factors (2,3).
Arachidonic acid metabolites, which are known as eicosanoids, are involved in regulating angiogenesis (4). Once released by cytosolic phospholipase A2 (5), arachidonic acid is metabolized via different pathways, including the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 pathways. Angiogenesis has been shown to be promoted by the metabolic products of COX2, prostaglandins (6) and the products of the lipoxygenase system, leukotrienes, and hydroxyeicosatetraenoic acids (HETEs) (7,8). LOXs are a group of closely related dioxygenases that catalyze the stereospecific oxygenation of arachidonic acid and other polyunsaturated fatty acids (PUFAs) and are classified as 5-, 8-, 12-, or 15-LOX, according to the site of oxygen insertion within arachidonic acid.
Three types of 12-LOX have been characterized: platelet, leukocyte, and epidermal 12-LOX (9), which are detected in various cell types, including smooth muscle cells (SMCs) (10), endothelial cells (10,11), and glial cells (12). The major product of 12-LOX metabolism of arachidonic acid, 12-HETE has a role in various biological processes, including atherogenesis, cancer cell growth, and neuronal apoptosis (13,14). In addition, 12-HETE has proinflammatory effects (15,16) and has been implicated in diabetic vascular complications (13). For example, high glucose treatment increases 12-HETE production in vascular endothelial cells and SMCs, and this increase is linked to vascular endothelial growth factor (VEGF) upregulation (17,18) and leukostasis (19) in the intracellular adhesion molecule-1–dependent pathway (15). Similarly, 12-HETE has been shown to contribute to tumor angiogenesis via a VEGF-dependent pathway (20) and to stimulate endothelial cell mitogenesis (7,8) and tube formation (21).
VEGF and PEDF are identified as key angiogenic factors whose altered production contributes to the development of retinal NV. They induce opposite effects in the retina, which causes vasculopathies associated with diabetic retinopathy and ROP. Although VEGF has angiogenic and permeability effects that were shown to be mediated via oxidative stress (22,23) and inflammatory pathways (24), PEDF elicits antiangiogenic and antipermeability effects in part through antioxidant (25,26) and anti-inflammatory mechanisms (27). There are multiple cellular sources for the growth factors involved in retinal NV. Müller cells (rMCs) are known to express several modulators of angiogenesis by responding to hypoxia or hyperglycemia and releasing VEGF (28,29). They also are shown to have an antiangiogenic background attributed to PEDF secretion (30). Moreover, VEGF and PEDF expression in rMCs are altered by a high glucose concentration, which contributes to retinal NV in diabetic retinopathy (31).
The role of lipoxygenases in general, and 12-LOX in particular, in the development of retinal NV has not been well investigated. The goal of this study was to explore the changes in 12-LOX expression and activity during retinal NV and to determine whether targeting 12-LOX activity impacts retinal NV perhaps through changes in the level of angiogenic factors.
The current study presents, for the first time, that oxygen-induced ischemic retinopathy (OIR) and proliferative diabetic retinopathy (PDR) are associated with increased 12-LOX expression and activity. Inhibition of the LOX pathway or 12-LOX deletion significantly abrogated retinal NV and VEGF expression, while preserving retinal PEDF levels during OIR.
RESEARCH DESIGN AND METHODS
Animals.
Wild-type C57BL/6J mice and 12-LOX–deficient mice (B6.129S2-Alox15 <tm1Fun>/J) were obtained from The Jackson Laboratory (Bar Harbor, ME). Backcrossing of 12-LOX knockout mice with wild-type C57BL/6, DNA extraction, and genotyping were performed according to the protocol provided by The Jackson Laboratory using PCR. Animal experiments were performed according to the Association of Research in Vision and Ophthalmology statement for the use of animals in vision research.
Vitreous samples.
Human vitreous samples were obtained from the Department of Ophthalmology, Medical College of Georgia, according to the tenets of the Helsinki Declaration. After obtaining patient consent, vitreous samples were collected from the eyes of individuals undergoing pars plana vitrectomy as a treatment for PDR with tractional retinal detachment. The control group comprised vitreous samples from eyes of patients who were undergoing vitrectomy for nondiabetic causes. Vitreous samples were centrifuged for 5 min and processed for biochemical analysis.
Mouse model of OIR.
Experiments were performed on wild-type C57BL/6J and 12-LOX–deficient mice. The groups comprised control, OIR wild type, OIR treated with the LOX pathway inhibitor baicalein, and OIR 12-LOX–deficient mice. Retinal NV was developed as previously described (32). Murine pups were incubated in high oxygen (75%) for 5 days, from postnatal day 7 to postnatal day 12, followed by 5 days in room air (postnatal days 12–17). One group of OIR mice was treated with baicalein (20 mg/kg/day i.p.) on postnatal days 12–16. All mice were killed on postnatal day 17, and one retina was collected from each animal for biochemical assays while the other eye was harvested whole for morphological study. Additional experiments were performed using db/db mice retinas. Six to eight-week-old mice received streptozotocin (55 mg/kg). Mice with a glucose level of ≥250 mg/dL were considered diabetic. The streptozotocin-induced db/db mice were maintained for 8 weeks, then one eye of each animal was processed for frozen sections and the retina of the other eye was frozen for protein analysis.
Liquid chromatography–mass spectrometry.
Liquid chromatography–mass spectrometry (LC/MS) was used to measure the amount of HETEs in murine retina and vitreous samples. Samples were spiked with 10 ng 15(S)-HETE-d8 (internal standard), acidified to pH <4 with dilute hydrochloric acid, applied to preconditioned SEP-Pak C18 cartridges (100 mg adsorbent; Waters), and washed with water followed by hexane. Eicosanoids were eluted with 500 μL ethyl acetate. The eluate was dried under nitrogen and reconstituted in methanol:25 mmol/L aqueous ammonium acetate (8:2). The extracted and reconstituted sample was subjected to high-performance liquid chromatography on a Max-RP C18 column (2 × 150 mm, 3μ; Phenomenex). The compounds were eluted isocratically with methanol:13 mmol/L aqueous ammonium acetate (8:2) at a flow rate of 0.4 mL/min. The eluent was monitored for HETEs by a mass spectrometer (QuattroLC, Micromass) in the negative ion mode using multiple-reaction monitoring for transitions of m/z 319 to m/z 115 for 5-HETE, m/z 179 for 12-HETE, and m/z 219 for 15-HETE (source block: 120°C, desolvation: 350°C, cone voltage: −24 V, collision energy: 14 eV, and collision gas pressure: 3.2 × 10−4). 15(S)-HETE-d8 (MRM transition of m/z 327 to 226 under identical conditions) was used as an internal standard for recovery and quantitation. Retention times for 15-HETE (and 15(S)-HETE-d8), 12-HETE, and 5-HETE were 2.6, 2.9, and 3.6 min, respectively, and the detection limit was 50 pg for each compound on the column.
12-LOX immunolocalization.
To localize the retinal expression of 12-LOX, retinal frozen sections were fixed by 2% paraformaldhyde, followed by blocking the nonspecific reaction using normal goat serum. The sections then were incubated with PBS containing the vascular marker isolectin-B4 (15 μg/mL vector) and anti–12-LOX (1:100; Santa Cruz) followed by incubation with PBS containing Texas red (25 μg/mL vector) and Oregon green secondary antibody (1:500). Images were collected by confocal microscopy.
Assessment of new vessel formation.
Retinal NV was assessed by labeling retinal vasculature with isolectin-B4. Briefly, the enucleated eye ball was fixed in 4% paraformaldhyde overnight, followed by dissecting the retina out of the eye cup. Then, the retina was incubated in PBS containing 0.2% Triton-X100 and 15 μg/mL isolectin-B4 overnight. This was followed by washing with PBS and incubation in PBS containing 25 μg/mL Texas red for 2 h. The retina was then washed and flat mounted on a slide. Vascular density was calculated using Metamorph Imaging software. Metamorph was used to measure the area of new blood-vessel formation as a percentage of total surface area in retinas.
Cell culture.
rMC-1 was obtained as a gift from Dr. V. Sarthy (Northwestern University, Chicago, IL). The cells were maintained in Dulbecco’s modified Eagle’s medium (Mediatech/Cellgro) supplemented with 4.5 g/L glucose, 2 mmol/L l-glutamine, sodium pyruvate (DMEM:F12), penicillin/streptomycin, and 10% FBS. To determine the effect of 12-HETE on VEGF and PEDF expression in rMC-1, different doses of 12-HETE (Cayman Chemical, Ann Arbor, MI) were added to the cultured rMC-1 (0.5 μmol/L and 1 μmol/L 12-S-HETE in serum-free DMEM:F12). By the end of the experiment (after 72 h), rMC-conditioned medium and cell homogenate were collected and processed for enzyme-linked immunosorbent assay (ELISA) and Western blotting analysis of VEGF and PEDF expression, respectively.
Additional experiments were performed using murine retinal astrocytes and retinal pigment epithelial (RPE) cells. Primary murine retinal astrocytes and RPE cells were prepared as previously described (33). To determine the effects of HETEs on VEGF and PEDF expression, retinal astrocytes and RPE cells were plated. The next day, cells were washed with serum-free medium and then incubated with 1.5 mL serum-free medium containing 1 μmol/L 5-, 12-, or 15-HETE. The next day, 0.5 mL of fresh HETE-containing medium was added to each dish without removing the preconditioned medium. The next day, the conditioned medium was collected for VEGF and PEDF measurements. The cells were used for preparation of total RNA and quantitative PCR analysis, as described below.
Western blotting.
Western blotting was used to assess the expression of 12-LOX, VEGF, and PEDF in retina and retinal cells homogenate. The samples were homogenized in a modified radioimmunoprecipitation assay buffer (20 mmol/L Tris-HCl, pH 7.4; 2.5 mmol/L ethylenediaminetetraacetic acid; 50 mmol/L NaF; 10 mmol/L Na4P2O7; 1% Triton X-100; 0.1% sodium dodecyl sulfate; 1% sodium deoxycholate; and 1 mmol/L phenylmethylsulfonyl fluoride). Homogenates (40 μg protein) were separated by electrophoresis on a precast Tris-HCl 4–20% gradient gel (Bio-Rad) and transferred to the nitrocellulose membrane. Retina homogenates or cell lysate were reacted with different primary antibodies, including platelet type 12-LOX (1:200; Santa Cruz Biotechnology), leukocyte-type 12-LOX (1:1000; Abcam), VEGF (1:500), or PEDF (1:200; Santa Cruz Biotechnology). Protein was detected by appropriate secondary horseradish peroxidase–conjugated antibody (Jackson Immuno Research) and enhanced chemiluminescence (Amersham). Membranes were stripped and reprobed for β-actin to demonstrate equal loading, and the results were quantified by densitometry analysis.
ELISA.
ELISA was used to assess the levels of VEGF in the conditioned medium using immunoassay kits as recommended by the supplier (Chemicon).
VEGF and PEDF quantitative PCR.
The total RNA from the cells was extracted by a mirVana PARIS kit (Ambion) according to the manufacturer’s instructions. cDNA synthesis was performed from 1 μg total RNA using a Sprint RT Complete-Double PrePrimed kit (Clontech). A total of 1 μL of each cDNA (dilution 1:10) was used as a template in the quantitative PCR assays, which were performed in triplicate on a Mastercycler Realplex (Eppendorf) using the SYBR qPCR Premix (Clontech). Amplification parameters were as follows: 95°C for 2 min; 40 cycles of amplification (95°C for 15 s, 60°C for 40 s); and dissociation curve step (95°C for 15 s, 60°C for 15 s, and 95°C for 15 s).
Standard curves were generated from known quantities for each target gene of linearized plasmid DNA. Ten times dilution series were used for each known target, which were amplified using SYBR green quantitative PCR. The linear regression line for each nanogram of DNA was determined from relative fluorescent units at a threshold fluorescence value (Ct) to quantify gene targets from cell extracts by comparing the relative fluorescent units at the Ct to the standard curve. Calnexin was used as a housekeeping gene to normalize all samples. The primer sequences are listed in Table 1.
TABLE 1.
Primer sequences of PEDF and VEGF
| Forward 5′ to 3′ | Reverse 5′ to 3′ | ||
|---|---|---|---|
| Calnexin |
NM_007597.3 |
GCTGCTGGTCCTTGGAACTG |
TCAATATCAATCGCGTCATCATC |
| PEDF |
NM_011340.3 |
GCCCAGATGAAAGGGAAGATT |
TGAGGGCACTGGGCATTT |
| VEGFA164 | M95200.1 | ATCTTCAAGCCGTCCTGTGTGC | CAAGGCTCACAGTGATTTTCTGG |
Statistical analysis.
Group differences were evaluated using the t test or ANOVA followed by a Tukey post hoc test. Results were considered significant when the P value was <0.05. For in vivo studies, age-matched controls were compared with the oxygen-treated mice treated with or without baicalein. For the human study, diabetic subjects with PDR were compared with patients who had a vitrectomy for other causes. For in vitro studies, four dishes were prepared for each treatment group, and each experiment was replicated with at least two different batches of retinal cells. Data were represented as means ± SE from at least six animals and subjects in each group and three experiments from the in vitro study.
RESULTS
Increased 12-LOX expression and activity in OIR.
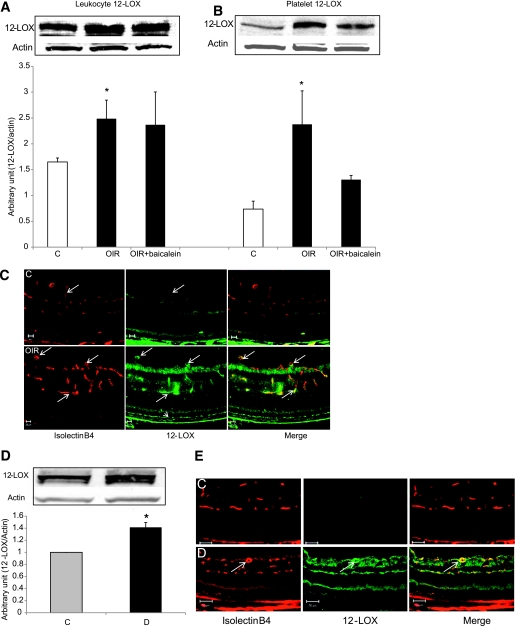
To determine whether 12-LOX plays a role in retinal NV, we investigated its expression and activity in the murine model of OIR, which is characterized by pathological retinal NV. Retinal levels of leukocyte and platelet isoforms of 12-LOX were significantly increased in OIR compared with the control (Fig. 1A and B). Immunolocalization indicates that 12-LOX is upregulated mainly in retinal vasculature and RPE cells (Fig. 1C). There also was a significant increase in 12-LOX expression in diabetic retinas compared with the control retinas (Fig. 1D) and, similar to OIR, 12-LOX was localized mainly in retinal vasculature (Fig. 1E).
FIG. 1.
Analysis of 12-LOX expression in the retina. Western blot analysis of leukocyte (A) and platelet (B) 12-LOX showed significant increases in OIR compared with the control (C). *P < 0.05 vs. control. C: Immunolocalization using vascular marker (isolectin-B4, red) and anti–12-LOX (green) showed a marked increase in 12-LOX immunoreactivity in retinal vessels (arrows) and RPE cells (arrowhead) of OIR compared with the control (C). D: Western blot analysis of 12-LOX expression showed a significant increase in retina of diabetic mice (D) compared with the control (C). *P < 0.05 vs. control. E: Immunolocalization of 12-LOX (green) indicated that it is upregulated in retinal vessels (red) of diabetic mice (D). (A high-quality digital representation of this figure is available in the online issue.)
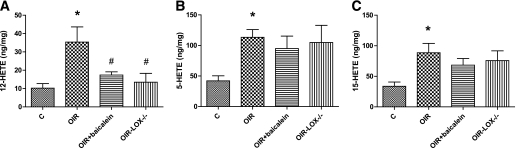
In addition, LC/MS analysis of 12-HETE in retinal homogenates from normal and OIR mice showed significant increases in OIR compared with the control (35.3 ± 6. ng/mg vs. 10.2 ± 2.0 ng/mg). This increase was significantly reduced in baicalein-treated and 12-LOX–deficient mice (18.3 ± 1.2 ng/mg and 13.5 ± 3.4 ng/mg, respectively) (Fig. 2A). The association between increased expression and activity of 12-LOX and retinal NV indicates that 12-LOX might be involved in the pathogenesis of ischemic retinopathy. Despite the fact that the primary focus of our study was on 12-LOX, we also noticed marked increases in the amounts of 5-HETE and 15-HETE in OIR compared with the control. Thus, 15- and 5-LOXs also may contribute to retinal NV. Although deletion of 12-LOX did not significantly affect the 5- or 15-HETEs level in OIR, we noticed a modest decrease in the amount of 5- and 15-HETEs by baicalein treatment (Fig. 2B and C).
FIG. 2.
LC/MS assay of HETEs production in mouse retina. There was a significant increase in the amount of 12-HETE (A), 5-HETE (B), and 15-HETE (C) in OIR compared with the control (C). The amount of 12-HETE was reduced in animals treated with baicalein or lacking 12-LOX (LOX−/−). *P < 0.05 vs. control; #P < 0.05 vs. OIR.
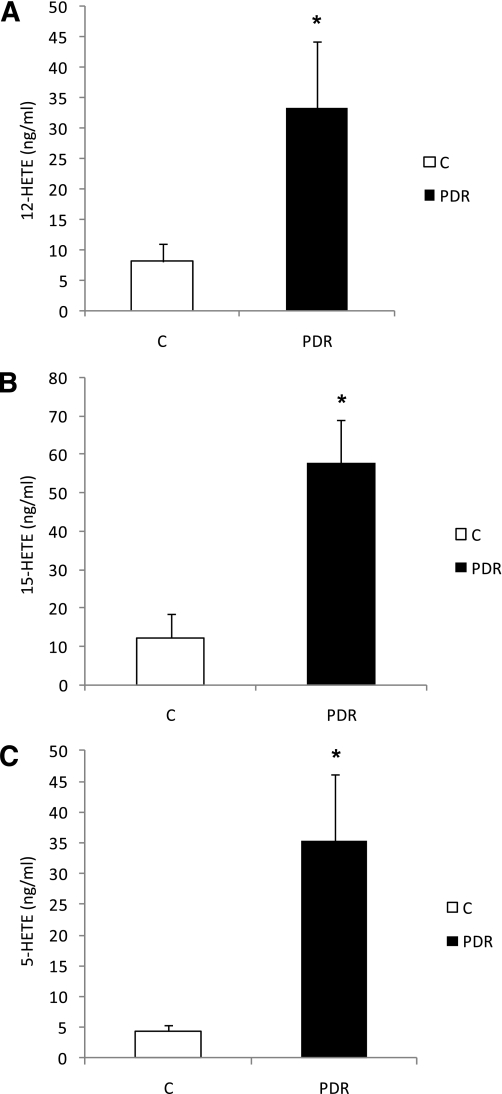
Increased 12-HETE production in the vitreous of patients with PDR.
To determine whether 12-LOX is involved in NV associated with diabetic retinopathy, we also tested the changes in 12-HETE production in vitreous samples of diabetic subjects with PDR compared with the amount produced in subjects without PDR. An LC/MS assay of HETEs (Table 2) demonstrated significantly higher levels of 12-HETE in diabetic subjects with PDR compared with control patients (33.4 ± 11.0 ng/mL vs. 8.0 ± 3.0 ng/mL) (Fig. 3A). We also tested the expression of 12-LOX in the retina of two diabetic and nondiabetic eye-donor subjects. We noticed a marked increase in the protein levels of both leukocyte and platelet 12-LOX in diabetic subjects compared with nondiabetic subjects (data not shown). In addition, 15-HETE and 5-HETE also were markedly increased in subjects with PDR compared with control subjects (57.8 ± 11.1 ng/mL vs. 12.3 ± 6.2 ng/mL and 35.4 ± 11.0 ng/mL vs. 4.5 ± 0.8 ng/mL), respectively (Fig. 3B and C). Taken together, our data from OIR and subjects with PDR suggest that along with 12-LOX, 5-LOX and 15-LOX also may play a role in retinal NV.
TABLE 2.
Levels of 12-, 15-, and 5-HETE in selected control subjects and diabetic subjects with PDR
| Patient diagnosis | Age (years)/sex (male or female) | 12-HETE (ng/mL) | 15-HETE (ng/mL) | 5-HETE (ng/mL) |
|---|---|---|---|---|
| Traumatic retinal detachment | 71/male | 4.16 | 8.78 | 7.8 |
| Epimacular membrane | 27/male | 20.7 | 55.3 | 6.2 |
| Dislocated lens secondary to trauma | 46/female | 3.18 | 8.28 | 5.7 |
| Idiopathic epimacular membrane | 65/male | 10.3 | 3.69 | 4.3 |
| PDR, traction retinal detachment/diabetes, hypertension | 60/male | 42.1 | 63.8 | 72 |
| PDR, traction retinal detachment/hypertension, heart disease | 52/female | 59 | 92.4 | 65 |
| PDR, traction retinal detachment/diabetes, hypertension, cerebello-spinal degeneration | 41/male | 12.8 | 47 | 25 |
| PDR, traction retinal detachment/diabetes, hypertension | 48/male | 67.9 | 102 | 205 |
| Advance PDR with traction retinal detachment and vitreous hemorrhage/diabetes, hypertension | 49/male | 11.0 | 38.2 | 20 |
FIG. 3.
LC/MS assay of HETE production in human vitreous samples. The amounts of 12-HETE (A), 15-HETE (B), and 5-HETE (C) were increased in diabetic subjects with PDR compared with the control (C). *P < 0.05.
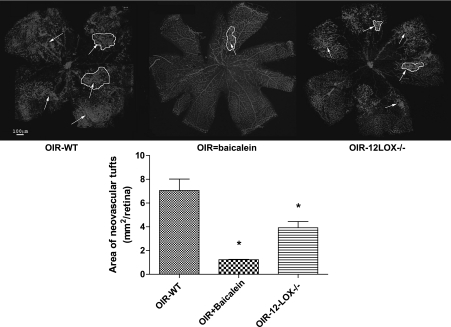
Attenuation of retinal NV by baicalein or deletion of 12-LOX.
To confirm the link between 12-LOX and retinal NV, we investigated the effect of baicalein or 12-LOX deletion on retinal NV during OIR. Our experiments demonstrated a significant decrease in the total area of new capillary tufts by baicalein or 12-LOX deletion compared with the nontreated group (1.3 ± 0.01 mm2/retina and 3.9 ± 0.5 mm2/retina, respectively, vs. 7.1 ± 0.3 mm2/retina) (Fig. 4), indicating that 12-LOX could be a target for therapeutic intervention in ischemic retinopathy.
FIG. 4.
Whole-mount retinas labeled with vascular marker (isolectin-B4). The total area of new capillary tufts (arrows, circled area) was reduced in baicalein-treated (OIR+baicalein) and 12-LOX knockout mice (OIR-12LOX−/−) compared with the nontreated group (OIR-WT). *P < 0.05 vs. the nontreated group. (A high-quality color representation of this figure is available in the online issue.)
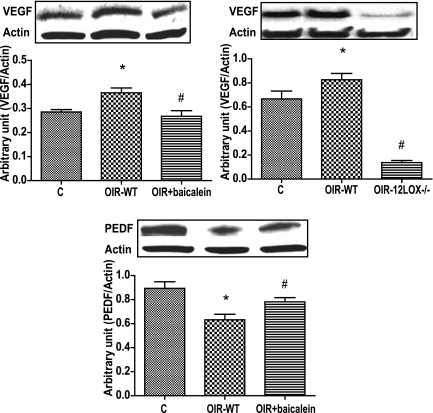
Effect of baicalein or 12-LOX deletion on VEGF and PEDF expression.
The VEGF and PEDF are key factors in retinal vascular homeostsis, and their alterations occur during retinal NV. We next determined the effects of baicalein or 12-LOX deletion on retinal expression of VEGF and PEDF during OIR. Retinal expression of VEGF (Fig. 5A and B) was significantly higher in OIR compared with age-matched control subjects. Administration of baicalein (Fig. 5A) or deletion of 12-LOX (Fig. 5B) attenuated the increase in VEGF levels. In addition, OIR was associated with a reduction in the retinal levels of PEDF in comparison with the age-matched control subjects. Baicalein treatment relatively restored the normal level of PEDF in oxygen-treated mice (Fig. 5C). However, deletion of 12-LOX did not affect retinal level of PEDF in OIR (data not shown).
FIG. 5.
Western blot analysis of VEGF and PEDF in the retina. A and B: OIR in wild-type (WT) mice was associated with significantly higher VEGF expression compared with the control. The change in VEGF expression was prevented by baicalein (OIR+baicalein) or deletion of 12-LOX (OIR-12LOX−/−). C: PEDF expression was reduced in the nontreated group (OIR-WT) and was restored by baicalein. *P < 0.05 vs. control; #P < 0.05 vs. the nontreated group.
Effect of 12-HETE on VEGF and PEDF levels in retinal cells.
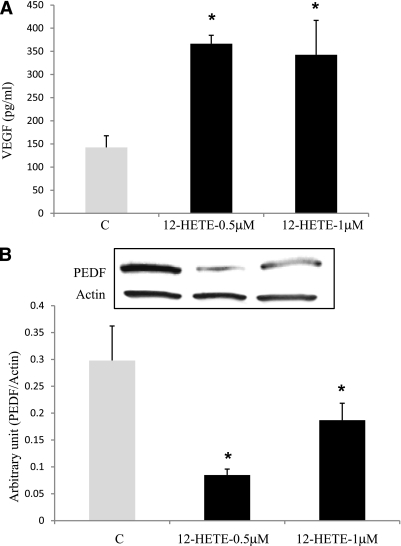
Because rMCs play a crucial role in retinal NV via secreting VEGF, we next tested the effect of 12-HETE on VEGF production in rMCs. There was a significant increase in VEGF production in the conditioned medium of rMCs incubated with different concentrations of 12-HETE (0.5 and 1 μmol/L) compared with vehicle-treated cells (366.5 ± 18 pg/mL and 342.6 ± 74 pg/mL vs. 142.5 ± 25 pg/mL, respectively) (Fig. 6A). The increase in VEGF production was observed as early as 12 h from the start of the treatment up to 72 h. Furthermore, normal levels of PEDF, as an angiostatic factor, may be required to prevent retinal NV. PEDF normally is expressed by different retinal cells, particularly RPE cells and rMCs. Thus, we also were interested in determining the effects of 12-HETE on PEDF expression in rMCs. There was a significant abrogation of PEDF expression by 0.5 and 1.0 μmol/L 12-HETE compared with the vehicle-treated group (0.09 ± 0.01 μmol/L and 0.19 ± 0.03 vs. 0.3 ± 06 μmol/L, respectively) (Fig. 6B). Taken together, our in vivo and in vitro data suggest that 12-HETE plays a significant role in retinal nevascularization in ischemic retinopathy through modulation of retinal VEGF and PEDF expression.
FIG. 6.
Effect of 12-HETE on the level of VEGF and PEDF expression in rMCs. A: VEGF level in rMC–conditioned medium was determined by ELISA. There was a marked increase in the amounts of VEGF by 12-HETE (0.5 and 1 μmol/L) compared with the control (C). *P < 0.05 vs. control. B: Western blot analysis of PEDF expression in rMCs. Note the decreased PEDF expression by 12-HETE (0.5 or 1.0 μmol/L) compared with the control (C). *P < 0.05 vs. control.
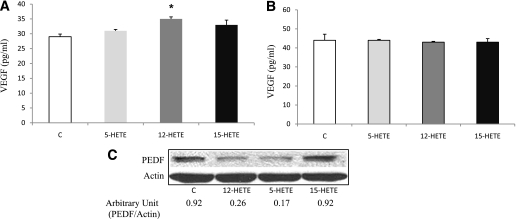
We also tested the effect of 12-, 5-, and 15-HETEs on VEGF and PEDF expression in murine astrocytes and RPE cells. Although 5-, 12-, or 15-HETE did not elicit significant changes on VEGF expression in the RPE cells (Fig. 7B), only 12-HETE significantly increased VEGF expression in the astrocytes (Fig. 7A). HETEs demonstrated similar effects on PEDF expression in murine RPE cells and astrocytes. 12- and 5-HETEs abrogated PEDF expression in RPE cells and astrocytes compared with the control and 15-HETE (Fig. 7C).
FIG. 7.
Effect of HETEs on VEGF and PEDF expression in primary mouse retinal astrocytes and RPE cells. ELISA assay of VEGF in cultured mouse retinal astrocytes (A) and RPE cells (B). 12-HETE induced a significant increase in VEGF expression of retinal astrocytes compared with the control and 5-HETE– and 15-HETE–treated cells (*P < 0.05 vs. control, 5-, and 15-HETE). There was no effect on VEGF expression in RPE cells incubated with any of the HETEs compared with the control. C: Western blot analysis of PEDF expression in RPE cells showed marked inhibition by 12- and 5-HETE compared with the control and 15-HETE.
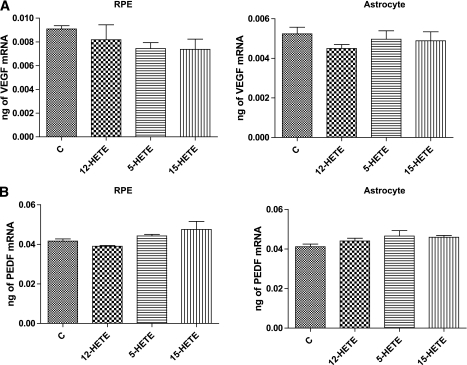
Quantitative PCR analysis of VEGF and PEDF mRNA expression demonstrated no significant changes by any HETE treatment in murine astrocytes and RPE cells (Fig. 8). Thus, the effects on the protein levels might be posttranscriptional.
FIG. 8.
Quantitative PCR analysis of VEGF (A) and PEDF (B) in murine RPE cells and retinal astrocytes. There was no significant change in VEGF or PEDF mRNA level of RPE cells or astrocytes incubated with different HETEs.
DISCUSSION
To the best of our knowledge, this is the first study to describe changes in the expression and activity of 12-LOX during pathological retinal NV such as in OIR and PDR. The major findings of our study include 1) increased expression of 12-LOX and production of 12-HETE, as well as 15- and 5-HETEs during OIR and PDR; 2) attenuation of VEGF expression and newly formed blood vessels during OIR by baicalein or 12-LOX deletion; and 3) induction and inhibition of VEGF and PEDF expression, respectively, in cultured glial cells (rMCs and primary murine astrocytes) by 12-HETE.
LOX products of arachidonate metabolism such as 12- and 15-HETE have been shown to have angiogenic properties (34,35). 12-LOX has been implicated in tumor angiogenesis (36) as well as endothelial cell proliferation and tube formation (7,8). However, the contribution of 12-HETE to ischemia-mediated retinal NV needs further investigation. The current study showed that OIR and PDR, which are characterized by retinal NV, were associated with increased 12-LOX expression and production of its metabolite 12-HETE. Interestingly, metabolites of 5- and 15-LOXs also were increased, indicating that the lipoxygenase pathway of arachidonic acid metabolism is implicated in the pathogenesis of ischemic retinopathy. Additional investigations are required to explore the specific role of each of the lipoxygenase products in mediating new vessel formation.
It recently was reported that retinas from nondiabetic or db/db mice neither produce leukotrieness nor 5-lipoxygenase mRNA (37). Of note, autooxidation of PUFAs leads to generation of various HETEs (38). Thus, increased reactive oxygen species production in ischemic or diabetic retina (39,40) also may contribute to the increased HETEs via oxidation of PUFAs. Increased production of 15- and 12-HETEs in cultured human retinal endothelial cells (HRECs) subjected to hypoxia recently has been reported (21). The same study also tested the effect of 5-, 12-, and 15-HETEs on HREC tube formation under normoxia. Although all three HETEs induced HREC tube formation, the effect of 12- and 15-HETEs was higher than that of 5-HETE. Consistent with this study, our in vivo data from the OIR model showed a significant increase in the amounts of HETEs in association with retinal NV. In addition, the amounts of HETEs also increased in the vitreous samples of diabetic subjects with PDR compared with those without PDR.
The mechanisms by which 12-HETE promotes angiogenesis remain elusive. In agreement with other reports, our studies demonstrated that the angiogenic effect of 12-HETE could be mediated via enhanced VEGF expression. This was further supported by the abrogation of new vessel growth and VEGF expression in the OIR model by baicalein or 12-LOX deletion. In addition, 12-HETE increased VEGF production in rMCs and murine astrocytes. Previous studies also have reported increased VEGF expression in vascular smooth muscle cells (17) and prostate cancer cells (36) by 12-HETE. Thus, VEGF seems to be crucial in mediating the angiogenesis-promoting effects of 12-HETE.
The cross-talk between 12-LOX and VEGF also has been demonstrated by increased production of 12-HETE and its receptor BLT2 expression in VEGF-activated endothelial cells. The use of 12-LOX siRNA or baicalein attenuated VEGF-induced angiogenesis, which was restored by the addition of supplemental 12-HETE (41). Thus, in the retina, a positive feedback between 12-HETE and VEGF derived from endothelial cells and rMCs may exist. Recently, 12-LOX has been shown to regulate hypoxia-inducible factor-1α, a transcription factor involved in regulating VEGF expression under hypoxic conditions via the phosphatidylinositol 3-kinase/AKT-dependent mechanism (42).
Retinal NV is tightly controlled by balanced VEGF and PEDF production. Disruption of this balance triggers new vessel formation in ischemic retinas. We therefore tested the effect of 12-HETE and baicalein on PEDF expression in rMCs and during OIR, respectively. In addition to VEGF, PEDF seemed to be another target of 12-LOX’s angiogenic effect. The current study showed abrogation of PEDF level in rMCs, murine astrocytes, and RPE cells by 12-HETE. Furthermore, baicalein restored the normal level of PEDF in retina of OIR, suggesting that 12-HETE’s angiogenic effect is mediated by disrupting VEGF/PEDF homeostasis. Despite the effect of HETE on VEGF and PEDF expression in retinal cells, there was no significant effect on either VEGF or PEDF mRNA, indicating that HETEs in general and 12-HETE in particular might modulate their expression at posttranscriptional level.
Baicalein is known to inhibit the lipoxygenase pathway. Our data demonstrate a significant reduction, in retinal HETEs particularly, of 12-HETE by baicalein. Although baicalein and deletion of 12-LOX reduced retinal NV in OIR, we noticed a more inhibitory effect for baicalein compared with 12-LOX deletion. In addition, although baicalein restored PEDF expression, 12-LOX deletion did not show a similar effect, indicating that in addition to targeting 12-LOX, baicalein might elicit its effect on retinal NV and PEDF expression through different pathways. Recently, baicalein administration to diabetic rats ameliorated diabetes-induced microglial activation, expression of proinflammatory cytokines, and VEGF and significantly reduced vascular permeability within the retina (43). Although 12-HETE induces a marked reduction in PEDF level in cultured retinal cells, deletion of 12-LOX did not restore the retinal level of PEDF. Whether this attributed to the effect of 5-HETE, which abrogates PEDF levels in vitro, needs to be clarified.
In addition to its role in angiogenesis, the LOX pathway plays a role in leukostasis and capillary degeneration associated with diabetic retinopathy. A recent study (44) demonstrated that 5- or 12-LOX deletion reduced leukostasis, an early sign of vascular injury in diabietic retinopathy. Although deletion of 5-LOX reduced capillary degeneration, 12-LOX deletion did not show the same effect (44). Of note, the neovascular stage of diabetic retinopathy is triggered by the hypoxia that develops in response to capillary degeneration. Under hypoxic conditions, there is more 12- and 15-HETEs production by HRECs compared with 5-HETE (21), suggesting that 5-, 12-, and 15-HETEs are each required for different stages of diabetic retinopathy. Taken together, we suggest that 12-HETE is more involved in mediating leukostasis and NV in diabetic retinopathy. Whether this mediation is via targeting circulating leukocytes or the recruiting hematopoetic stem cells needs further investigation. In particular, hematopoetic stem cells have been identified in the epiretinal membrane of PDR patients (45) and could contribute to retinal NV. 12-LOX products also upregulate key growth-related signaling kinases (46), which are involved in leukostasis and angiogenesis. For example, activation of nuclear factor-κB (15), P38 mitogen-activated protein kinase, and NADPH oxidase (47) are important mechanisms linking 12-LOX to vascular complications of diabetes, such as retinopathy and nephropathy.
In summary, the lipoxygenase pathway of arachidonic acid metabolism is involved in mediating retinal NV via the VEGF/PEDF balance disruption. Additional investigation is required to identify the mechanism by which 12-HETE modulates VEGF and PEDF expression in ischemic/diabetic retinas. Interruption of the lipoxygenase pathway could be a novel therapeutic intervention for prevention and treatment of ischemic retinopathy.
ACKNOWLEDGMENTS
M.A.-S. was supported by the American Heart Association (Grant AHA00104) and the Medical College of Georgia (Grant PSRP00026 and Vision Discovery Institute); V.S. was supported by the National Institutes of Health (NIH) (EY-019125) and by unrestricted funds from Research to Prevent Blindness, Inc.; and N.S. was supported by the NIH (EY-016695) and the Retina Research Foundation.
No potential conflicts of interest relevant to this article were reported.
M.A.-S. designed the experiments, researched data, contributed to discussion, and wrote and revised the manuscript. R.M. researched data and contributed to discussion. K.K. researched data and contributed to discussion. A.T. researched data and contributed to discussion. M.E. researched data and contributed to discussion. V.S. provided the rMC and contributed to discussion. J.N. provided vitreous samples and contributed to discussion. A.E.-M. researched data and contributed to discussion. S.Y.P. researched data. Z.G. researched data. N.S. researched data, contributed to discussion, and reviewed the manuscript. K.R.M. researched data, contributed to discussion, and reviewed the manuscript.
The authors thank N. Al-Rasheed, Research Chair, and Stacy Deppeler at King Saud University for the editing help.
REFERENCES
- 1.Bussolino F, Mantovani A, Persico G. Molecular mechanisms of blood vessel formation. Trends Biochem Sci 1997;22:251–256 [DOI] [PubMed] [Google Scholar]
- 2.Miller JW, Adamis AP, Aiello LP. Vascular endothelial growth factor in ocular neovascularization and proliferative diabetic retinopathy. Diabetes Metab Rev 1997;13:37–50 [DOI] [PubMed] [Google Scholar]
- 3.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science 1999;285:245–248 [DOI] [PubMed] [Google Scholar]
- 4.Nie D, Honn KV. Cyclooxygenase, lipoxygenase and tumor angiogenesis. Cell Mol Life Sci 2002;59:799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy RC, Gijón MA. Biosynthesis and metabolism of leukotrienes. Biochem J 2007;405:379–395 [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Wang H, Brown J, et al. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med 2006;203:941–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nie D, Hillman GG, Geddes T, et al. Platelet-type 12-lipoxygenase in a human prostate carcinoma stimulates angiogenesis and tumor growth. Cancer Res 1998;58:4047–4051 [PubMed] [Google Scholar]
- 8.Tang DG, Renaud C, Stojakovic S, Diglio CA, Porter A, Honn KV. 12(S)-HETE is a mitogenic factor for microvascular endothelial cells: its potential role in angiogenesis. Biochem Biophys Res Commun 1995;211:462–468 [DOI] [PubMed] [Google Scholar]
- 9.Funk CD, Keeney DS, Oliw EH, Boeglin WE, Brash AR. Functional expression and cellular localization of a mouse epidermal lipoxygenase. J Biol Chem 1996;271:23338–23344 [DOI] [PubMed] [Google Scholar]
- 10.Kim JA, Gu JL, Natarajan R, Berliner JA, Nadler JL. A leukocyte type of 12-lipoxygenase is expressed in human vascular and mononuclear cells. Evidence for upregulation by angiotensin II. Arterioscler Thromb Vasc Biol 1995;15:942–948 [DOI] [PubMed] [Google Scholar]
- 11.Funk CD, Funk LB, FitzGerald GA, Samuelsson B. Characterization of human 12-lipoxygenase genes. Proc Natl Acad Sci USA 1992;89:3962–3966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiyama M, Watanabe T, Ueda N, Tsukamoto H, Watanabe K. Arachidonate 12-lipoxygenase is localized in neurons, glial cells, and endothelial cells of the canine brain. J Histochem Cytochem 1993;41:111–117 [DOI] [PubMed] [Google Scholar]
- 13.Natarajan R, Gerrity RG, Gu JL, Lanting L, Thomas L, Nadler JL. Role of 12-lipoxygenase and oxidant stress in hyperglycaemia-induced acceleration of atherosclerosis in a diabetic pig model. Diabetologia 2002;45:125–133 [DOI] [PubMed] [Google Scholar]
- 14.Lebeau A, Terro F, Rostene W, Pelaprat D. Blockade of 12-lipoxygenase expression protects cortical neurons from apoptosis induced by beta-amyloid peptide. Cell Death Differ 2004;11:875–884 [DOI] [PubMed] [Google Scholar]
- 15.Bolick DT, Orr AW, Whetzel A, et al. 12/15-lipoxygenase regulates intercellular adhesion molecule-1 expression and monocyte adhesion to endothelium through activation of RhoA and nuclear factor-kappaB. Arterioscler Thromb Vasc Biol 2005;25:2301–2307 [DOI] [PubMed] [Google Scholar]
- 16.Li SL, Dwarakanath RS, Cai Q, Lanting L, Natarajan R. Effects of silencing leukocyte-type 12/15-lipoxygenase using short interfering RNAs. J Lipid Res 2005;46:220–229 [DOI] [PubMed] [Google Scholar]
- 17.Natarajan R, Bai W, Lanting L, Gonzales N, Nadler J. Effects of high glucose on vascular endothelial growth factor expression in vascular smooth muscle cells. Am J Physiol 1997;273:H2224–H2231 [DOI] [PubMed] [Google Scholar]
- 18.Natarajan R, Gu JL, Rossi J, et al. Elevated glucose and angiotensin II increase 12-lipoxygenase activity and expression in porcine aortic smooth muscle cells. Proc Natl Acad Sci USA 1993;90:4947–4951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patricia MK, Kim JA, Harper CM, et al. Lipoxygenase products increase monocyte adhesion to human aortic endothelial cells. Arterioscler Thromb Vasc Biol 1999;19:2615–2622 [DOI] [PubMed] [Google Scholar]
- 20.Duh E, Aiello LP. Vascular endothelial growth factor and diabetes: the agonist versus antagonist paradox. Diabetes 1999;48:1899–1906 [DOI] [PubMed] [Google Scholar]
- 21.Bajpai AK, Blaskova E, Pakala SB, et al. 15(S)-HETE production in human retinal microvascular endothelial cells by hypoxia: novel role for MEK1 in 15(S)-HETE induced angiogenesis. Invest Ophthalmol Vis Sci 2007;48:4930–4938 [DOI] [PubMed] [Google Scholar]
- 22.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem 2004;264:85–97 [DOI] [PubMed] [Google Scholar]
- 23.Ushio-Fukai M, Tang Y, Fukai T, et al. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res 2002;91:1160–1167 [DOI] [PubMed] [Google Scholar]
- 24.Ishida S, Yamashiro K, Usui T, et al. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med 2003;9:781–788 [DOI] [PubMed] [Google Scholar]
- 25.Yamagishi S, Nakamura K, Matsui T, et al. Pigment epithelium-derived factor inhibits advanced glycation end product-induced retinal vascular hyperpermeability by blocking reactive oxygen species-mediated vascular endothelial growth factor expression. J Biol Chem 2006;281:20213–20220 [DOI] [PubMed] [Google Scholar]
- 26.Amano S, Yamagishi S, Inagaki Y, et al. Pigment epithelium-derived factor inhibits oxidative stress-induced apoptosis and dysfunction of cultured retinal pericytes. Microvasc Res 2005;69:45–55 [DOI] [PubMed] [Google Scholar]
- 27.Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J 2006;20:323–325 [DOI] [PubMed] [Google Scholar]
- 28.Fletcher EL, Phipps JA, Ward MM, Puthussery T, Wilkinson-Berka JL. Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr Pharm Des 2007;13:2699–2712 [DOI] [PubMed] [Google Scholar]
- 29.Behzadian MA, Wang XL, Al-Shabrawey M, Caldwell RB. Effects of hypoxia on glial cell expression of angiogenesis-regulating factors VEGF and TGF-beta. Glia 1998;24:216–225 [PubMed] [Google Scholar]
- 30.Yafai Y, Lange J, Wiedemann P, Reichenbach A, Eichler W. Pigment epithelium-derived factor acts as an opponent of growth-stimulatory factors in retinal glial-endothelial cell interactions. Glia 2007;55:642–651 [DOI] [PubMed] [Google Scholar]
- 31.Mu H, Zhang XM, Liu JJ, Dong L, Feng ZL. Effect of high glucose concentration on VEGF and PEDF expression in cultured retinal Müller cells. Mol Biol Rep 2009;36:2147–2151 [DOI] [PubMed] [Google Scholar]
- 32.Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 1994;35:101–111 [PubMed] [Google Scholar]
- 33.Scheef E, Wang S, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal astrocytes. Mol Vis 2005;11:613–624 [PubMed] [Google Scholar]
- 34.Laniado-Schwartzman M, Lavrovsky Y, Stoltz RA, et al. Activation of nuclear factor kappa B and oncogene expression by 12(R)-hydroxyeicosatrienoic acid, an angiogenic factor in microvessel endothelial cells. J Biol Chem 1994;269:24321–24327 [PubMed] [Google Scholar]
- 35.Setty BN, Graeber JE, Stuart MJ. The mitogenic effect of 15- and 12-hydroxyeicosatetraenoic acid on endothelial cells may be mediated via diacylglycerol kinase inhibition. J Biol Chem 1987;262:17613–17622 [PubMed] [Google Scholar]
- 36.Nie D, Krishnamoorthy S, Jin R, et al. Mechanisms regulating tumor angiogenesis by 12-lipoxygenase in prostate cancer cells. J Biol Chem 2006;281:18601–18609 [DOI] [PubMed] [Google Scholar]
- 37.Talahalli R, Zarini S, Sheibani N, Murphy RC, Gubitosi-Klug RA. Increased synthesis of leukotrienes in the mouse model of diabetic retinopathy. Invest Ophthalmol Vis Sci; 2010;51:1699–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yin H, Davis T, Porter NA. Simultaneous analysis of multiple lipid oxidation products in vivo by liquid chromatographic-mass spectrometry (LC-MS). Methods Mol Biol 2010;610:375–386 [DOI] [PubMed] [Google Scholar]
- 39.Al-Shabrawey M, Bartoli M, El-Remessy AB, et al. Inhibition of NAD(P)H oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol 2005;167:599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Shabrawey M, Rojas M, Sanders T, et al. Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci 2008;49:3239–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim GY, Lee JW, Cho SH, Seo JM, Kim JH. Role of the low-affinity leukotriene B4 receptor BLT2 in VEGF-induced angiogenesis. Arterioscler Thromb Vasc Biol 2009;29:915–920 [DOI] [PubMed] [Google Scholar]
- 42.Krishnamoorthy S, Jin R, Cai Y, et al. 12-Lipoxygenase and the regulation of hypoxia-inducible factor in prostate cancer cells. Exp Cell Res 2010;316:1706–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang LP, Sun HL, Wu LM, et al. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Invest Ophthalmol Vis Sci 2009;50:2319–2327 [DOI] [PubMed] [Google Scholar]
- 44.Gubitosi-Klug RA, Talahalli R, Du Y, Nadler JL, Kern TS. 5-Lipoxygenase, but not 12/15-lipoxygenase, contributes to degeneration of retinal capillaries in a mouse model of diabetic retinopathy. Diabetes 2008;57:1387–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abu El-Asrar AM, Struyf S, Verbeke H, Van Damme J, Geboes K. Circulating bone-marrow-derived endothelial precursor cells contribute to neovascularization in diabetic epiretinal membranes. Acta Ophthalmol. 18 September 2009 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Rao GN, Baas AS, Glasgow WC, Eling TE, Runge MS, Alexander RW. Activation of mitogen-activated protein kinases by arachidonic acid and its metabolites in vascular smooth muscle cells. J Biol Chem 1994;269:32586–32591 [PubMed] [Google Scholar]
- 47.Wen Y, Gu J, Vandenhoff GE, Liu X, Nadler JL. Role of 12/15-lipoxygenase in the expression of MCP-1 in mouse macrophages. Am J Physiol Heart Circ Physiol 2008;294:H1933–H1938 [DOI] [PubMed] [Google Scholar]