Abstract
Rationale
Tramadol is an atypical, mixed-mechanism analgesic involving both opioid and catecholamine processes that appears to have low abuse potential and may be useful as a treatment for opioid dependence.
Objectives
The current study assessed the level of physical dependence and opioid blockade efficacy produced by daily maintenance on oral tramadol.
Methods
Nine residential opioid-dependent adults were maintained on two doses of daily oral tramadol (200 and 800 mg) for approximately 4-week intervals in a randomized, double-blind, crossover design. The acute effects of intramuscular placebo, naloxone (0.25, 0.5, and 1.0 mg), and hydromorphone (1.5, 3.0, and 6.0 mg) were tested under double-blind, randomized conditions. Outcomes included observer- and subject-rated measures and physiologic indices.
Results
Challenge doses of naloxone resulted in significantly higher mean peak withdrawal scores compared to placebo. Withdrawal intensity from naloxone was generally greater during 800 versus 200 mg/day tramadol maintenance. Mean peak ratings of agonist effects were elevated at higher hydromorphone challenge doses, but did not differ significantly between tramadol doses. Physiologic measures were generally affected by challenge conditions in a dose-dependent manner, with few differences between tramadol maintenance dose conditions.
Conclusions
Chronic tramadol administration produces dose-related opioid physical dependence, without producing dose-related attenuation of agonist challenge effects. Tramadol may be a useful treatment for patients with low levels of opioid dependence or as a treatment for withdrawal during opioid detoxification, but does not appear to be effective as a maintenance medication due to a lack of opioid cross-tolerance.
Keywords: Tramadol, Opioid, Dependence, Withdrawal, Naloxone, Treatment, Hydromorphone, Blockade, Abuse
Introduction
Tramadol is an atypical, centrally acting synthetic analgesic used to treat moderate to severe pain, with antinociceptive effects that are mediated by a combination of mu-opioid agonist effects and norepinephrine and serotonin reuptake inhibition (Kayser et al. 1992; Raffa et al. 1992; Driessen et al. 1993; Desmeules et al. 1996). In its parent form, tramadol exists as a racemic mixture of two active enantiomers which undergo hepatic biotransformation to form N- and O-demethylated compounds (Raffa et al. 1993). The O-demethylated metabolite, (+)-O-demethyltramadol (known as M1), has greater affinity for the mu-opioid receptor than the parent compound and is primarily responsible for tramadol’s mu-opioid activity; however, this activity is relatively weak in comparison to full mu-agonists (Gillen et al. 2000; Raffa 2008). M1 has been shown to possess one tenth the affinity for the mu-receptor as morphine (Frink et al. 1996; Gillen et al. 2000), and in humans parenteral tramadol is approximately one tenth as potent as parenteral morphine in producing analgesia (Gutstein and Akil 2001) and 1/20 as potent as parenteral morphine in producing prototypic subjective opioid agonist effects (Epstein et al. 2006; Preston et al. 1991).
Tramadol has been widely prescribed for over 40 years in many countries with limited evidence of diversion and abuse. Despite its mu-opioid activity, tramadol was approved as an unscheduled analgesic in the USA in 1994 based largely on epidemiologic experience, and a number of animal (Miranda and Pinardi 1998; Murano et al. 1978; Yanagita 1978) and human (Cami et al. 1994; Jasinski et al. 1993; Preston and Jasinski 1989; Preston et al. 1991; Richter et al. 1985) studies that suggested, it had low abuse potential. Postmarketing surveillance in the USA has shown that abuse and diversion of tramadol has remained low even as new formulations and generic versions have become available (Cicero et al. 1999, 2005; Inciardi et al. 2006; Woody et al. 2003).
Although tramadol is viewed as being a relatively weak opioid with a low potential for abuse, studies in humans suggest that it does possess mu-agonist activity. Studies in experienced, but nondependent drug users have shown that doses of tramadol ranging from the therapeutic to supratherapeutic can induce miosis, a prototypic mu-agonist effect, and result in significantly elevated opiate-like reinforcing subjective effects (Epstein et al. 2006; Preston et al. 1991; Zacny 2005). Retrospective, prospective, and controlled clinical designs have indicated that tramadol may be effective in alleviating spontaneous opioid withdrawal, although doses higher than those typically prescribed for analgesia may be required (Salehi et al. 2005; Sobey et al. 2003; Tamaskar et al. 2003; Threlkeld et al. 2006; Carroll et al. 2006; Lofwall et al. 2007).
Despite evidence that repeated use of tramadol may lead to physical dependence, no well-controlled studies assessing tramadol’s ability to produce opioid physical dependence in humans have been conducted. There have been case reports of patients with apparent physical dependence on tramadol, as manifested by withdrawal upon cessation of tramadol use (e.g., Barsotti et al. 2003; Yates et al. 2001; Freye and Levy 2000; Ehrenreich and Poser 1993; Senay et al. 2003). Tramadol’s physical dependence capacity is important to quantify, as it provides one index of tramadol’s opioid agonist effects, is informative regarding tramadol’s abuse potential when used as an analgesic, and also can provide data relevant to its potential development as a treatment for opioid dependence. If tramadol is to be developed as a potential treatment for opioid dependence, an assessment of its ability to attenuate opioid agonist effects is also warranted. Effective opioid treatment medications such as methadone provide both suppression of withdrawal and cross-tolerance to the effects of other opioids. The purpose of the current study was to evaluate the level of physical dependence and opioid blockade efficacy produced by daily administration of tramadol, utilizing a randomized, placebo-controlled, within-subject, crossover design.
Materials and methods
Participants
The study was approved by the Institutional Review Board for human research, and all participants provided written informed consent and were paid for their participation. All tested positive for opiates on urine toxicology and were diagnosed with Opioid Dependence using the Structured Clinical Interview for DSM-IV (Spitzer et al. 1992). Volunteers underwent routine medical screening, which included a history and physical examination, ECG, serum chemistry and hematology, and urinalysis testing. Exclusion criteria included current physical dependence on alcohol or a sedative/hypnotic drug, major mental illness (e.g., schizophrenia), significant medical problem (e.g., history of seizure or hypertension), and pregnancy. Participants were informed that the study was testing whether tramadol might be helpful for treatment of opioid dependence and that they would be maintained on morphine for about a week and a half and tramadol for the remainder of the study, and they were informed that their morphine and tramadol doses might change without their knowledge. Twenty participants enrolled; nine completed (Table 1) and 11 left the study for a variety of reasons, including withdrawal discomfort, personal reasons, inconsistent data responses, and minor medical issues.
Table 1.
Participant characteristics
| Variablea | Total (N=9) |
|---|---|
| Age (years) | 35.7 (± 2.4) |
| Sex (% male) | 78 |
| Race (% Caucasian) | 78 |
| Education (years) | 11.3 (± 0.6) |
| Marital status (% single) | 67 |
| Opiate use | |
| Years since 1st heroin use | 11.7 (± 1.8) |
| Days of heroin use in the last 30 days | 28.9 (± 0.7) |
| I.V. users (%) | 89 |
| Amount spent on heroin/day ($) | 18.9 (± 4.6) |
| Lifetime use (years) | 6.6 (± 2.2) |
| Cocaine useb | |
| Years since 1st cocaine use | 15.0 (± 2.2) |
| Days of cocaine use in the past 30 days | 12.2 (± 4.2) |
| I.V. users (%) | 44 |
Values shown are means (± SEM) for continuous measures, except where otherwise indicated
N=8; one participant did not use cocaine
Study setting
Participants resided on a closed 14-bed residential unit for approximately 10 weeks. Random urine and breath samples were collected and tested with an on-site enzyme multiplied immunoassay technique toxicology system (Behring Diagnostics, San Jose, CA, USA) and an Alco-Sensor IV breathalyzer (AlcoPro, Knoxville, TN, USA) for illicit drugs and alcohol, respectively. No tests were positive. Participants were maintained on a caffeine free diet and were allowed to smoke ad lib up to 45 min prior to sessions.
Study design and procedure
This was a randomized, placebo-controlled, within-subjects crossover study consisting of two phases. In phase 1, participants were administered subcutaneous (SC) morphine 15 mg QID (0700, 1200, 1700, and 2200 hours). Following at least 7 days of morphine administration, participants underwent two experimental sessions to verify their capacity to report measurable precipitated opioid withdrawal effects. During these sessions, participants received either placebo or 0.5 mg intramuscular (IM) naloxone, in double-blind, randomized order. Evidence of precipitated withdrawal was based on results from three measures: (1) pupil diameter increase >0.5 mm from baseline, (2) visual analog scale (VAS) ratings of Bad Effects peak score ≥30, and (3) observer ratings of withdrawal signs ≥5 (this measure is described below). Participants who met criteria for two of the three measures in response to naloxone and not in response to placebo entered the second study phase. Criteria for entering phase 2 were not known by participants or by staff performing assessments. One of the 20 participants did not qualify for phase 2 due to inconsistent data responses during phase 1. In phase 2, participants were maintained for approximately 4-week durations on two different dose levels of daily oral tramadol; the order of tramadol doses was randomized. The doses tested were 200 mg/day (50 mg QID), a typical analgesic dose, and 800 mg/day (200 mg QID), a relatively high dose that is double the recommended daily dose, and was expected to provide discernable opioid agonist effects in opioid-dependent participants. Participants were maintained on each maintenance tramadol dose for at least 7 days before starting a series of seven experimental sessions conducted at least 48 h apart (i.e., maximum of three sessions per week). Each session tested one of seven conditions via IM injection: naloxone (0.25, 0.5, 1.0 mg), hydromorphone (1.5, 3.0, 6.0 mg), and placebo. An additional eighth session was permitted if methodological problems (e.g., missing data or computer error) required repeating a session. While category of challenge medication (i.e., naloxone, hydromorphone, or placebo) was randomized, naloxone and hydromorphone were administered in ascending doses, permitting discontinuation if strong antagonist or agonist effects were experienced at lower doses. Only two participants failed to receive all seven experimental conditions; one participant did not receive the 1.0-mg naloxone challenge and the other did not receive the 6.0-mg hydromorphone challenge, both while on 800-mg/day tramadol.
Drugs
Tramadol HCl (Ortho-McNeil Pharmaceuticals, Raritan, NJ, USA), naloxone HCl (Endo Laboratories, Chadds Ford, PA, USA), hydromorphone (Abbott Laboratories, Abbott Park, IL, USA), and morphine sulfate (Baxter Healthcare Corporation, Deerfield, IL, USA) were obtained from commercial sources. Tramadol was administered under an investigator-sponsored Investigational New Drug Application (IND) from the Food and Drug Administration (IND #69,537). Tramadol tablet(s) (50 mg each) were overencapsulated along with lactose monohydrate powder, N.F. (Ruger Chemical Co., Irvington, NJ, USA) in size 0 capsules (Capsugel, Greenwood, SC, USA). Each tramadol dose (50 and 200 mg) contained the same number of capsules (one capsule for each dose; four capsules per day) to maintain the blind. Capsules without tramadol (placebo) contained lactose monohydrate powder, N.F. (Ruger Chemical Co., Irvington, NJ, USA). All injected drugs were aseptically prepared under a laminar flow hood by filtering the solution through a 0.22-micron Millex-GS Millipore filter (Millipore Products Division, Bedford, MA, USA) into a sterile, pyrogen-free vial (American Pharmaceutical Partners, Los Angeles, CA, USA). Naloxone doses were prepared from a 0.4-mg/ml solution, and morphine doses were prepared from a 15-mg/ml solution; both were diluted with bacteriostatic 0.9% saline for injection (Hospira, Inc., Lake Forest, IL, USA) using sterile pyrogen-free plastic syringes (Becton Dickinson & Company, Franklin Lakes, NJ, USA) to achieve the desired doses in a 1-ml volume.
Experimental sessions
Sessions took place in a quiet room separate from the residential unit, and participants were monitored continuously throughout the test session by a research assistant and/or nursing staff. The room contained two chairs, an Apple computer, circular lights apparatus, and physiologic monitoring equipment. Subjective and observer measures along with cognitive performance measures were presented on the computer screen, and responses were entered using a key pad or mouse. Physiologic measures were recorded directly to the computer.
Sessions lasted 4 h and ran from approximately 0830 to 1230 hours; at 0900 hours, one of the two (phase 1) or seven (phase 2) experimental drug conditions was administered (on session days, morphine or tramadol doses normally scheduled at 1200 hours were moved to 1330 hours). Thirty minutes of data were collected prior to drug administration; data from 15 min prior to drug administration served as baseline data for analyses. Data collection continued for 210 min after drug administration. Measures described below were collected every 15 min throughout each session except for heart rate, systolic and diastolic blood pressure, oxygen saturation, and skin temperature, which were collected every minute and then averaged across 15-min intervals.
Measures
Measures collected included physiologic assessments (e.g., blood pressure, pupil diameter) and VAS items, participant- and observer-rated adjective scales, a participant-rated Behavioral Pharmacology Research Unit (BPRU) opioid withdrawal scale (BOWS), and an observer-rated pharmacologic class questionnaire (these have been described in detail in prior studies; Lofwall et al. 2007; Strain et al. 2000).
Data analysis
Separate analyses were conducted for the morphine and tramadol maintenance phases. Depending upon the specific dependent variable, either mean maximum values or mean minimum values were derived and used in statistical analyses. Data for the initial morphine maintenance phase were analyzed with a repeated measures regression model with a spherical covariance structure since only two conditions were being compared. Data for the subsequent randomized-dose tramadol maintenance phase were analyzed using a repeated measures regression model with an autoregressive covariance structure with a lag of 1, which models a dose-related relationship. Tukey’s post hoc tests were used to compare the 200- and 800-mg tramadol maintenance conditions at each challenge dose and to compare placebo versus active challenge doses within each tramadol maintenance condition.
Analyses were conducted using SAS PROC Mixed, version 9.1. The repeated measures regression model allows for the inclusion of participants with incomplete data. p values below 0.05 were considered statistically significant.
Results
Visual analog scale items
Mean peak values for the VAS items are shown in Fig. 1 for the morphine and tramadol maintenance phases. Results of challenge with 0.5 mg of naloxone during morphine maintenance indicated that participants experienced strong precipitated opioid withdrawal effects, as evidenced by significant increases versus placebo in the items Any Drug Effects (p=0.009), Bad Effects (p=0.001), and Feel Sick (p=0.01).
Fig. 1.
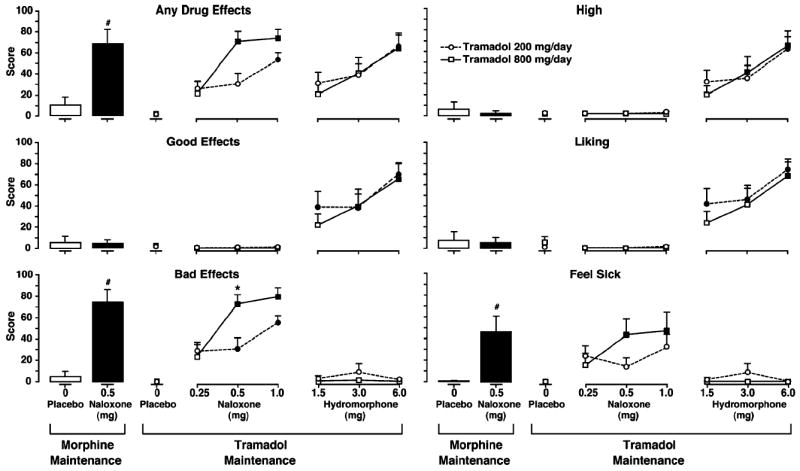
Mean peak VAS drug effect scores. Results following IM placebo and naloxone challenges during SC morphine maintenance are shown on the left, and results following IM naloxone and hydromorphone challenges during 200 and 800 mg/day oral tramadol maintenance are shown on the right. Tramadol 200 mg/day is represented by circles, and tramadol 800 mg/day is represented by squares. A number sign indicates a significant difference from placebo following naloxone challenge during morphine maintenance. A darkened symbol indicates a significant difference from placebo during tramadol maintenance, and an asterisk indicates a significant difference between tramadol dosing conditions. Statistical significance was set at p<0.05. Vertical bars represent the SEM
During tramadol maintenance, VAS results indicated that withdrawal intensity was related to both naloxone challenge dose and tramadol maintenance dose. As shown in Fig. 1, the highest mean peak VAS ratings of Any Drug Effects, Bad Effects, and Feel Sick occurred following challenge with 0.5 and 1.0 mg naloxone during tramadol 800 mg/day, and these scores were similar in magnitude to those observed following 0.5 mg naloxone during morphine maintenance. Mean peak ratings of Any Drug Effects, Bad Effects, and Feel Sick were significantly elevated versus placebo for both 0.5 and 1.0 mg naloxone during 800 mg/day tramadol (all p≤0.004). However, during 200 mg/day tramadol, only Bad Effects was significantly increased following both 0.5 and 1.0 mg naloxone doses (p≤0.026), while Any Drug Effects was significantly elevated only in response to 1.0 mg naloxone (p=0.004). Mean peak scores of Any Drug Effects, Bad Effects, and Feel Sick were all numerically higher following 0.5 and 1.0 mg naloxone for the 800- versus 200-mg/day conditions, although the only significant difference between the two maintenance doses was observed for 0.5 mg naloxone on the Bad Effects item.
Peak values for VAS items indicative of agonist effects were elevated in a clear dose–response fashion, as successively higher doses of hydromorphone resulted in higher scores of Any Drug Effects, Good Effects, High, and Liking (Fig. 1). During 200 mg/day tramadol, relative to placebo challenge, mean peak ratings of Good Effects and Liking were significantly elevated following all three hydromorphone challenge doses (p≤0.046), and High and Any Drug Effects were significantly elevated following only 6.0 mg hydromorphone (p≤0.0001). During 800 mg/day tramadol, Good Effects and High were significantly elevated versus placebo following 3.0 and 6.0 mg hydromorphone (p≤0.035), and Liking and Any Drug Effects were significantly elevated following only 6.0 mg hydromorphone (p≤0.001). Ratings on these items following hydromorphone challenges did not significantly differ between the two tramadol maintenance doses.
Opioid adjective rating questionnaire and BOWS
Mean maximum and minimum values and results of post hoc analyses for participant- and observer-rated and physiological measures are presented in Table 2. During morphine maintenance, 0.5 mg naloxone produced significantly elevated participant- and observer-rated antagonist scale scores relative to placebo challenge (p=0.001 and p=0.007, respectively), and significantly increased the observer-rated BOWS (p<0.001; Fig. 2, top panel), indicating substantial precipitated withdrawal effects.
Table 2.
Summary of mean maximum and minimum values following drug challenge
| Measure | Drug condition (mg) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morphine |
Tramadol 200 |
Tramadol 800 |
||||||||||||||
| Placebo | Naloxone | Placebo | Naloxone |
Hydromorphone |
Placebo | Naloxone |
Hydromorphone |
|||||||||
| 0 | 0.5 | 0 | 0.25 | 0.5 | 1.0 | 1.5 | 3.0 | 6.0 | 0 | 0.25 | 0.5 | 1.0 | 1.5 | 3.0 | 6.0 | |
| Maximum values | ||||||||||||||||
| Antagonist adjective scale | ||||||||||||||||
| Observer rated | 2.1 | 19.7a | 1.8 | 7.8 | 10.0 | 14.3d,e | 1.8 | 2.6 | 2.9 | 2.0 | 10.1 | 19.0d | 26.1d,e | 1.6 | 2.2 | 1.8 |
| Participant rated | 2.2 | 26.3a | 2.0 | 9.8 | 11.3 | 17.7d | 1.7 | 4.2 | 3.8 | 1.2 | 10.6 | 18.3d | 24.8d | 1.8 | 2.3 | 1.7 |
| Agonist adjective scale | ||||||||||||||||
| Observer rated | 12.7 | 12.4 | 12.6 | 13.0 | 13.4 | 12.3 | 16.0 | 17.1 | 22.6d | 12.7 | 12.8 | 13.7 | 10.8 | 14.4 | 15.6 | 22.7d |
| Participant rated | 15.8 | 16.0 | 13.4 | 13.9 | 12.8 | 14.2 | 18.6 | 18.1 | 24.3d | 11.8 | 13.0 | 13.3 | 13.0 | 15.3 | 17.0 | 20.9d |
| Observer-rated BOWS | ||||||||||||||||
| Lacrimation | 0.2 | 1.4a | 0.1 | 1.6d | 1.2d | 1.6d | 0 | 0.2 | 0.4 | 0 | 1.2d | 1.9d | 2.0d | 0 | 0.2 | 0 |
| Rhinorrhea | 0.1 | 1.6a | 0 | 0.9d | 0.8d,e | 1.7d | 0 | 0 | 0.2 | 0 | 0.9d | 1.8d,e | 2.0d | 0.2 | 0 | 0 |
| Perspiration | 0.1 | 1.2a | 0.2 | 0.4 | 0.7 | 0.8 | 0.2 | 1.1 | 0.2 | 0.2 | 0.6 | 1.4 | 1.4 | 0.2 | 0.6 | 0.7 |
| Gooseflesh | 0 | 0.8a | 0 | 0.3 | 0.6 | 1.1d | 0 | 0 | 0.2 | 0 | 0.1 | 0.9d | 2.0d | 0 | 0.2 | 0 |
| Bowel sounds | 1.7 | 1.7 | 1.2 | 1.6 | 1.4 | 1.6 | 1.2 | 1.6 | 1.3 | 1.4 | 1.6 | 1.8 | 1.4 | 1.4 | 1.4 | 1.5 |
| Yawning | 0.8 | 1.9a | 0.9 | 1.4 | 1.8d | 1.9d | 0.1 | 0.0d | 0.4 | 0.7 | 1.4 | 2.0d | 2.0d | 0.2 | 0.1 | 0 |
| Restlessness | 0.7 | 1 | 0.2 | 1.0 | 1.1d | 0.9 | 0.1 | 0.3 | 0.7 | 0.4 | 0.6 | 1.3d | 1.7d | 0.4 | 0.3 | 0.3 |
| Total BOWS | 2.4 | 8.6a | 2.2 | 5.7d | 6.6d,e | 7.8d,e | 1.6 | 3.1 | 2.6 | 2.4 | 5.9d | 10.1d,e | 11.9d,e | 2.0 | 2.4 | 2.2 |
| Visual analog scale | ||||||||||||||||
| High | 6.1 | 2.2 | 0.8 | 0 | 0 | 1.0 | 29.8 | 33.0d | 60.8d | 0 | −1.1 | 0 | −2.3 | 17.7 | 38.3d | 62.2d |
| Drug Effect | 10.3 | 68.6a | 1.2 | 25.3 | 30.2e | 53.1d | 31.0 | 38.6 | 66.3d | 1.6 | 20.0 | 70.7d,e | 72.6d | 20.6 | 40.2d | 62.4d |
| Good Effects | 5.4 | 4.3 | 1.1 | 0.1 | 0.3 | 0.9 | 38.7d | 38.4d | 70.1d | 1.6 | −1.3 | 0 | −2.6 | 21.8 | 40.0d | 64.1d |
| Bad Effects | 4.8 | 75.2a | 0 | 28.1d | 30.2d,e | 55.2d | 2.6 | 8.6 | 1.4 | 0.0 | 22.6 | 72.6d,e | 80.3d | 0.4 | 0.9 | −0.6 |
| Liking | 7.4 | 5.11 | 0.8 | 0.2 | 0 | 1.3 | 41.3d | 45.6d | 73.6d | 5.3 | −1.2 | 0 | −3.0 | 23.3 | 40.3d | 65.9d |
| Feel sick | 0.4 | 46.0a | 0 | 23.2 | 13.8 | 31.8d | 1.9 | 8.2 | 0.0 | 0 | 14.2 | 43.2d | 48.6d | 0 | 0 | −0.9 |
| Physiological measures | ||||||||||||||||
| Pupil diameterb (mm) | 1.0 | 1.3 | 0.2 | 0.7 | 0.8 | 0.6 | 0 | −0.6 | −1.6d | 0.7 | 0.7 | 1.2 | 0.9 | −0.2d | −0.3d | −1.2d |
| Pulse (bpm) | 77.8 | 85.1a | 85.2 | 86.8 | 84.5e | 88.1 | 98.3 | 98.4 | 98.0 | 87.5 | 86.3 | 92.5e | 95.2 | 84.7 | 89.4 | 94.8 |
| Skin temperature (°F) | 86.9 | 86.5 | 87.3 | 86.0 | 87.8 | 84.9 | 84.2 | 85.0 | 87.4 | 88.3 | 85.9 | 85.8 | 87.0 | 89.6 | 90.1 | 90.3 |
| Systolic BP (mmHg) | 111.4 | 120.1a | 115.2 | 118.6 | 118.6e | 122.0e | 89.1 | 90.6 | 92.0d | 121.7 | 123.4 | 129.1e | 138.3d,e | 120.3 | 125.3 | 129.4 |
| Diastolic BP (mmHg) | 61.2 | 67.3a | 66.9 | 65.9e | 70.0e | 71.4e | 117.6 | 118.5 | 125.2 | 72.2 | 73.5e | 79.5d,e | 85.0d,e | 72.0 | 75.5 | 76.5 |
| Minimum values | ||||||||||||||||
| Physiological measures | ||||||||||||||||
| Pupil diameterc (mm) | −0.2 | 0 | −0.7 | −0.5 | −0.5 | −0.6 | −1.3 | −1.9d | −2.5d | −0.6 | −0.4 | −0.3 | −0.7 | −1.4 | −2.0d | −2.2d |
| Oxygen saturation (%) | 97.3 | 97.4 | 97.4 | 97.3 | 97.2 | 97.5 | 96.9 | 96.8 | 95.8d | 97.2 | 97.5 | 96.9 | 96.9 | 96.9 | 96.6 | 96.0 |
| Respiratory rate (bpm) | 13.6 | 14.4 | 13.6 | 14.2 | 13.6 | 13.6 | 12.2 | 11.6 | 11.6 | 12.7 | 13.0 | 12.7 | 12.6 | 12.4 | 12.0 | 9.9 |
Routes of drug administration for daily maintenance and challenge sessions were as follows: morphine 60 mg/day (15 mg QID)—subcutaneous injection; tramadol 200 mg/day (50 mg QID) and 800 mg/day (200 mg QID)—oral; placebo, naloxone, and hydromorphone challenges—intramuscular injection. Variance results were not included due to space limitations but are available upon request
Significant difference (p<0.05) from placebo challenge during morphine maintenance
The mean maximum increase in pupil diameter from baseline
The mean maximum decrease in pupil diameter from baseline
Significant difference (p<0.05) from placebo challenge during tramadol maintenance
Significant difference (p<0.05) between tramadol dose conditions
Fig. 2.
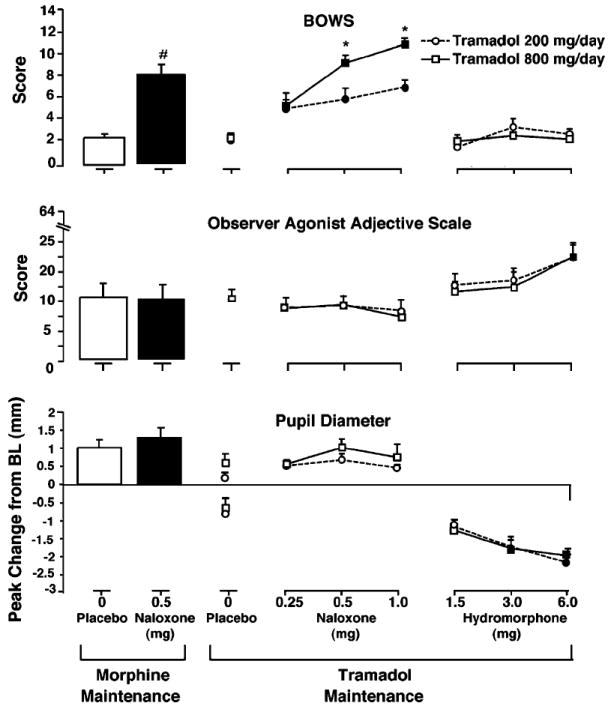
Mean peak scores on the BPRU opioid withdrawal scale (BOWS) and observer agonist adjective scale are shown in the top and middle panels, respectively. Mean peak increase (above abscissa) and decrease (below abscissa) from baseline in pupil diameter is shown in the bottom panel. Results following IM placebo and naloxone challenges during SC morphine maintenance are shown on the left, and results following IM naloxone and hydromorphone challenges during 200 and 800 mg/day oral tramadol maintenance are shown on the right. Tramadol 200 mg/day is represented by circles, and tramadol 800 mg/day is represented by squares. A number sign indicates a significant difference from placebo following naloxone challenge during morphine maintenance. A darkened symbol indicates a significant difference from placebo during tramadol maintenance, and an asterisk indicates a significant difference between tramadol dosing conditions. Statistical significance was set at p<0.05. Vertical bars represent the SEM
During tramadol maintenance, participant-rated antagonist scale scores indicated that withdrawal intensity was related to both naloxone challenge dose and tramadol maintenance dose. The highest mean peak participant-rated antagonist scale scores occurred following challenge with 0.5 and 1.0 mg naloxone during tramadol 800 mg/day (Table 2), and these scores were similar in magnitude to the mean peak score observed following 0.5 mg naloxone during morphine maintenance. Participant-rated antagonist scale scores were significantly elevated versus placebo in response to 0.5 and 1.0 mg naloxone (both p<0.001) during 800 mg/day tramadol; however, only 1.0 mg naloxone resulted in a significant elevation of scores (p=0.001) during 200 mg/day tramadol. Although mean peak scores following each dose of naloxone tested were numerically higher for 800 versus 200 mg/day tramadol, there were no statistically significant differences between the two tramadol maintenance doses.
Observer-rated antagonist scale scores followed a similar pattern as the participant-rated scores (see Table 2). Mean peak scores on this scale during 800 mg/day tramadol were numerically higher than 200 mg/day tramadol at each dose of naloxone tested and increased as naloxone dose increased. During 800 mg/day tramadol, mean peak scores following 0.5 and 1.0 mg naloxone were similar in magnitude to the mean peak score observed following 0.5 mg naloxone during morphine maintenance. Following 0.5 and 1.0 mg naloxone, there were significant increases versus placebo (both p<0.001) for 800 mg/day tramadol; however, for 200 mg/day tramadol, only the highest dose of naloxone resulted in a significant increase in peak score (p=0.005). Further, mean peak observer-rated antagonist scale score following 1.0 mg naloxone during 800 mg/day tramadol was significantly elevated as compared to 200 mg/day tramadol (p=0.023). There were no significant differences between the two doses of tramadol at either of the lower doses of naloxone.
As shown in Fig. 2 (top panel), observer-rated total BOWS scores were also related to naloxone and tramadol dose. For both tramadol maintenance doses, total BOWS scores were significantly elevated versus placebo following each of the three challenge doses of naloxone (all p≤0.023). Although mean peak BOWS scores for 800 and 200 mg/day tramadol were practically identical following the 0.25-mg naloxone dose, challenge with 0.5 and 1.0 mg naloxone resulted in significantly elevated BOWS scores for 800 versus 200 mg/day tramadol (p=0.013 and p= 0.005, respectively). Total BOWS scores for 800 mg/day tramadol following 0.5 and 1.0 mg naloxone were slightly higher in magnitude than the mean peak BOWS score observed following 0.5 mg naloxone during morphine maintenance, which itself was slightly higher than the scores observed for 200 mg/day tramadol.
Participant- and observer-rated agonist adjective scale results for 200 and 800 mg/day tramadol followed similar patterns in response to hydromorphone challenges. Relative to placebo, participant- and observer-rated scores (Fig. 2, middle panel) increased modestly in response to the two lowest doses of hydromorphone (1.5 and 3.0 mg), with no statistical differences observed between 200 and 800 mg/day tramadol. Administration of 6.0 mg hydromorphone resulted in significant and practically identical increases in agonist adjective scale scores versus placebo during each maintenance dose of tramadol, on both the participant- and observer-rated scales (all p≤0.002).
Physiologic measures
Mean maximum and minimum data for physiologic measures are summarized in Table 2. Physiological measures were generally affected by challenge conditions in a dose-dependent manner, with few differences between tramadol maintenance dose conditions. As shown in Fig. 2 (bottom panel), pupil diameter (calculated as maximum increases and decreases from baseline) was not significantly affected by any challenge doses of naloxone for either tramadol dose condition, relative to placebo. Challenge with hydromorphone resulted in dose-dependent maximum decreases in pupil diameter that were similar for both tramadol dose conditions, with significant decreases relative to placebo challenge observed following 3.0 and 6.0 mg hydromorphone (all p≤0.001).
Compared with placebo challenge, administration of 0.5 mg naloxone during morphine maintenance significantly elevated pulse and systolic and diastolic blood pressure (see Table 2). During 800 mg/day tramadol dosing, challenge with 0.5 and 1.0 mg naloxone resulted in several significant differences relative to placebo challenge and to 200 mg/day tramadol in these variables, particularly in peak systolic and diastolic blood pressure.
For all challenge conditions (naloxone and hydromorphone), there were no significant differences between the two tramadol maintenance conditions in pupil diameter, oxygen saturation, skin temperature, and respiration rate. Oxygen saturation decreased significantly relative to placebo in response to the 6.0-mg hydromorphone challenge during 200 mg/day tramadol (p≤0.001), but not during the 800-mg/day tramadol maintenance dose.
Discussion
This study evaluated the level of physical dependence and blockade efficacy produced by maintenance on two dose levels of the mixed-mechanism analgesic tramadol in opioid-dependent volunteers. Overall, naloxone-precipitated withdrawal was observed to occur, and its intensity was related to both tramadol maintenance dose and naloxone challenge dose. Elevations in participant- and observer-rated measures of antagonist effects that were observed in response to dosing with 0.5 and 1.0 mg naloxone during 800 mg/day tramadol maintenance tended to be similar in magnitude to scores observed following 0.5 mg naloxone during maintenance on 60 mg/day (15 mg QID) SC morphine. However, neither maintenance dose of tramadol appeared to provide discernible opioid agonist blockade effects in response to challenges with hydromorphone. Scores on participant- and observer-rated measures of agonist effects were elevated in a clear dose–response manner following successively higher doses of hydromorphone, with no significant differences observed between tramadol maintenance conditions following any hydromorphone dose.
Unlike previous work performed by Jasinski et al. (1993) and Preston et al. (1991) that focused exclusively on characterizing the acute effects of parenterally and orally administered tramadol in nondependent opioid abusers, the current investigation is the first to systematically assess the ability of different doses of tramadol to produce opioid physical dependence and opioid blockade effects in human volunteers when administered daily for a sustained period. The results suggest that although tramadol may exhibit weak opioid-like subjective effects in humans, it does possess the potential for physical dependence at a daily dose typically prescribed for the treatment of pain (i.e., 200 mg/day). The results of this study emphasize the importance of acute agonist effects, as opposed to physical dependence, as the critical determinant of abuse liability. Epidemiological experience with tramadol has confirmed it to be a drug of low abuse potential, yet the present human laboratory data (and some epidemiological data) show that repeated use can lead to physical dependence. Thus, the low abuse of tramadol is consistent with its modest and delayed opioid agonist effects more than with its physical dependence potential.
Lofwall et al. (2007) reported that acute administration of oral tramadol at doses of 200 and 400 mg produced evidence of opioid withdrawal suppression with a delayed onset of action relative to morphine in opioid-dependent volunteers experiencing spontaneous withdrawal. Other investigators have also reported that tramadol administration results in a slow onset and lack of robust opioid-like subjective, observer-rated, and physiological effects (Carroll et al. 2006; Epstein et al. 2006; Preston et al. 1991). Although previous studies have shown a relative lack of acute opioid-like effects in human participants, the results of the current study indicate that administration of daily tramadol at a typical analgesic dose of 200 mg/day (50 mg QID) can lead to pronounced precipitated opioid withdrawal effects following challenge with naloxone and that opioid physical dependence can result from daily dosing with tramadol. Further, daily dosing with 800 mg/day (200 mg QID) tramadol, a relatively high dose that is similar to the reported maximum acute dose administered to humans (700 mg; Jasinski et al. 1993), resulted in discernibly greater precipitated withdrawal effects following challenge with 0.5 and 1.0 mg naloxone, and these effects were quite similar in magnitude to those observed following 0.5 mg naloxone challenge during dosing with 60 mg/day morphine. Thus, the development of opioid physical dependence from tramadol administration appears to be dose-related, and administration of 800 mg/day tramadol appears to lead to similar levels of opioid physical dependence as 60 mg/day morphine. It is possible that acute dosing regimens are not sufficient to produce pronounced opioid-like effects but that a sustained dosing regimen of tramadol results in the development of neural adaptations characteristic of other mu-agonists.
It was observed anecdotally by staff that, during the period between the morphine maintenance phase to either of the tramadol doses, some participants complained of mild spontaneous opioid withdrawal symptoms. No data were collected to support these observations, and no volunteers withdrew from the study due to these effects. Following this initial period, most volunteers described feeling no withdrawal during the remainder of the study and generally appeared to tolerate both doses of tramadol very well.
A lack of cross-tolerance was observed in this study, as evidenced by challenge doses of hydromorphone producing robust subjective increases in measures of liking and high and significant miosis regardless of daily tramadol dose. These results are in agreement with data from other studies suggesting that tramadol does not effectively block the effects of selective mu-agonists (Friderichs et al. 1978; Cami et al. 1994; Carroll et al. 2006). Taken together, the findings that daily tramadol administration leads to dose-dependent physical dependence with no evidence of opioid cross-tolerance indicate that tramadol may be useful in treating opioid withdrawal in individuals with low levels of physical dependence or as a detoxification agent, but would not be effective as a maintenance treatment.
There are limitations to this study. One limitation was not drawing blood samples for pharmacokinetic evaluations of tramadol and M1 levels. A more definitive relationship between levels of M1 in the blood and the magnitude of observed precipitated opioid withdrawal responses could have been derived from such analyses. A second limitation of the study was the omission of a hydromorphone challenge dose during the morphine maintenance phase. Therefore, no relative comparison can be made between the apparent lack of opioid blockade effects observed during tramadol dosing and what might have occurred following hydromorphone challenge during maintenance on morphine. A third limitation was that only nine of 20 consented participants completed the study, potentially limiting the generalizability of the results. However, previously published human laboratory within-subjects studies of similar design have clearly shown that sample sizes of five to eight subjects are adequate to produce consistent statistically significant effects across subject-rated, observer-rated, and physiologic measures (e.g., see Cami et al. 1994; Strain et al. 2000; Carroll et al. 2006). Lastly, subjects were opioid dependent prior to study enrollment, and daily tramadol dosing may have perpetuated physical dependence rather than produced it. The present study does not permit a distinction to be made between the production versus maintenance of opioid physical dependence.
In conclusion, this study is the first to show under controlled laboratory conditions that human volunteers can become physically dependent upon tramadol, that this dependence is mediated through opioid receptors, and that the level of physical dependence is related to the dose of tramadol administered. Evidence of physical dependence was observed following daily maintenance on a dose of tramadol typically prescribed to treat mild to moderate pain. The unique pharmacology of tramadol, in which hepatic biotransformation of the parent compound to an active metabolite is necessary to produce significant mu-agonist effects, likely explains why studies of acute dosing show minimal opioid-like effects, yet chronic dosing results in opioid physical dependence and withdrawal upon discontinuation. An accumulation of M1, which has a significant affinity for the mu-receptor likely leads to CNS adaptations that are typical of other mu-agonists. The lack of opioid cross-tolerance observed in this study indicates that tramadol’s potential usefulness as a treatment for opioid dependence may be limited to patients with a relatively low level of physical dependence and who do not require a significant acute opioid agonist effect to maintain medication compliance or as a treatment for withdrawal during opioid detoxification. Although tramadol does appear to have minimal abuse potential compared to more efficacious full mu-agonists such as hydromorphone, oxycodone, or heroin, it is apparent that repeated dosing with tramadol can produce opioid physical dependence that is characteristic of other opioids and that caution should be used when prescribing tramadol to those at risk of substance abuse.
Acknowledgments
The authors thank Elliot Joseph, Jessica Vanderhoff, Sonia Bansal, Mary Misenhimer, Sarah Ilk, John Yingling, Linda Felch, and the nursing staff at the Behavioral Pharmacology Research Unit for their assistance in volunteer recruitment, data collection, and analysis. This study complies with current laws of the USA.
Funding This work was supported by the National Institutes of Health/National Institute on Drug Abuse Grant R01DA018125 to Johns Hopkins University (ECS), Midcareer Investigator Award in Patient-Oriented Research K24D023186 (ECS), and Training Grant T32DA07209 to Johns Hopkins University.
Footnotes
Disclosure Tramadol was developed by Grünenthal. Dr. Strain is a paid consultant to Grünenthal. This arrangement is being managed by the Johns Hopkins University in accordance with its conflict of interest policies. In recent years, Dr. Bigelow has received consulting payments from Abbott Laboratories, Takeda Pharmaceuticals, and Teva Pharmaceuticals and through his university has received research support from Titan Pharmaceuticals and Pain Therapeutics, Inc. Dr. Lanier is now an employee of Rock Creek Pharmaceuticals.
Contributor Information
Ryan K. Lanier, Email: rlanier1@gmail.com, Rock Creek Pharmaceuticals, Inc., 55 Blackburn Center, Gloucester, MA 01930, USA.
Michelle R. Lofwall, Department of Psychiatry, College of Medicine, University of Kentucky, Lexington, KY, USA
Miriam Z. Mintzer, Department of Psychiatry and Behavioral Sciences, School of Medicine, Johns Hopkins University, Baltimore, MD, USA
George E. Bigelow, Department of Psychiatry and Behavioral Sciences, School of Medicine, Johns Hopkins University, Baltimore, MD, USA
Eric C. Strain, Department of Psychiatry and Behavioral Sciences, School of Medicine, Johns Hopkins University, Baltimore, MD, USA
References
- Barsotti CE, Mycyk MB, Reyes J. Withdrawal syndrome from tramadol hydrochloride. Am J Emerg Med. 2003;21:87–88. doi: 10.1053/ajem.2003.50039. [DOI] [PubMed] [Google Scholar]
- Cami J, Lamas X, Farre M. Acute effects of tramadol in methadone-maintained volunteers. Drugs. 1994;47(Suppl 1):39–43. doi: 10.2165/00003495-199400471-00007. [DOI] [PubMed] [Google Scholar]
- Carroll CP, Walsh SL, Bigelow GE, Strain EC, Preston KL. Assessment of agonist and antagonist effects of tramadol in opioid-dependent humans. Exp Clin Psychopharmacol. 2006;14:109–120. doi: 10.1037/1064-1297.14.2.109. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Adams EH, Geller A, Inciardi JA, Munoz A, Schnoll SH, Senay EC, Woody GE. A postmarketing surveillance program to monitor Ultram (tramadol hydrochloride) abuse in the United States. Drug Alcohol Depend. 1999;57:7–22. doi: 10.1016/s0376-8716(99)00041-1. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Inciardi JA, Adams EH, Geller A, Senay EC, Woody GE, Munoz A. Rates of abuse of tramadol remain unchanged with the introduction of new branded and generic products: results of an abuse monitoring system, 1994–2004. Pharmacoepidemiol Drug Saf. 2005;14:851–859. doi: 10.1002/pds.1113. [DOI] [PubMed] [Google Scholar]
- Desmeules JA, Piguet V, Collart L, Dayer P. Contribution of monoaminergic modulation to the analgesic effect of tramadol. Br J Clin Pharmacol. 1996;41:7–12. doi: 10.1111/j.1365-2125.1996.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Driessen B, Reimann W, Giertz H. Effects of the central analgesic tramadol on the uptake and release of noradrenaline and dopamine in vitro. Br J Pharmacol. 1993;108:806–811. doi: 10.1111/j.1476-5381.1993.tb12882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Poser W. Dependence on tramadol. Clin Investig. 1993;72:76. doi: 10.1007/BF00231123. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Jasinski DR. Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: lessons from tramadol. Biol Psychol. 2006;73:90–99. doi: 10.1016/j.biopsycho.2006.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freye E, Levy J. Acute abstinence syndrome following abrupt cessation of long-term use of tramadol (Ultram): a case study. Eur J Pain. 2000;4:307–311. doi: 10.1053/eujp.2000.0187. [DOI] [PubMed] [Google Scholar]
- Friderichs E, Felgenhauer F, Jongschaap P, Osterloh G. Pharmacological studies on analgesia, dependence on and tolerance of tramadol, a potent analgetic drug (author’s transl) Arzneimittelforschung. 1978;28:122–134. [PubMed] [Google Scholar]
- Frink M, Hennies HH, Englberger W, Haurand M, Wilffert B. Influence of tramadol on neurotransmitter systems of the rat brain. Arzneimittelforschung. 1996;46:1029–1036. [PubMed] [Google Scholar]
- Gillen C, Haurand M, Kobelt DJ, Wnendt S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human muopioid receptor. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:116–121. doi: 10.1007/s002100000266. [DOI] [PubMed] [Google Scholar]
- Gutstein H, Akil H. Opioid Analgesics. In: Hardman J, Limbird L, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 10. McGraw-Hill; New York: 2001. pp. 569–619. [Google Scholar]
- Inciardi JA, Cicero TJ, Munoz A, Adams EH, Geller A, Senay EC, Woody GE. The diversion of Ultram, Ultracet, and generic tramadol HCL. J Addict Dis. 2006;25:53–58. doi: 10.1300/J069v25n02_08. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Preston KL, Sullivan JT, Testa M. Abuse potential of oral tramadol. NIDA Res Monogr. 1993;132:103. [PubMed] [Google Scholar]
- Kayser V, Besson JM, Guilbaud G. Evidence for a noradrenergic component in the antinociceptive effect of the analgesic agent tramadol in an animal model of clinical pain, the arthritic rat. Eur J Pharmacol. 1992;224:83–88. doi: 10.1016/0014-2999(92)94822-d. [DOI] [PubMed] [Google Scholar]
- Lofwall MR, Walsh SL, Bigelow GE, Strain EC. Modest opioid withdrawal suppression efficacy of oral tramadol in humans. Psychopharmacology (Berl) 2007;194:381–393. doi: 10.1007/s00213-007-0847-3. [DOI] [PubMed] [Google Scholar]
- Miranda HF, Pinardi G. Antinociception, tolerance, and physical dependence comparison between morphine and tramadol. Pharmacol Biochem Behav. 1998;61:357–360. doi: 10.1016/s0091-3057(98)00123-3. [DOI] [PubMed] [Google Scholar]
- Murano T, Yamamoto H, Endo N, Kudo Y, Okada N, Masuda Y, Yano I. Studies on dependence on tramadol in rats. Arzneimittelforschung. 1978;28:152–158. [PubMed] [Google Scholar]
- Preston KL, Jasinski DR. Effects of tramadol in humans: assessment of its abuse potential. NIDA Res Monogr. 1989;95:392. [PubMed] [Google Scholar]
- Preston KL, Jasinski DR, Testa M. Abuse potential and pharmacological comparison of tramadol and morphine. Drug Alcohol Depend. 1991;27:7–17. doi: 10.1016/0376-8716(91)90081-9. [DOI] [PubMed] [Google Scholar]
- Raffa RB. Basic pharmacology relevant to drug abuse assessment: tramadol as an example. J Clin Pharm Ther. 2008;33:101–108. doi: 10.1111/j.1365-2710.2008.00897.x. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992;260:275–285. [PubMed] [Google Scholar]
- Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL, Jacoby HI, Selve N. Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J Pharmacol Exp Ther. 1993;267:331–340. [PubMed] [Google Scholar]
- Richter W, Barth H, Flohe L, Giertz H. Clinical investigation on the development of dependence during oral therapy with tramadol. Arzneimittelforschung. 1985;35:1742–1744. [PubMed] [Google Scholar]
- Salehi M, Amanatkar M, Barekatain M. Tramadol versus methadone for the management of acute opioid withdrawal: an add-on study. J Res Med Sci. 2005;11:185–189. [Google Scholar]
- Senay EC, Adams EH, Geller A, Inciardi JA, Munoz A, Schnoll SH, Woody GE, Cicero TJ. Physical dependence on Ultram (tramadol hydrochloride): both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol Depend. 2003;69:233–241. doi: 10.1016/s0376-8716(02)00321-6. [DOI] [PubMed] [Google Scholar]
- Sobey PW, Parran TV, Jr, Grey SF, Adelman CL, Yu J. The use of tramadol for acute heroin withdrawal: a comparison to clonidine. J Addict Dis. 2003;22:13–25. doi: 10.1300/j069v22n04_03. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The structured clinical interview for DSM-III-R (SCID). I. History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stoller K, Walsh SL, Bigelow GE. Effects of buprenorphine versus buprenorphine/naloxone tablets in non-dependent opioid abusers. Psychopharmacology (Berl) 2000;148:374–383. doi: 10.1007/s002130050066. [DOI] [PubMed] [Google Scholar]
- Tamaskar R, Parran TV, Jr, Heggi A, Brateanu A, Rabb M, Yu J. Tramadol versus buprenorphine for the treatment of opiate withdrawal: a retrospective cohort control study. J Addict Dis. 2003;22:5–12. doi: 10.1300/j069v22n04_02. [DOI] [PubMed] [Google Scholar]
- Threlkeld M, Parran TV, Adelman CA, Grey SF, Yu J. Tramadol versus buprenorphine for the management of acute heroin withdrawal: a retrospective matched cohort controlled study. Am J Addict. 2006;15:186–191. doi: 10.1080/10550490500528712. [DOI] [PubMed] [Google Scholar]
- Woody GE, Senay EC, Geller A, Adams EH, Inciardi JA, Schnoll S, Munoz A, Cicero TJ. An independent assessment of MED-Watch reporting for abuse/dependence and withdrawal from Ultram (tramadol hydrochloride) Drug Alcohol Depend. 2003;72:163–168. doi: 10.1016/s0376-8716(03)00198-4. [DOI] [PubMed] [Google Scholar]
- Yanagita T. Drug dependence potential of 1-(m-methoxyphenyl)-2-dimethylaminomethyl)-cyclohexan-1-ol hydrochloride (tramadol) tested in monkeys. Arzneimittelforschung. 1978;28:158–163. [PubMed] [Google Scholar]
- Yates WR, Nguyen MH, Warnock JK. Tramadol dependence with no history of substance abuse. Am J Psychiatry. 2001;158:964. doi: 10.1176/appi.ajp.158.6.964. [DOI] [PubMed] [Google Scholar]
- Zacny JP. Profiling the subjective, psychomotor, and physiological effects of tramadol in recreational drug users. Drug Alcohol Depend. 2005;80:273–278. doi: 10.1016/j.drugalcdep.2005.05.007. [DOI] [PubMed] [Google Scholar]


