Abstract
OBJECTIVE
We have developed a new antihyperglycemic agent (teglicar) through the selective and reversible inhibition of the liver isoform of carnitine palmitoyl-transferase 1 (L-CPT1).
RESEARCH DESIGN AND METHODS
Glucose production was investigated in isolated hepatocytes and during pancreatic clamps in healthy rats. Chronic treatments on C57BL/6J, db/db, high-fat fed mice, and rats were performed to understand glucose metabolism and insulin sensitivity.
RESULTS
In isolated hepatocytes, teglicar concentration dependently reduced ketone bodies and glucose production up to 72 and 50%, respectively. In rats, teglicar reduced the endogenous glucose production (−62%) without affecting peripheral glucose utilization. Heart 2-[3H]deoxyglucose uptake in mice was also not affected, confirming in vivo the drug selectivity toward L-CPT1. Chronic treatment in db/db mice (50 mg/kg/bid; 45 days) reduced postabsorptive glycemia (−38%), water consumption (−31%), and fructosamine (−30%). Such antidiabetic activity was associated with an improved insulin sensitivity assessed by the insulin tolerance test. A significant 50% increase in hepatic triglyceride content (HTGC) was found, although plasma alanineaminotransferase was not altered. In addition, long-term teglicar administration to high-fat fed C57BL/6J mice normalized glycemia (−19%) and insulinemia (−53%). Long-term teglicar administration (30 days, 80 mg/kg) in healthy overnight-fasted rats slightly reduced basal glycemia (−20%, ns), reduced basal insulin levels by 60%, doubled triglycerides, and increased free-fatty acids (+53%). HTGC was markedly increased, but liver and peripheral insulin sensitivity assessed by hyperinsulinemiceuglycemic clamp were not affected.
CONCLUSIONS
Teglicar, in vitro and in animal models, reduces gluconeogenesis and improves glucose homeostasis, refreshing the interest in selective and reversible L-CPT1 inhibition as a potential antihyperglycemic approach.
Fasting hyperglycemia presented by severe type 2 diabetic patients (glucose >9 mmol/L) is largely a function of increased endogenous glucose production (EGP) (1–3).
Pharmacological reduction of hepatic gluconeogenesis (GNG), which markedly contributes to high EGP, is considered one of the main targets in treating diabetes (4); in fact, the therapeutic effect of the most widely prescribed drug, metformin, is mainly due to its inhibitory effect on GNG (5).
Various studies have shown that inhibition of fatty acid oxidation in humans by blocking carnitine palmitoyl-transferase 1 (CPT1) (6–8) or by decreasing lipolysis (9,10) may reduce EGP and fasting glycemia. The oxidation of fatty acids provides energy (ATP) and reducing equivalents (NADH), and it stimulates GNG through structural changes in pyruvate carboxylase via increased levels of acetyl-CoA (11).
CPT1 (12) is a ubiquitous enzyme that plays a pivotal role in mitochondrial fatty acid β-oxidation. It is part of the carnitine palmitoyl-transferase system, which enables the activated acyl-CoA to be transported inside the matrix, crossing the inner mitochondrial membrane. It catalyzes the trans-esterification of acyl-CoA into acyl-carnitine, which permeates the mitochondrial membrane by a specific carrier mechanism and reacts with a matrix pool of CoA in a reaction catalyzed by CPT2 on the inner face of the inner membrane. The reformed acyl-CoA then enters the β-oxidation pathway, while the released carnitine returns to the extramitochondrial compartment. At a more complex level, growing evidence (13–15) supports the hypothesis of CPT1 activity as being part of the hypothalamic machinery involved in sensing nutrient availability, which in turns affects feeding behavior and endogenous glucose production.
Etomoxir, a CPT1 inhibitor with hypoglycemic activity in diabetic patients, was abandoned because of its inability to distinguish between the liver (L-CPT1) and the muscle (M-CPT1) isoform of CPT1, causing undesirable cardiac effects (16) and, possibly, muscle insulin resistance (17). Therefore, selectivity between the two isoforms of CPT1 is a major issue.
We have previously reported the discovery of the new substrate mimetic aminocarnitine derivative teglicar (ST1326) (18), as a more selective and reversible L-CPT1 inhibitor (half-maximal inhibitory concentration [IC50] = 0.68 ± 0.13 μmol/L, Ki = 0.36 ± 0.04, enhanced CPT1 selectivity ratio [∼70]), with respect to other previously described compounds (19–21).
The presently reported studies are aimed at the assessment of the effects of teglicar on glucose production, insulin sensitivity, and heart glucose metabolism, both in vitro and in animal models of diabetes in vivo, to address not only its hypoglycemic properties but also the potential consequences of sustained liver CPT1 inhibition.
RESEARCH DESIGN AND METHODS
Animals and diets.
SD 12-week-old male rats were purchased from Charles River (Calco, Italy). BKS db/db and C57BL/6J 8-week-old male mice were purchased from Jackson Laboratory (Bar Harbor, ME). SD rats and BKS db/db mice were fed ad libitum with a standard diet (No. 4RF21 Mucedola; Settimo Milanese, Italy), whereas high-fat-fed C57BL/6J mice received a fatty diet (58% fat kcal, No. 12331-Research Diets; New Brunswick, NJ) ad libitum for 7 months. All animal experiments were conducted in accordance with European Directive nr 86/609 and Italian D.L. nr 116, 27 January 1992. All procedures were reviewed and approved by our internal animal studies committee and comply with the Principles of Laboratory Animal Care (National Institutes of Health publication No. 85-23, Revised 1985).
Blood and tissue collection, serum and tissue analysis.
Blood samples in mice were taken by a tail-tip withdrawal. Tissues were quickly isolated, frozen in liquid nitrogen, and stored at −80°C for subsequent analysis. Glucose was measured by the hexokinase method (HK125, ABX Diagnostics), and ketone bodies, fructosamine, triglycerides, free fatty acids (FFA), cholesterol, and serum liver enzyme activities were measured by commercial colorimetrics kits. Insulin was measured by radioimmunoassay using rat insulin standards, which shows 100% cross-reaction with mouse insulin (Biotrack RPA-547, Amersham). Tissue triglycerides were extracted with isopropanol (22) and measured as above. Glycogen content was determined by the Mauvais-Jarvis method (23).
Hepatocyte isolation and incubation.
Hepatocytes were isolated by collagenase digestion (24) from rats fasted for 16 h. Because rat liver glycogen stores are almost absent (24) after a 16-h fast, the quantity of cumulative glucose and ketones bodies released into Krebs medium by fresh isolated hepatocytes is a good indicator of GNG and ketogenesis, respectively. Cells (1*106/mL) were incubated (25) in closed vials in an O2/CO2 (19:1) atmosphere at 37°C in a shaking water bath in a Krebs bicarbonate buffer containing: 10 mmol/L lactate and 1 mmol/L pyruvate, which guarantee GNG; 1 mmol/L glucose; 2% BSA and 2 mmol/L l-carnitine. After 30 min of preincubation with teglicar at different concentrations, 1 mmol/L sodium oleate was added to activate β-oxidation and pyruvate carboxylase. The effect of selected concentrations of teglicar on hepatocytes exposed to different concentrations of lactate and pyruvate, always in a 10:1 ratio, was also tested. Incubation was stopped 2 h later by precipitation in 3% perchloric acid. Once neutralized by KOH and centrifuged, medium-accumulated glucose and ketone bodies were measured.
Clamp procedure in rats.
One week before study, male SD rats underwent surgery to implant indwelling catheters in the internal jugular vein and carotid artery. Recovery was monitored by measuring daily food consumption and weight gain for 4 to 5 days after surgery.
Pancreatic clamp.
Pancreatic clamps were performed at 10:00 A.M. in conscious, 7-h-fasted, unrestrained SD rats. At time 0, a prime continuous infusion of [3-3H]glucose (NEN Dupont, Boston, MA [40 μCi bolus, 0.4 μCi/min for the duration of the study]), insulin (0.7 mU/kg/min), somatostatin (15 μg bolus, 1.5 μg/kg/min), and saline (0.5 mL/h) was started. The rate of insulin infusion was designed to replace normal basal levels in postabsorptive rats. Once steady state was achieved (EGP1) (after 2 h), in the treated group the saline was switched to teglicar (5.3 mg/kg/h) for further 3 h (EGP2). Euglycemia (7.5 mmol/L) was maintained by a variable infusion of 25% glucose solution. Sampling frequency, for determination of [3-3H]glucose–specific activity, was increased at 5-min intervals 20 min before end points EGP1 and EGP2.
Hyperinsulinemic-euglycemic clamp.
To assess the effect of chronic treatment with the drug on hepatic and peripheral insulin sensitivity, SD rats were orally treated once a day with teglicar (80 mg/kg) for 30 days. Clamps were performed at 10:00 A.M. After an overnight fast, conscious unrestrained rats received a primed continuous infusion of [3-3H]glucose (40 μCi bolus, 0.4 μCi/min) to set basal condition. At 2 h, a primed continuous infusion of insulin (15 mU bolus, 3 mU/kg/min) and somatostatin (15 μg bolus, 1.5 μg/kg/min) was started and continued for 3 h. The euglycemia was maintained with a variable infusion of 25% glucose solution. The determination of [3-3H]glucose–specific activity was performed as described above. Following these procedures, rats were anesthetized (55 mg/kg body wt iv, pentobarbital) and killed, and liver tissue samples were excised and cooled in liquid nitrogen. Samples were stored at −80°C for subsequent analysis.
Heart 2-[3H]deoxyglucose uptake.
Postabsorptive C57BL6/J mice were acutely treated by gavage with 50 mg/kg teglicar or etomoxir. The long-term treatment was performed by administering teglicar at 100 mg/kg/day for 30 days. Three hours postdose, 2-[3H]deoxyglucose (2-DG) was injected into the tail vein. Heart glucose uptake was measured by the Kraegen method (26).
Long-term treatment of db/db mice and insulin tolerance test.
Teglicar at the dose of 50 mg/kg twice a day was administered by gavage to 8-week-old db/db mice. Daily food intake and water consumption were measured and reported as average over 45 days. Postabsorptive glycemia was measured after 28, 35, and 45 days of treatment. Animals were then killed, after 8-h fast, and liver tissue was collected, frozen, and stored at −80°C.
The insulin tolerance test (ITT) was perfomed on the 40th day of treatment on fed mice by injection of 1 U/kg body wt ip insulin (Humulin R, Eli-Lilly, Indianapolis, IN). Glucose in tail vein blood was measured immediately before injection (time 0) and at 20, 40, and 60 min after injection (One Touch Ultra glucometer; Lifescan, Milpitas, CA). The blood glucose area under the curve (AUC) was calculated using the trapezoidal rule.
Long-term treatment of high-fat diet C57BL/6J mice.
C57BL/6/J male mice, previously fed for 7 months with high-fat diet (starting from their 8th week of life), were treated for 26 days with 30 mg/kg teglicar, or vehicle, twice a day. Glucose, insulin, triglycerides, FFAs, and cholesterol serum levels were then estimated in postabsorption state (fasting from 11:00 A.M. to 4:30 P.M.), 8 h from the last treatment. After 15 days of treatment, animals were subjected to an oral glucose tolerance test (OGTT), receiving a single bolus of 30% glucose solution (3 g/kg) by gavage. Blood was withdrawn 5 h from last treatment and after 5 h fasting, at 0, 30, 60, 90, and 120 min.
Evaluation of proliferator-activated receptor α activation after long-term treatment.
To exclude the hypothesis of a counterregulatory effect of prolonged teglicar treatment on proliferator–activated receptor α (PPAR-α), PPAR-α and its target gene product medium chain acyl-CoA dehydrogenase (MCAD), were quantified by Western blot densitometric analysis using rabbit anti-MCAD polyclonal antibody (Alexis Corporation, San Diego, CA) and mouse monoclonal anti-PPAR-α antibody (Affinity BioReagents, Golden, CO), respectively, in tissues of db/db mice after 45 days of treatment (50 mg/kg twice per day). Fresh crude peroxisomal fractions (livers were homogenated in buffered 0.32 mol/L sucrose; once pelleted the cellular debris, crude peroxisomes were pelleted at 15,000 × g per 15 min and resuspended in sucrose buffer) were assayed using [14C]U-Palmitoyl-CoA as substrate. Potassium cyanide was added in the assay buffer to detect only β-oxidation from peroxisomes as described by Lazarow (27).
Statistical analysis.
All values are presented as the mean ± SE. The Student t test was used to determine the difference between control and treatment groups. Significance was accepted as P < 0.05. For multiple comparisons between groups, a one-way ANOVA followed by Dunnett test was performed.
RESULTS
Effects of teglicar on ketone bodies and β-oxidation-dependent glucose production in isolated hepatocytes.
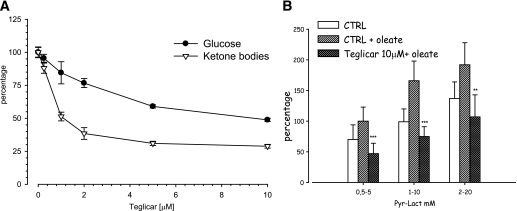
Teglicar induced a concentration-dependent reduction of ketone bodies and glucose production by hepatocytes incubated with 10 mmol/L lactate and 1 mmol/L pyruvate (Fig. 1A). At 10 μmol/L teglicar, ketone bodies production was reduced by 72% (effective dose 50 = 1.17 ± 0.12 μmol/L), whereas cumulative glucose in the medium diminished up to 50% with respect to the untreated cells. The residual glucose production, independent from β-oxydation (we obtained similar results with etomoxir and 2-tetradecylglycidic acid (TDGA), data not shown), may be affected by changing lactate and pyruvate concentrations (Fig. 1B).
FIG. 1.
A: Teglicar-dependent suppression of glucose and ketone bodies production by hepatocytes isolated from 16-h-fasted rats and incubated in presence of 10:1 lactate/pyruvate and 1 mmol/L oleate. B: Relationship between hepatocytes glucose production and the concentration of pyruvate/lactate in Krebs medium containing 1 mmol/L oleate in the presence or absence of 10 μmol/L teglicar. Each point represents the mean ± SE from eight different hepatocyte preparations in triplicate: ***P < 0.001; **P < 0.01 vs. control (CTRL) + oleate.
Effects of teglicar on endogenous glucose production in SD rats.
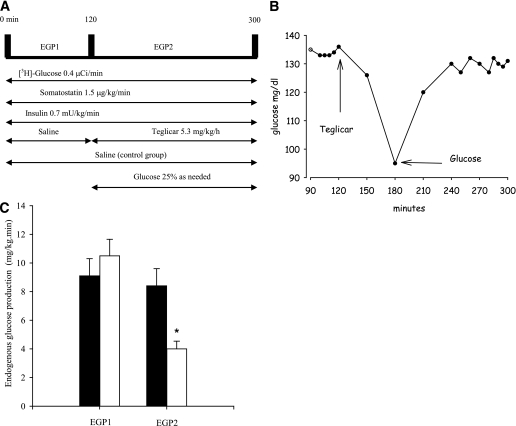
The pancreatic clamp was set up as shown in Fig. 2A to assess the effect of the drug on EGP. After basal EGP (EGP1) during the first 120 min of clamp (9.1 ± 1.2 and 10.5 ± 1.15 mg/kg/min, in saline and treatment group, respectively) was measured, a 3-h teglicar infusion was administered. In the first 60 min the drug infusion determined a rapid drop in glycemia (Fig. 2B), requiring a coinfusion of 25% glucose to restore and maintain previously measured glucose values. At the end of the clamp session, teglicar-suppressed EGP (EGP2) diminished by 62% (Fig. 2C), whereas peripheral glucose utilization (GU) was not affected (8.4 ± 1.2 saline vs. 9.69 ± 1.31 mg/kg/min teglicar).
FIG. 2.
A: Pancreatic clamp procedure involves the infusion of 0.4 μCi/min [3-3H]glucose, 1.5 μg/kg/min somatostatin, and 0.7 mU/kg/min insulin. Control rats received saline throughout the experiment, whereas teglicar infusion was started at 120'. Rats receiving the drug also received glucose to prevent hypoglycemia. B: Representation of teglicar-induced drop in glycemia during pancreatic clamp. Euglycemia is restored by glucose coinfusion. C: Rates of glucose production before (EGP1) and during (EGP2) the infusion of teglicar (white bars) or saline (black bars) in postabsorptive SD rats. The box plots indicate the mean ± SE from five different pancreatic clamps/group. *P < 0.05 vs. saline.
Effects of teglicar on insulin sensitivity in SD rats.
To better define the impact of long-term L-CPT1 inhibition on insulin resistance, healthy SD rats were treated with 80 mg/kg/day teglicar for 30 days; treatment did not produce differences in food intake or final weight. Teglicar reduced basal insulin levels; a nonsignificant trend toward a reduction of basal blood glucose was also observed, whereas EGP, glucose infusion rate (GIR), and peripheral insulin sensitivity (GU), assessed with a hyperinsulinemic-euglycemic clamp, did not differ between treated animals and controls. In contrast, plasma FFA and triglycerides increased but quickly return to normal values in hyperinsulinemic conditions. After death, treated rats showed a higher triglyceride and lower glycogen content in the liver, without any change in liver weight (Table 1).
TABLE 1.
Effect of 30 days of teglicar treatment before and during insulin infusion (3 mU/min/kg) on plasma glucose, lipid, and liver parameters in overnight-fasted, conscious, unrestrained SD rats
| Control | Teglicar | |
|---|---|---|
| Glucose (mg/dL) | ||
| Basal | 114.3 ± 9.2 | 92.0 ± 4.9 |
| Clamp | 91.5 ± 5.2 | 87.0 ± 3.8 |
| Insulin (ng/mL) | ||
| Basal | 1.12 ± 0.44 | 0.45 ± 0.04* |
| Clamp | 4.14 ± 0.35 | 4.02 ± 0.36 |
| FFA (μmol/L) | ||
| Basal | 1214 ± 112 | 1868 ± 212* |
| Clamp | 214 ± 70 | 184 ± 17 |
| Triglycerides (mg/dL) | ||
| Basal | 33.9 ± 1.4 | 70.9 ± 18* |
| Clamp | 13.8 ± 3.7 | 12 ± 1.2 |
| EGP (mg/kg/min) | ||
| Basal | 7.41 ± 0.45 | 7.82 ± 0.46 |
| Clamp | 0.21 ± 0.11 | 0.15 ± 0.09 |
| GU (mg/kg/min) | 26.7 ± 1.12 | 28.4 ± 1.51 |
| GIR (mg/kg/min) | 26.9 ± 1.05 | 28.3 ± 1.63 |
| Liver parameters at the end of clamp session | ||
| Liver weight (g) | 17.2 ± 1.2 | 18.1 ± 1.4 |
| Liver triglyceride content (mg/100 mg wt) | 0.42 ± 0.1 | 5.3 ± 0.7* |
| Liver glycogen (μmol/g wt) | 50.5 ± 4.1 | 20.1 ± 1.4* |
Data are means ± SE of 6 rats/group.
*P < 0.05 vs. controls.
Effects of teglicar on heart glucose uptake in vivo in C57BL/6J mice.
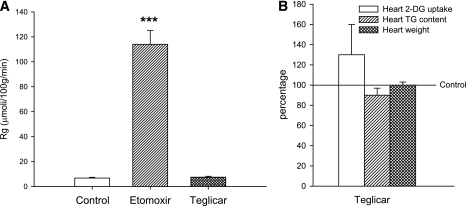
To rule out the possibility of any effect of teglicar heart glucose metabolism, heart 2-DG uptake was measured after both single dose (50 mg/kg) and long-term treatment (30 days at 100 mg/kg/day) in C57BL/6J mice; oral etomoxir (50 mg/kg) was used as positive control in the single-dose experiment. Figure 3A clearly shows that etomoxir causes a massive switch in cardiomyocyte metabolism. Although etomoxir-induced M-CPT1 inhibition caused a 12-fold increase in heart 2-DG uptake, teglicar did not modify this parameter (Fig. 3A). Long-term, effective treatment with teglicar failed to determine significant changes in 2-DG heart uptake, heart weights, and triglyceride content (Fig. 3B).
FIG. 3.
A: Heart 2-DG uptake in C57BL/6J mice after a single administration of teglicar (50 mg/kg) or etomoxir (50 mg/kg). The box plots are expressed as micromoles of 2-DG incorporated per 100 grams wet tissue per minute ± SE obtained from eight animals/group. ***P < 0.001 vs. controls. B: Effect of long-term (30 days) teglicar administration (100 mg/kg/day) on heart 2-DG uptake, heart weight, and heart triglyceride (TG) content in C57BL/6J mice. The box plots are expressed in percentage with respect to controls ± SE obtained from six animals/group.
Long-term effects of teglicar on murine models of diabetes—db/db mice
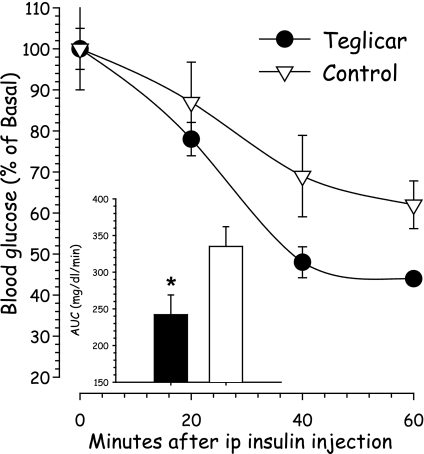
Teglicar treatment (50 mg/kg twice per day for 45 days) induced a significant reduction (up to 50%) of postabsorptive serum glucose, which was evident after 28 days of treatment and lasted throughout the experiment. Consistent with the improvement of glucose control, serum fructosamine and average daily water consumption were also reduced, underlining an improvement in long-term glycemic control. Serum FFAs were slightly increased, 10% more than controls, whereas insulin levels, triglycerides, alanine aminotransferase, and cholesterol did not change. Food intake showed a nonsignificant trend toward reduction, but body weight was not affected in treated mice. After death, hepatic triglyceride content, but not liver weight, was moderately increased (Table 2). Teglicar also induced a significant reduction of glucose AUC during ITT in comparison with controls (Fig. 4).
TABLE 2.
Effect of teglicar treatment on different parameters of db/db mice after long-term treatment (45 days unless otherwise specified)
| Control | Teglicar | |
|---|---|---|
| Body weight (g) | 40.0 ± 1.1 | 40.4 ± 2.2 |
| Food intake (g/day) | 6.71 ± 0.2 | 5.46 ± 0.4 |
| Water intake (mL/day) | 13.5 ± 0.2 | 9.38 ± 1.1* |
| Serum parameters | ||
| Glycemia (day 28) (mg/dL) | 495 ± 28 | 250 ± 20* |
| Glycemia (day 35) (mg/dL) | 570 ± 30 | 322 ± 25* |
| Glycemia (mg/dL) | 576 ± 32 | 352 ± 25* |
| Fructosamine (mmol/L) | 4.8 ± 0.1 | 3.36 ± 0.3* |
| Insulin (ng/mL) | 2.81 ± 0.2 | 2.32 ± 0.3 |
| Triglycerides (mg/dL) | 80.7 ± 5.5 | 82.2 ± 6.1 |
| FFA (μmol/L) | 1.98 ± 0.15 | 2.29 ± 0.1* |
| Cholesterol (mg/dL) | 110.4 ± 10.1 | 112.4 ± 6.1 |
| ALT (U/L) | 82.9 ± 4.9 | 102.6 ± 7.2 |
| Liver parameters | ||
| Liver weight (g) | 1.94 ± 0.08 | 1.93 ± 0.09 |
| Liver triglyceride content (mg/100 mg wt) | 5.71 ± 0.9 | 8.54 ± 0.7* |
| Liver glycogen (μmol/g wt) | 329 ± 54 | 255 ± 46 |
Data are means ± SE of eight mice.
*P < 0.05 vs. controls.
FIG. 4.
ITT in db/db mice after teglicar treatment. The small bar plot indicates the AUC (0–60 min) of glycemia during the ITT. Teglicar, black bar and circles; control, white bar and triangles. Data are expressed as percentage variation from initial glucose and in the bar plot as milligrams per deciliters per minute. Each point indicates the mean ± SE of eight mice. *P < 0.05 vs. controls.
Long-term effects of teglicar on murine models—high-fat diet C57BL/6J mice.
The effects of teglicar were also studied in a further model of insulin resistance and hyperglycemia, induced by feeding C57BL/6J mice with a high-fat diet; a low-fat diet (8% Kcal from fat) untreated control group was also included.
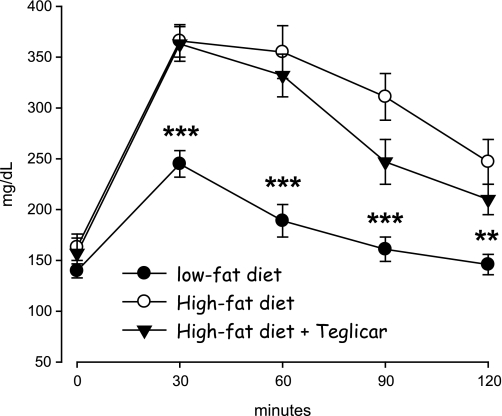
Treating mice fed high-fat diet with teglicar (30 mg/kg twice per day for 26 days) did not affect food intake and body weight (not shown). Serum FFAs and triglycerides, unlike the previous data on db/db mice, did not change. The diabetogenic effects of high-fat diet on glucose and insulin serum levels were abolished by teglicar (−19% glycemia and −53% insulinemia; Table 3). As expected, mice fed high-fat diet were overtly glucose intolerant during the OGTT test with respect to mice fed low-fat diet. Teglicar did not affect this parameter although a slight trend toward reduction was seen (Fig. 5).
TABLE 3.
Effect of teglicar on postabsorptive serum parameters of C57BL/6J mice fed with a high-fat diet
| High-fat diet |
Low-fat diet |
||
|---|---|---|---|
| Control | Teglicar | Control | |
| Glucose (mg/dL) | 216.7 ± 12.9 | 174.8 ± 12.5* | 178.7 ± 11.8* |
| Insulin (ng/mL) | 3.01 ± 0.10 | 1.39 ± 0.34** | 1.46 ± 0.29** |
| FFA (μmol/L) | 725 ± 58 | 780 ± 31 | 690 ± 46 |
| Triglycerides (mg/dL) | 69.5 ± 6.9 | 56.9 ± 3.9 | 43.5 ± 2.2** |
| Cholesterol (mg/dL) | 220.0 ± 5.1 | 241.7 ± 8.9 | 156.4 ± 6.8** |
Data are means ± SE of five (teglicar treated) and six (high-fat and low-fat control groups) mice group.
*P < 0.05;
**P < 0.01 vs. high-fat diet group.
FIG. 5.
Blood glucose levels during OGTT test on 5-h fasted male C57BL/6J mice fed with a high-fat diet and treated with teglicar (ST1326, 30 mg/kg, twice per day) for 15 days, at 5 h from last treatment. Means are ± SE (n = 6 for high-fat diet and low-fat diet; n = 5 for high-fat diet + teglicar). ***P < 0.001; **P < 0.01 vs. high-fat diet group.
Long-term effects of teglicar on PPAR-α activation and hepatic peroxisomal β-oxydation.
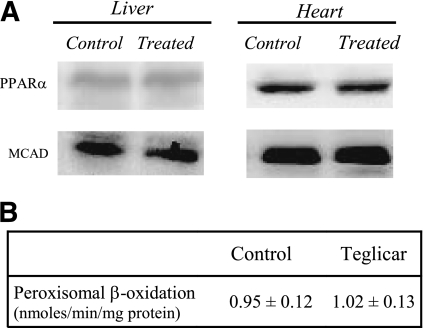
Teglicar treatment for 45 days did not induce any variation in the content of PPAR-α and its target gene product MCAD in liver and heart of db/db mice (Fig. 6A). Measurements of peroxisomal β-oxidation in the liver also did not show any significant differences between groups (Fig. 6B).
FIG. 6.
A: Representative Western blot of the reported target proteins. Protein extracts were obtained from tissue of control or teglicar-treated db/db mice (n = 8) subjected to analysis. B: Liver peroxisomal β-oxidation activity in controls and teglicar-treated mice.
DISCUSSION
Teglicar, a selective and reversible inhibitor of liver CPT1, reduced both ketogenesis and glucose production in freshly isolated hepatocytes prepared from fasted rats. This cellular effect on glucose production was further corroborated by in vivo experiments with pancreatic clamp, showing that teglicar acutely reduces hepatic glucose production without interfering with peripheral GU, as expected by its selectivity for liver isoform. In fact, an increase of peripheral GU would be indicative of an ongoing reversed Randle effect (28,29) through inhibition of M-CPT1. A more accurate analysis of cardiac muscle metabolism in mice revealed that, even after full-dose long-term treatment, heart weight, triglyceride content, and glucose uptake were not affected, further excluding the concern of heart hypertrophy and muscle insulin resistance due to M-CPT1 inhibition as reported for etomoxir (16,17,30).
The chronic administration of the drug confirmed its antihyperglycemic action in db/db mice, a well-established murine model of type 2 diabetes, with a reduction of fasting plasma glucose, serum fructosamine, and daily water intake. Body weight did not change but a slight not significant reduction of food intake was observed, mostly explained by a decreased calories waste through glycosuria. The improvement of insulin sensitivity observed in treated animals with the ITT could be interpreted as an indirect consequence of ameliorated metabolic control, with reduced glucose toxicity. The effects of teglicar were investigated also in a different animal model, the hyperglycemic high-fat-fed C57BL/6J mice, which are characterized by obesity, hyperinsulinemia, hyperleptinemia, and hyperglycemia and resemble more closely human type 2 diabetes. Being insulin resistance and hyperglycemia determined by prolonged high-fat feeding, this model can be considered as particularly challenging for a compound interfering with fatty acid oxidation. The antidiabetic activity of the compound was confirmed in this model, which yielded similar results as those obtained in db/db mice, in terms of glycemia and insulin reduction, but without further compromising the serum lipid asset.
The inhibition of L-CPT1 induced, as expected, a significant increase in fat liver content (marked in fasted healthy rats, moderate in db/db mice). The marked increase observed in healthy rats may anyway be confined to the prolonged fasting condition, where teglicar, by reducing ketogenesis and gluconeogenesis, puts at risk glucose homeostasis. However, fasting normoglycemia was maintained by the important reduction of plasma insulin, which consequently was responsible for the increase of FFAs and triglycerides, contributing to the instauration of liver steatosis.
Despite this fact, teglicar does not induce insulin resistance.
Even if increased hepatic triglyceride content is commonly thought to be associated with reduced insulin sensitivity (31), our evidence are in agreement with the results of studies in which liver steatosis induced by acute treatment with TDGA in C57BL/6J mice, or with methylpalmoxirate in hyperlipidemic APO3*Leiden mice, does not induce liver insulin resistance (32,33). In addition, Buettner et al. (34) perfusing ex vivo livers from high-fat-fed Wistar rats showed that increased intracellular lipids per se do not induce defects in insulin action, reporting that fasting-induced hepatic steatosis is not associated with an impairment of liver insulin sensitivity in healthy rodents (35), suggesting alternative extrahepatic regulators of liver insulin sensitivity other than triglycerides. This last concept was also recently proposed by Wendel et al. (36), which clearly showed that preventing hepatic steatosis in ob/ob mice, by deleting glycerol-3-phosphate acyltransferase-1 (Gpat1), does not improve insulin sensitivity. Hormones from adipose tissue and a primary central nervous system control may be responsible for this alternative regulation.
In this regard, it has been reported (Obici et al. [37]) that by reducing the activity of the hypothalamic L-CPT1 through injection of a ribozyme-containing plasmid into the third ventricle of the rat, designed to reduce the expression of the enzyme, or by infusing teglicar (5 or 25 pmol), a decrease in food intake and glucose production was induced. More recently, the structure of feeding behavior and satiation time course were examined in mice after teglicar intracerebroventricular treatment, evidencing that the significant anorectic response is mirrored by an early occurrence of satiety onset (38). Anyway, because of its chemico-physical characteristics, teglicar is not likely to cross the blood-brain barrier, and the results of the presently reported experiments failed to support the evidence of such central control of glucose production and appetite. However, in our opinion at least a slight hypothalamic L-CPT1 inhibition during chronic therapy in humans cannot be completely excluded without further investigation, since in the long term the hypothalamic concentration of the drug could achieve the extremely low levels required.
The results of our research sustain the hypothesis that selectively targeting L-CPT1 could represent an effective approach to lower fasting hyperglycemia in type 2 diabetes. This is supported also by the fact that a rare disorder of L-CPT1 deficiency in the Canadian Hutterite population is not easily recognized, due to lack of disease-specific metabolic markers other than hypoketotic hypoglycemia during fasting (39,40). Another L-CPT1 mutation was identified in humans (41), which causes total inactivation of L-CPT1, leading to life-threatening nocturnal hypoglycemia without other signs of illness. These data, if from one side confirm the potential of L-CPT inhibition as a mean for reducing gluconeogenesis in humans, on the other end could raise concern about the possibility of fasting-induced hypoglycemia. In our experiments in diabetic mice or normal rats fasting, hypoglycemia was not recorded. This may be ascribed to the reversible nature of teglicar inhibition, compared with the constitutional lack of enzymatic activity. We also have evidence that even if in 36-h fasting healthy volunteers no hypoglycemia was observed (data not shown).
In conclusion the reported data demonstrate that teglicar, in vitro and in animal models, reduces gluconeogenesis and is able to improve glucose homeostasis, refreshing the interest in selective and reversible L-CPT1 inhibition as a potential antihyperglycemic approach. The recent discovery (42) that metformin reduces hepatic gluconeogenesis independently of the LKB1/AMPK pathway, which, instead, blunts metformin efficacy, opens up the possibility of an advantageous association with L-CPT1 inhibitors.
Further studies are needed to assess the real potential of teglicar, alone and in combination with other agents, in the therapy of type 2 diabetes.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
R.C. researched data and wrote the article. E.M. contributed to discussion and reviewed and edited the article. P.P. researched data. E.T. researched data. P.C. contributed to discussion. F.G. researched data and wrote the article. A.A. contributed to discussion and reviewed and edited the article.
Parts of the data were presented as posters at the 61st Annual Meeting of the American Diabetes Association, June 2001, Philadelphia, Pennsylvania, and at the 63rd Annual Meeting of the American Diabetes Association, 13–17 June 2003, New Orleans, Louisiana.
The authors thank Drs. A.F. Sciarroni, F.M. Milazzo, and M. Bandera for the evaluation of PPAR-α activation.
Footnotes
A.A. is currently affiliated with CoreQuest Sagl, Bioggio, Switzerland.
P.P. is currently retired.
†Deceased.
REFERENCES
- 1.Basu R, Schwenk WF, Rizza RA. Both fasting glucose production and disappearance are abnormal in people with “mild” and “severe” type 2 diabetes. Am J Physiol Endocrinol Metab 2004;287:E55–E62 [DOI] [PubMed] [Google Scholar]
- 2.Boden G, Chen X, Stein TP. Gluconeogenesis in moderately and severely hyperglycemic patients with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2001;280:E23–E30 [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care 1992;15:318–368 [DOI] [PubMed] [Google Scholar]
- 4.Proietto J, Andrikopoulos S. Molecular mechanisms of increased glucose production: identifying potential therapeutic targets. J Investig Med 2004;52:389–393 [DOI] [PubMed] [Google Scholar]
- 5.McCormack JG, Westergaard N, Kristiansen M, Brand CL, Lau J. Pharmacological approaches to inhibit endogenous glucose production as a means of anti-diabetic therapy. Curr Pharm Des 2001;7:1451–1474 [DOI] [PubMed] [Google Scholar]
- 6.Mandarino L, Tsalikian E, Bartold S, et al. Mechanism of hyperglycemia and response to treatment with an inhibitor of fatty acid oxidation in a patient with insulin resistance due to antiinsulin receptor antibodies. J Clin Endocrinol Metab 1984;59:658–664 [DOI] [PubMed] [Google Scholar]
- 7.Ratheiser K, Schneeweiss B, Waldhäusl W, et al. Inhibition by etomoxir of carnitine palmitoyltransferase I reduces hepatic glucose production and plasma lipids in non-insulin-dependent diabetes mellitus. Metabolism 1991;40:1185–1190 [DOI] [PubMed] [Google Scholar]
- 8.Hübinger A, Knode O, Susanto F, Reinauer H, Gries FA. Effects of the carnitine-acyltransferase inhibitor etomoxir on insulin sensitivity, energy expenditure and substrate oxidation in NIDDM. Horm Metab Res 1997;29:436–439 [DOI] [PubMed] [Google Scholar]
- 9.Bajaj M, Suraamornkul S, Kashyap S, Cusi K, Mandarino L, DeFronzo RA. Sustained reduction in plasma free fatty acid concentration improves insulin action without altering plasma adipocytokine levels in subjects with strong family history of type 2 diabetes. J Clin Endocrinol Metab 2004;89:4649–4655 [DOI] [PubMed] [Google Scholar]
- 10.Boden G, Chen X, Capulong E, Mozzoli M. Effects of free fatty acids on gluconeogenesis and autoregulation of glucose production in type 2 diabetes. Diabetes 2001;50:810–816 [DOI] [PubMed] [Google Scholar]
- 11.Walter P. Pyruvate carboxylase: intracellular localization and regulation. In Gluconeogenesis: Its Regulation in Mammalian Species. Hanson RW, Mehlman MA, Eds. New York, John Wiley, 1976, pp. 239–265 [Google Scholar]
- 12.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem 1997;244:1–14 [DOI] [PubMed] [Google Scholar]
- 13.Loftus TM, Jaworsky DE, Frehywot GL, et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 2000;288:2379–2381 [DOI] [PubMed] [Google Scholar]
- 14.Hu Z, Cha SH, Chohnan S, Lane MD. Hypothalamic malonyl-CoA as a mediator of feeding behavior. Proc Natl Acad Sci USA 2003;100:12624–12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obici S, Rossetti L. Minireview: nutrient sensing and the regulation of insulin action and energy balance. Endocrinology 2003;144:5172–5178 [DOI] [PubMed] [Google Scholar]
- 16.Bressler R, Gay R, Copeland JG, Bahl JJ, Bedotto J, Goldman S. Chronic inhibition of fatty acid oxidation: new model of diastolic dysfunction. Life Sci 1989;44:1897–1906 [DOI] [PubMed] [Google Scholar]
- 17.Dobbins RL, Szczepaniak LS, Bentley B, Esser V, Myhill J, McGarry JD. Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes 2001;50:123–130 [DOI] [PubMed] [Google Scholar]
- 18.Giannessi F, Pessotto P, Tassoni E, et al. Discovery of a long-chain carbamoyl aminocarnitine derivative, a reversible carnitine palmitoyltransferase inhibitor with antiketotic and antidiabetic activity. J Med Chem 2003;46:303–309 [DOI] [PubMed] [Google Scholar]
- 19.Anderson RC, Balestra M, Bell PA, et al. Antidiabetic agents: a new class of reversible carnitine palmitoyltransferase I inhibitors. J Med Chem 1995;38:3448–3450 [DOI] [PubMed] [Google Scholar]
- 20.Deems RO, Anderson RC, Foley JE. Hypoglycemic effects of a novel fatty acid oxidation inhibitor in rats and monkeys. Am J Physiol 1998;274:R524–R528 [DOI] [PubMed] [Google Scholar]
- 21.Anderson RC. Carnitine palmitoyltransferase: a viable target for the treatment of NIDDM? Curr Pharm Des 1998;4:1–16 [PubMed] [Google Scholar]
- 22.Oakes ND, Thalén PG, Jacinto SM, Ljung B. Thiazolidinediones increase plasma-adipose tissue FFA exchange capacity and enhance insulin-mediated control of systemic FFA availability. Diabetes 2001;50:1158–1165 [DOI] [PubMed] [Google Scholar]
- 23.Mauvais-Jarvis F, Virkamaki A, Michael MD, et al. A model to explore the interaction between muscle insulin resistance and beta-cell dysfunction in the development of type 2 diabetes. Diabetes 2000;49:2126–2134 [DOI] [PubMed] [Google Scholar]
- 24.Berry MN, Edwards AM, Barrit GJ. Isolated Hepatocytes Preparation, Properties and Applications. Amsterdam, Elsevier, 1991 [Google Scholar]
- 25.Rigoulet M, Leverve XM, Plomp PJ, Meijer AJ. Stimulation by glucose of gluconeogenesis in hepatocytes isolated from starved rats. Biochem J 1987;245:661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraegen EW, James DE, Jenkins AB, Chisholm DJ. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol 1985;248:E353–E362 [DOI] [PubMed] [Google Scholar]
- 27.Lazarow PB. Assay of peroxisomal beta-oxidation of fatty acids. Methods Enzymol 1981;72:315–319 [DOI] [PubMed] [Google Scholar]
- 28.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1963;1:785–789 [DOI] [PubMed] [Google Scholar]
- 29.Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 1998;14:263–283 [DOI] [PubMed] [Google Scholar]
- 30.Schmidt-Schweda S, Holubarsch C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci (Lond) 2000;99:27–35 [PubMed] [Google Scholar]
- 31.Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 2002;87:3023–3028 [DOI] [PubMed] [Google Scholar]
- 32.Grefhorst A, Hoekstra J, Derks TGJ, et al. Acute hepatic steatosis in mice by blocking β-oxidation does not reduce insulin sensitivity of very-low-density lipoprotein production. Am J Physiol Gastrointest Liver Physiol 2005;289:G592–G598 [DOI] [PubMed] [Google Scholar]
- 33.Duivenvoorden I, Teusink B, Rensen PC, et al. Acute inhibition of hepatic beta-oxidation in APOE*3Leiden mice does not affect hepatic VLDL secretion or insulin sensitivity. J Lipid Res 2005;46:988–993 [DOI] [PubMed] [Google Scholar]
- 34.Buettner R, Ottinger I, Schölmerich J, Bollheimer LC. Preserved direct hepatic insulin action in rats with diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab 2004;286:E828–E833 [DOI] [PubMed] [Google Scholar]
- 35.Heijboer AC, Donga E, Voshol PJ, et al. Sixteen hours of fasting differentially affects hepatic and muscle insulin sensitivity in mice. J Lipid Res 2005;46:582–588 [DOI] [PubMed] [Google Scholar]
- 36.Wendel AA, Li LO, Li Y, Cline GW, Shulman GI, Coleman RA. Glycerol-3-phosphate acyltransferase 1 deficiency in ob/ob mice diminishes hepatic steatosis but does not protect against insulin resistance or obesity. Diabetes 2010;59:1321–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med 2003;9:756–761 [DOI] [PubMed] [Google Scholar]
- 38.Coccurello R, Caprioli A, Bellantuono S, et al. Effects of the increase in neuronal fatty acids availability on food intake and satiety in mice. Psychopharmacology (Berl) 2010;210:85–95 [DOI] [PubMed] [Google Scholar]
- 39.Bennett MJ, Boriack RL, Narayan S, Rutledge SL, Raff ML. Novel mutations in CPT 1A define molecular heterogeneity of hepatic carnitine palmitoyltransferase I deficiency. Mol Genet Metab 2004;82:59–63 [DOI] [PubMed] [Google Scholar]
- 40.Prip-Buus C, Thuillier L, Abadi N, et al. Molecular and enzymatic characterization of a unique carnitine palmitoyltransferase 1A mutation in the Hutterite community. Mol Genet Metab 2001;73:46–54 [DOI] [PubMed] [Google Scholar]
- 41.Stoler JM, Sabry MA, Hanley C, Hoppel CL, Shih VE. Successful long-term treatment of hepatic carnitine palmitoyltransferase I deficiency and a novel mutation. J Inherit Metab Dis 2004;27:679–684 [DOI] [PubMed] [Google Scholar]
- 42.Foretz M, Hébrard S, Leclerc J, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 2010;120:2355–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]